Abstract
A series of NaTaO3 photocatalysts were prepared with Ta2O5 and NaOH via a hydrothermal method. CuO was loaded onto the surface of NaTaO3 as a cocatalyst by successive impregnation and calcination. The obtained photocatalysts were characterized by XRD, SEM, UV–vis, EDS and XPS and used to photocatalytically reduce CO2 in isopropanol. This worked to both absorb CO2 and as a sacrificial reagent to harvest CO2 and donate electrons. Methanol and acetone were generated as the reduction product of CO2 and the oxidation product of isopropanol, respectively. NaTaO3 nanocubes loaded with 2 wt % CuO and synthesized in 2 mol/L NaOH solution showed the best activity. The methanol and acetone yields were 137.48 μmol/(g·h) and 335.93 μmol/(g·h), respectively, after 6 h of irradiation. Such high activity could be attributed to the good crystallinity, morphology and proper amount of CuO loading, which functioned as reductive sites for selective formation of methanol. The reaction mechanism was also proposed and explained by band theory.
Introduction
Global warming is one of the most major environmental problems that we are facing in the 21st century [1]. Carbon dioxide (CO2) contributes significantly to global climate change as it is the main greenhouse gas present in the atmosphere and primarily formed from the consumption of fossil fuels [2]. To date, many methods have been proposed to reduce the emitted CO2 concentration. A particularly advantageous approach is the capture of CO2 from the atmosphere for the conversion to fuel by using a sustainable source of energy like sunlight. In this way, global warming and energy shortage problems can be solved simultaneously [3-7]. For this purpose, the photocatalytic conversion of CO2 to fuel is particularly emphasized.
In 1979, Inoue et al. [8] first reported the photocatalytic reduction of CO2 in aqueous solution using several semiconductor materials (WO3, TiO2, ZnO, CdS, GaP and SiC), producing CH3OH, HCOOH, HCHO and trace amounts of CH4. In the 1990s, Ta oxide photocatalysts began to draw attention in the field of water splitting. A series of Ta catalysts, such as LiTaO3 [9], NaTaO3 [10], KTaO3 [11], AgTaO3 [12], CaTa2O6 [13], SrTa2O6 [13], KBa2Ta3O10 [14], were proved to efficiently split water. In the 21st century, the study of Ta catalysts for the reduction of CO2 began. Kentaro Teramura et al. [15] prepared ATaO3 (A = Li, Na, K) compounds using a solid state reaction (SSR) method to reduce CO2 in the presence of H2. The only product was CO and the order of photocatalytic activity was LiTaO3 > NaTaO3 > KTaO3, which was consistent with that of the Eg (band gap) values. However, the highest yield of CO in LiTaO3 was 0.42 μmol/g after 24 h of photoirradiation, which was still far from satisfactory. Ye et al. [16] synthesized a series of noble-metal-loaded NaTaO3 samples to reduce CO2 with water. H2 was introduced into this process as an electron donor. Ru/NaTaO3 was found to have the best activity (CH4 51.8 μmol/(g·h)) and product selectivity in converting CO2 to CH4. Junwang Tang and his team [17] prepared KTaO3 nanoflakes by a solvothermal method in a hexane–water mixture and reduced CO2 using pure water as an electron donor. The activity was quite high for both H2 and CO production, achieving 20× (H2) and 7× (CO) higher than that of the cubic sample prepared by the solid state reaction. This was an indication that the catalyst morphology played a crucial role in activity. Jeffrey C. S. Wu et al. [18] prepared NiO-loaded InTaO4 photocatalysts by a sol–gel method and carried out the photocatalytic reduction of CO2 in a self-made optical fiber reactor filled with 0.2 mol/L NaOH solution. The formation rate of methanol was 11.1 μmol/(g·h) under halogen lamp irradiation at 25 °C. Ru-Shi Liu and co-workers [19] prepared a series of nanostructured core–shell materials (Ni@NiO/N-doped InTaO4 photocatalysts) for the reduction of CO2 to methanol in pure water. In these structures, the core–shell nanostructure might offer a new reaction center transferred from the surface of the InTaO4 material.
In this paper, we report the photocatalytic reduction of CO2 to methanol using CuO-loaded NaTaO3 catalysts. NaTaO3 nanocubes were synthesized via a hydrothermal method using Ta2O5 and NaOH. CuO was loaded onto the surface of NaTaO3 by impregnation, where CuO acts as a cocatalyst for CO2 reduction, promoting charge transfer and limiting the fast recombination of electrons and holes [20,21]. According to the literature, Cu oxides and Cu cations are active cocatalysts for CO2 reduction and could serve as reductive sites for selective reduction of CO2 to methanol [22-27]. Isopropanol was employed as both an absorber and a sacrificial reagent due to its good capability to absorb CO2 and donate electrons [28-30]. Acetone, an important industrial material, was generated as the oxidation product of isopropanol.
Experimental
Catalyst preparation
Tantalum oxide (Ta2O5, 99.99%), sodium hydroxide (NaOH, 96%) and isopropanol (iPrOH, 99.9%) were purchased from Aladdin Industrial Corporation. Copper nitrate (Cu(NO3)2·3H2O, AR) was purchased from Tianjin Guangfu Chemical Reagent Company. All reagents were used as received without any further purification.
The NaTaO3 nanocubes were synthesized by a hydrothermal method as reported by Li et al. [31]. In a typical procedure, 0.442 g of Ta2O5 and a sufficient amount of NaOH were added into a Teflon-lined autoclave with a total volume of 50 mL, and deionized water was filled up to 40 mL. The autoclave temperature was held at 140 °C for 12 h then cooled to room temperature in air. The obtained product was washed with deionized water several times before being dried at 80 °C in an oven overnight. The as-prepared catalysts were denoted as 1M-NaTaO3, 2M-NaTaO3, 3M-NaTaO3, 4M-NaTaO3, corresponding to a NaOH concentration of 1 mol/L, 2 mol/L, 3 mol/L, 4 mol/L, respectively.
CuO was loaded onto the surface of NaTaO3 by impregnation. 0.1 g of 2M-NaTaO3 and a given amount of Cu(NO3)2·3H2O were mixed in a crucible with 3 mL deionized water. After stirring for 10 min, the crucible was transferred into a muffle furnace and held for 4 h at 450 °C. After cooling down to room temperature, the resulting product was washed and dried at 80 °C overnight. The as-prepared CuO–NaTaO3 catalysts were denoted as 1wt-NaTaO3, 2wt-NaTaO3, 3wt-NaTaO3, 4wt-NaTaO3 and 5wt-NaTaO3 corresponding to 1 wt %, 2 wt %, 3 wt %, 4 wt % and 5 wt % CuO loading on NaTaO3, respectively.
Catalyst characterization
The catalysts were characterized by X-ray diffraction (XRD, Bruke/D8-Advance, Cu Kα radiation, λ = 0.154056 nm) at a scanning rate of 4°/min ranging from 15° to 70°. The morphology was observed with a Hitachi S-4800 field emission scanning electron microscope (SEM) with an accelerating voltage of 3.0 kV. The surface composition of the catalysts was investigated using a Thermo Scientific energy dispersion X-ray (EDX) fluorescence analyzer (with a Mg Kα ADES (hν = 1253.6 eV) source) as an addition to the SEM and XPS (PHA-5400, SPECS, America). Light absorbance was measured with a Shimadzu UV-2550 spectrometer using BaSO4 as a reference in the wavelength region of 190–600 nm.
Photocatalytic reaction
The photocatalytic reduction of carbon dioxide was carried out in a transparent batch reactor with a slurry bed with cooling jacket. The light source was a 250 W high-pressure mercury lamp with an irradiation peak at about 365 nm. The reaction temperature was controlled by a thermostatic water bath at 25 ± 3 °C. The reactor, in which 12 mg of catalyst was dispersed in 12 mL of isopropanol, was tightly sealed during the reaction. A magnetic stirrer agitated at the bottom of the suspension until the reaction ended. Before irradiation, CO2 (99.99% purity) was bubbled through the reactor for 30 min to eliminate air and saturate the suspension. A typical run was 6 h.
After reaction, the suspension was centrifuged and the liquid sample was examined by a GC-MS (Agilent 5975C) and quantified by a GC (Agilent 7890A, FID, HP-WAX 60 m column). Control experiments were also carried out to confirm that methanol generation was complete in the CO2 reduction. Neither methanol nor acetone was detected in dark or in the absence of catalyst. When N2 was bubbled into the reactor instead of CO2, only acetone was found after the reaction, indicating the likelihood that the isopropanol was oxidized to acetone.
Results and Discussions
Catalyst characterization
Figure 1 shows the XRD patterns of NaTaO3 nanocubes prepared with different NaOH concentrations. All diffraction peaks can be indexed to the orthorhombic phase NaTaO3 structure according to JCPDS#25-0836 with the space group belonging to I, and lattice parameters a = 5.513 Å, b = 7.750 Å, and c = 5.494 Å. As Ta2O5 could not completely convert to NaTaO3 under conditions of low NaOH concentration during the hydrothermal treatment [32,33], sufficient NaOH was used to ensure that pure NaTaO3 was obtained. As calculated by Jade 5.0 software, all the samples had good crystallinity (>98%), which was attributed to the hydrothermal method of catalyst preparation.
![[2190-4286-7-69-1]](/bjnano/content/figures/2190-4286-7-69-1.png?scale=2.0&max-width=1024&background=FFFFFF)
Figure 1: XRD patterns of NaTaO3 nanocubes.
Figure 1: XRD patterns of NaTaO3 nanocubes.
Figure 2 shows the SEM images of NaTaO3 nanocubes synthesized with different NaOH concentrations. When the NaOH concentration was 1 mol/L, only a small percentage of the NaTaO3 grew into cubes. As the NaOH concentration was increased to 2 mol/L, almost all of the particles became larger cubes with an average size of about 300 nm. When the NaOH concentration was increased to 3 mol/L and 4 mol/L, the ideal morphology of the nanocubes was disrupted and fewer nanocubes were observed. Generally, the SEM image of 2M-NaTaO3 presents the best morphology. He et al. [34] reported a hydrothermal synthesis of NaTaO3 with Ta2O5 powder and NaOH followed a dissolution–precipitation mechanism, where the concentration of the NaOH solution played a crucial role on the morphology of the crystal. This was confirmed in our work.
![[2190-4286-7-69-2]](/bjnano/content/figures/2190-4286-7-69-2.jpg?scale=2.0&max-width=1024&background=FFFFFF)
Figure 2: SEM images of NaTaO3 nanocubes: (a) 1M-NaTaO3, (b) 2M-NaTaO3, (c) 3M-NaTaO3, and (d) 4M-NaTaO3.
Figure 2: SEM images of NaTaO3 nanocubes: (a) 1M-NaTaO3, (b) 2M-NaTaO3, (c) 3M-NaTaO3, and (d) 4M-NaTaO3.
Figure 3 shows UV–vis diffuse reflectance spectra and optical absorption edges of NaTaO3 nanocubes prepared with different concentrations of NaOH. From Figure 3a, it can be observed that the main absorption peaks are around 300 nm, which means the powders have an apparent absorption of UV light. The band gap energy (Eg) of each catalyst, prepared with different NaOH concentrations from 1 mol/L to 4 mol/L, can be seen in Figure 3b where the Eg values of these NaTaO3 samples range from 4.06 to 4.12 eV.
![[2190-4286-7-69-3]](/bjnano/content/figures/2190-4286-7-69-3.png?scale=2.0&max-width=1024&background=FFFFFF)
Figure 3: UV–vis diffuse reflectance spectra (a) and optical absorption band edges (b) of NaTaO3 nanocubes.
Figure 3: UV–vis diffuse reflectance spectra (a) and optical absorption band edges (b) of NaTaO3 nanocubes.
Figure 4 shows XRD patterns of 2M-NaTaO3 nanocubes loaded with different amounts of CuO. Comparing with a pure NaTaO3 catalyst, the XRD patterns of the CuO-loaded materials seemed not to change, indicating that the crystalline phase of NaTaO3 was not affected by CuO loading. CuO was also not detected because the loading amount was relatively low [35].
![[2190-4286-7-69-4]](/bjnano/content/figures/2190-4286-7-69-4.png?scale=2.0&max-width=1024&background=FFFFFF)
Figure 4: XRD patterns of 2M-NaTaO3 nanocubes loaded with different amounts of CuO.
Figure 4: XRD patterns of 2M-NaTaO3 nanocubes loaded with different amounts of CuO.
SEM images of CuO–NaTaO3 nanocubes are shown in Figure 5. It can be seen that the surface of pure NaTaO3 nanocubes was flat and smooth (Figure 5a). With moderate loadings of 1 wt % and 2 wt % CuO, CuO particles were dispersed on the surface of the NaTaO3 nanocubes with an average size of tens of nanometers (Figure 5b and Figure 5c). When the loading reached 5 wt %, the CuO nanoparticles began to aggregate and large clusters were formed (Figure 5d).
![[2190-4286-7-69-5]](/bjnano/content/figures/2190-4286-7-69-5.jpg?scale=2.0&max-width=1024&background=FFFFFF)
Figure 5: SEM images of CuO–NaTaO3 nanocubes: (a) 2M-NaTaO3, (b) 1wt-NaTaO3, (c) 2wt-NaTaO3, and (d) 5wt-NaTaO3.
Figure 5: SEM images of CuO–NaTaO3 nanocubes: (a) 2M-NaTaO3, (b) 1wt-NaTaO3, (c) 2wt-NaTaO3, and (d) 5wt-NaTaO...
The UV–vis diffuse reflectance spectra of CuO–NaTaO3 are shown in Figure 6. With a large energy gap, it can be found that pure NaTaO3 had low light absorbance in the visible region. After CuO was loaded, the absorbance of the CuO–NaTaO3 catalysts in the visible light region (λ > 400 nm) became much stronger with the increase of CuO loading. The increase in visible light absorbance correlates with the formation rate and increase in electrons and holes on the photocatalyst surface [35].
![[2190-4286-7-69-6]](/bjnano/content/figures/2190-4286-7-69-6.png?scale=2.0&max-width=1024&background=FFFFFF)
Figure 6: UV–vis diffuse reflectance spectra of CuO-loaded 2M-NaTaO3 nanocubes.
Figure 6: UV–vis diffuse reflectance spectra of CuO-loaded 2M-NaTaO3 nanocubes.
Energy-dispersive X-ray spectroscopy (EDS) and X-ray photoelectron spectroscopy (XPS) were carried out to confirm that CuO was loaded onto the surface of NaTaO3 nanocubes. Figure 7 presents the EDS analysis of 5wt-NaTaO3, which was performed over a single nanoparticle on the catalyst surface. It can be seen that the main compositional elements of the nanoparticle were Cu and O. Figure 8 demonstrates the Cu 2p XPS peak of 2M-NaTaO3, 2wt-NaTaO3 and 5wt-NaTaO3. The two peaks located at 933.20 eV and 953.20 eV corresponded to Cu 2p3/2 and Cu 2p1/2 and a satellite peak was also observed at about 944 eV. These peaks were characteristic for Cu2+, which indicated that Cu existed in the form of CuO [36-39]. The peak intensity increases with increasing loading.
![[2190-4286-7-69-8]](/bjnano/content/figures/2190-4286-7-69-8.png?scale=2.0&max-width=1024&background=FFFFFF)
Figure 8: Smoothed Cu 2p XPS peaks 2M-NaTaO3, 2wt-NaTaO3 and 5wt-NaTaO3.
Figure 8: Smoothed Cu 2p XPS peaks 2M-NaTaO3, 2wt-NaTaO3 and 5wt-NaTaO3.
Photocatalytic reduction of CO2
The photocatalytic activity of CuO–NaTaO3 samples was evaluated by photocatalytic reduction of CO2 in isopropanol under UV light irradiation for 6 h. Methanol and acetone were generated as the reduction product of CO2 and the oxidation product of isopropanol, respectively. 2M-NaTaO3 was chosen for CuO loading because of its good morphology. In our experiments, there was no methanol generation in the absence of copper, which was consistent with Hirato’s report [40].
Figure 9 represents the methanol and acetone yield for 2M-NaTaO3 loaded with different amounts of CuO after 6 h of irradiation. CuO nanoparticles loaded on the surface of 2M-NaTaO3 functioned as reductive sites on which CO2 was reduced to methanol. Below the optimal amount of CuO (2 wt %), the activity was promoted with the increase of CuO loading. When the loading was greater than 2 wt %, the activity began to decrease.
![[2190-4286-7-69-9]](/bjnano/content/figures/2190-4286-7-69-9.png?scale=2.0&max-width=1024&background=FFFFFF)
Figure 9: Methanol and acetone yields for 2M-NaTaO3 loaded with different amounts of CuO after 6 h of irradiation.
Figure 9: Methanol and acetone yields for 2M-NaTaO3 loaded with different amounts of CuO after 6 h of irradia...
In our experiments, 2 wt % CuO loaded 2M-NaTaO3 showed the highest activity, which was attributed to its good crystallinity, morphology and proper amount of CuO loading. According to the XRD results of non-loaded NaTaO3, all samples had good crystallinity, which was beneficial to photocatalytic activity. The high crystalline quality correlates to a low number of defects. The defects usually function as recombination centers where photogenerated electrons and holes recombine fast, resulting in poor photocatalytic activity [41]. Among these catalysts, 2M-NaTaO3 had the best morphology, which played a crucial role in this case. A regular morphology is helpful to the electron transmission process, as it shortens the pathway through which generated electrons transfer from the bulk to the surface of the crystal, thus making the electrons more efficient for the reaction. The CuO loading amount was another important factor. Below the optimal amount of CuO (2 wt %), the activity increased with the increase of CuO loading. For CuO loading greater than 2 wt %, the activity decreased with increasing CuO loading. An explanation for this is that the CuO nanoparticles provide more reductive sites and could reduce the recombination of photogenerated electron–hole pairs with an increase in (moderate) loading, as shown in Figure 5b and Figure 5c [42]. When excessive CuO was loaded, the CuO nanoparticles aggregated to form larger ones (shown in Figure 5d), which decreases the number of effective reductive sites. On the other hand, the large CuO particles could also decrease the efficient separation of electron–hole pairs as compared with smaller ones. Both of these situations could lead to a poor activity.
Reaction mechanism
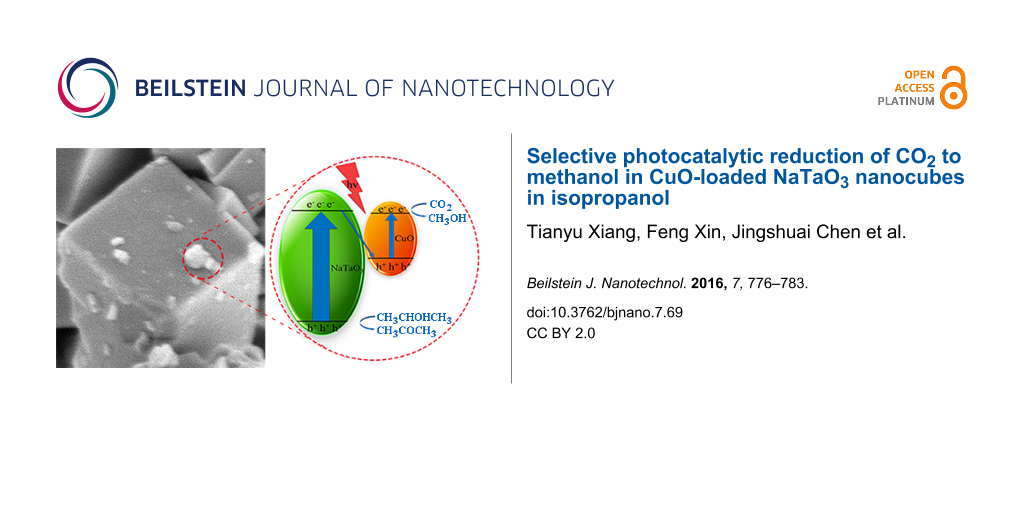
The mechanism for photocatalytic reduction of CO2 to methanol in isopropanol is shown in Figure 10. When the CuO–NaTaO3 catalysts were irradiated by UV light, photogenerated electron–hole pairs were created. The electrons in the conduction band (CB) of NaTaO3 (ECB = −0.92 V vs NHE at pH 7, the same below) [41] could recombine with holes in the valence band (VB) of CuO, enhancing the separation and prolonging the lifespan of photogenerated electron–hole pairs. It was believed that the reduction reaction happened in the CB of CuO (−0.78 V) [35], where CO2 reacted with electrons and protons to generate methanol (ECO2/CH3OH = −0.38 V) [43], as no methanol was detected using pure NaTaO3. The valence band (VB) potential of NaTaO3 is 3.13 V, which is more positive than the potential of isopropanol oxidation to acetone (about 0.47 V) [44,45], thus the oxidation reaction could happen in the VB of NaTaO3.
The reaction in the CB of CuO was as follows:
The isopropanol was oxidized into acetone and protons by holes in the valence band of NaTaO3, which was illustrated by G. R. Dey [46]:
and the overall reaction was
![[2190-4286-7-69-10]](/bjnano/content/figures/2190-4286-7-69-10.png?scale=2.0&max-width=1024&background=FFFFFF)
Figure 10: Schematic diagram for photocatalytic reduction of CO2 to methanol in CuO–NaTaO3 photocatalyst under UV light irradiation.
Figure 10: Schematic diagram for photocatalytic reduction of CO2 to methanol in CuO–NaTaO3 photocatalyst under...
Theoretically, one mole of methanol and three moles of acetone were generated simultaneously. But in our experiments, the mole ratio of acetone to methanol ranged from 2.33 to 2.55. This was attributed to the generation of acetone that was sequentially oxidized into small molecules, which were not detectable by GC.
Conclusion
NaTaO3 nanocubes were synthesized with Ta2O5 powder and NaOH solution via a hydrothermal method. CuO was loaded onto the surface of NaTaO3 by impregnation to suppress the electron–hole recombination and functioned as a reductive site for methanol formation. Acetone was also generated as the oxidation product of isopropanol. With 2 wt % CuO loading, NaTaO3 prepared by 2 mol/L NaOH solution showed the best performance. The highest yields of methanol and acetone were 137.48 μmol/(g·h) and 335.93 μmol/(g·h), respectively, after 6 h of irradiation. These good yields were attributed to the good crystallinity and morphology of NaTaO3 and the proper loading amount of CuO on NaTaO3. The mechanism for photocatalytic reduction of CO2 in isopropanol to methanol was also proposed and explained by band theory.
References
-
Roy, S. C.; Varghese, O. K.; Paulose, M.; Grimes, C. A. ACS Nano 2010, 4, 1259–1278. doi:10.1021/nn9015423
Return to citation in text: [1] -
Izumi, Y. Coord. Chem. Rev. 2013, 257, 171–186. doi:10.1016/j.ccr.2012.04.018
Return to citation in text: [1] -
Ahmed, N.; Shibata, Y.; Taniguchi, T.; Izumi, Y. J. Catal. 2011, 279, 123–135. doi:10.1016/j.jcat.2011.01.004
Return to citation in text: [1] -
Ahmed, N.; Morikawa, M.; Izumi, Y. Catal. Today 2012, 185, 263–269. doi:10.1016/j.cattod.2011.08.010
Return to citation in text: [1] -
Kubacka, A.; Fernández-García, M.; Colón, G. Chem. Rev. 2012, 112, 1555–1614. doi:10.1021/cr100454n
Return to citation in text: [1] -
Morris, A. J.; Meyer, G. J.; Fujita, E. Acc. Chem. Res. 2009, 42, 1983–1994. doi:10.1021/ar9001679
Return to citation in text: [1] -
Wang, S.; Wang, X. Appl. Catal., B 2015, 162, 494–500. doi:10.1016/j.apcatb.2014.07.026
Return to citation in text: [1] -
Inoue, T.; Fujishima, A.; Konishi, S.; Honda, K. Nature 1979, 277, 637–638. doi:10.1038/277637a0
Return to citation in text: [1] -
Kato, H.; Kudo, A. J. Phys. Chem. B 2001, 105, 4285–4292. doi:10.1021/jp004386b
Return to citation in text: [1] -
Kato, H.; Kudo, A. Catal. Today 2003, 78, 561–569. doi:10.1016/S0920-5861(02)00355-3
Return to citation in text: [1] -
Kato, H.; Kudo, A. Chem. Phys. Lett. 1998, 295, 487–492. doi:10.1016/S0009-2614(98)01001-X
Return to citation in text: [1] -
Kato, H.; Kobayashi, H.; Kudo, A. J. Phys. Chem. B 2002, 106, 12441–12447. doi:10.1021/jp025974n
Return to citation in text: [1] -
Kato, H.; Kudo, A. Chem. Lett. 1999, 28, 1207–1208. doi:10.1246/cl.1999.1207
Return to citation in text: [1] [2] -
Kim, H. G.; Hwang, D. W.; Kim, J.; Kim, Y. G.; Lee, J. S. Chem. Commun. 1999, 12, 1077–1078. doi:10.1039/a902892g
Return to citation in text: [1] -
Teramura, K.; Okuoka, S.-i.; Tsuneoka, H.; Shishido, T.; Tanaka, T. Appl. Catal., B 2010, 96, 565–568. doi:10.1016/j.apcatb.2010.03.021
Return to citation in text: [1] -
Li, M.; Li, P.; Chang, K.; Wang, T.; Liu, L.; Kang, Q.; Ouyang, S.; Ye, J. Chem. Commun. 2015, 51, 7645–7648. doi:10.1039/C5CC01124H
Return to citation in text: [1] -
Li, K.; Handoko, A. D.; Khraisheh, M.; Tang, J. Nanoscale 2014, 6, 9767–9773. doi:10.1039/C4NR01490A
Return to citation in text: [1] -
Wang, Z.-Y.; Chou, H.-C.; Wu, J. C. S.; Tsai, D. P.; Mul, G. Appl. Catal., A 2010, 380, 172–177. doi:10.1016/j.apcata.2010.03.059
Return to citation in text: [1] -
Tsai, C.-W.; Chen, H. M.; Liu, R.-S.; Asakura, K.; Chan, T.-S. J. Phys. Chem. C 2011, 115, 10180–10186. doi:10.1021/jp2020534
Return to citation in text: [1] -
Wang, S.; Ding, Z.; Yao, W.; Lin, J.; Wang, X. Angew. Chem., Int. Ed. 2014, 53, 1034–1038. doi:10.1002/anie.201309426
Return to citation in text: [1] -
Wang, S.; Ding, Z.; Wang, X. Chem. Commun. 2015, 51, 1517–1519. doi:10.1039/C4CC07225A
Return to citation in text: [1] -
Liu, D.; Fernández, Y.; Ola, O.; Mackintosh, S.; Maroto-Valer, M.; Parlett, C. M. A.; Lee, A. F.; Wu, J. C. S. Catal. Commun. 2012, 25, 78–82. doi:10.1016/j.catcom.2012.03.025
Return to citation in text: [1] -
Núñez, J.; de la Peña O’Shea, V. A.; Jana, P.; Coronado, J. M.; Serrano, D. P. Catal. Today 2013, 209, 21–27. doi:10.1016/j.cattod.2012.12.022
Return to citation in text: [1] -
Wu, J.; Saito, M.; Takeuchi, M.; Watanabe, T. Appl. Catal., A 2001, 218, 235–240. doi:10.1016/S0926-860X(01)00650-0
Return to citation in text: [1] -
Slamet; Nasution, H. W.; Purnama, E.; Riyani, K.; Gunlazuardi, J. World Appl. Sci. J. 2009, 6, 112–122.
Return to citation in text: [1] -
Fujitani, T.; Nakamura, J. Appl. Catal., A 2000, 191, 111–129. doi:10.1016/S0926-860X(99)00313-0
Return to citation in text: [1] -
Wang, S.; Hou, Y.; Wang, X. ACS Appl. Mater. Interfaces 2015, 7, 4327–4335. doi:10.1021/am508766s
Return to citation in text: [1] -
Kaneco, S.; Shimizu, Y.; Ohta, K.; Mizuno, T. J. Photochem. Photobiol., A: Chem. 1998, 115, 223–226. doi:10.1016/S1010-6030(98)00274-3
Return to citation in text: [1] -
Dey, G. R.; Belapurkar, A. D.; Kishore, K. J. Photochem. Photobiol., A: Chem. 2004, 163, 503–508. doi:10.1016/j.jphotochem.2004.01.022
Return to citation in text: [1] -
Dey, G. R.; Pushpa, K. K. Res. Chem. Intermed. 2007, 33, 631–644. doi:10.1163/156856707781749883
Return to citation in text: [1] -
Li, X.; Zang, J. J. Phys. Chem. C 2009, 113, 19411–19418. doi:10.1021/jp907334z
Return to citation in text: [1] -
Xiong, P.; Tan, G.; Zhang, W.; Xia, A.; Ren, H. J. Cluster Sci. 2013, 24, 515–522. doi:10.1007/s10876-013-0557-4
Return to citation in text: [1] -
Shi, J.; Liu, G.; Wang, N.; Li, C. J. Mater. Chem. 2012, 22, 18808–18813. doi:10.1039/c2jm33470d
Return to citation in text: [1] -
He, Y.; Zhu, Y.; Wu, N. J. Solid State Chem. 2004, 177, 3868–3872. doi:10.1016/j.jssc.2004.07.011
Return to citation in text: [1] -
Qin, S.; Xin, F.; Liu, Y.; Yin, X.; Ma, W. J. Colloid Interface Sci. 2011, 356, 257–261. doi:10.1016/j.jcis.2010.12.034
Return to citation in text: [1] [2] [3] -
Liu, L.; Gao, F.; Zhao, H.; Li, Y. Appl. Catal., B 2013, 134–135, 349–358. doi:10.1016/j.apcatb.2013.01.040
Return to citation in text: [1] -
Huang, L.; Peng, F.; Ohuchi, F. S. Surf. Sci. 2009, 603, 2825–2834. doi:10.1016/j.susc.2009.07.030
Return to citation in text: [1] -
Li, G.; Dimitrijevic, N. M.; Chen, L.; Rajh, T.; Gray, K. A. J. Phys. Chem. C 2008, 112, 19040–19044. doi:10.1021/jp8068392
Return to citation in text: [1] -
Colón, G.; Maicu, M.; Hidalgo, M. C.; Navío, J. A. Appl. Catal., B 2006, 67, 41–51. doi:10.1016/j.apcatb.2006.03.019
Return to citation in text: [1] -
Liu, B.-J.; Torimoto, T.; Yoneyama, H. J. Photochem. Photobiol., A 1998, 115, 227–230. doi:10.1016/S1010-6030(98)00272-X
Return to citation in text: [1] -
Kudo, A.; Miseki, Y. Chem. Soc. Rev. 2009, 38, 253–278. doi:10.1039/B800489G
Return to citation in text: [1] [2] -
Ganesh, I. Renewable Sustainable Energy Rev. 2014, 31, 221–257. doi:10.1016/j.rser.2013.11.045
Return to citation in text: [1] -
Habisreutinger, S. N.; Schmidt-Mende, L.; Stolarczyk, J. K. Angew. Chem., Int. Ed. 2013, 52, 7372–7408. doi:10.1002/anie.201207199
Return to citation in text: [1] -
Markiewicz, M. E. P.; Hebert, D. M.; Bergens, S. H. J. Power Sources 2006, 161, 761–767. doi:10.1016/j.jpowsour.2006.05.002
Return to citation in text: [1] -
Mitoraj, D.; Kisch, H. J. Phys. Chem. C 2009, 113, 20890–20895. doi:10.1021/jp903893w
Return to citation in text: [1] -
Dey, G. R. J. Nat. Gas Chem. 2007, 16, 217–226. doi:10.1016/S1003-9953(07)60052-8
Return to citation in text: [1]
| 42. | Ganesh, I. Renewable Sustainable Energy Rev. 2014, 31, 221–257. doi:10.1016/j.rser.2013.11.045 |
| 35. | Qin, S.; Xin, F.; Liu, Y.; Yin, X.; Ma, W. J. Colloid Interface Sci. 2011, 356, 257–261. doi:10.1016/j.jcis.2010.12.034 |
| 1. | Roy, S. C.; Varghese, O. K.; Paulose, M.; Grimes, C. A. ACS Nano 2010, 4, 1259–1278. doi:10.1021/nn9015423 |
| 9. | Kato, H.; Kudo, A. J. Phys. Chem. B 2001, 105, 4285–4292. doi:10.1021/jp004386b |
| 18. | Wang, Z.-Y.; Chou, H.-C.; Wu, J. C. S.; Tsai, D. P.; Mul, G. Appl. Catal., A 2010, 380, 172–177. doi:10.1016/j.apcata.2010.03.059 |
| 8. | Inoue, T.; Fujishima, A.; Konishi, S.; Honda, K. Nature 1979, 277, 637–638. doi:10.1038/277637a0 |
| 19. | Tsai, C.-W.; Chen, H. M.; Liu, R.-S.; Asakura, K.; Chan, T.-S. J. Phys. Chem. C 2011, 115, 10180–10186. doi:10.1021/jp2020534 |
| 3. | Ahmed, N.; Shibata, Y.; Taniguchi, T.; Izumi, Y. J. Catal. 2011, 279, 123–135. doi:10.1016/j.jcat.2011.01.004 |
| 4. | Ahmed, N.; Morikawa, M.; Izumi, Y. Catal. Today 2012, 185, 263–269. doi:10.1016/j.cattod.2011.08.010 |
| 5. | Kubacka, A.; Fernández-García, M.; Colón, G. Chem. Rev. 2012, 112, 1555–1614. doi:10.1021/cr100454n |
| 6. | Morris, A. J.; Meyer, G. J.; Fujita, E. Acc. Chem. Res. 2009, 42, 1983–1994. doi:10.1021/ar9001679 |
| 7. | Wang, S.; Wang, X. Appl. Catal., B 2015, 162, 494–500. doi:10.1016/j.apcatb.2014.07.026 |
| 16. | Li, M.; Li, P.; Chang, K.; Wang, T.; Liu, L.; Kang, Q.; Ouyang, S.; Ye, J. Chem. Commun. 2015, 51, 7645–7648. doi:10.1039/C5CC01124H |
| 17. | Li, K.; Handoko, A. D.; Khraisheh, M.; Tang, J. Nanoscale 2014, 6, 9767–9773. doi:10.1039/C4NR01490A |
| 14. | Kim, H. G.; Hwang, D. W.; Kim, J.; Kim, Y. G.; Lee, J. S. Chem. Commun. 1999, 12, 1077–1078. doi:10.1039/a902892g |
| 46. | Dey, G. R. J. Nat. Gas Chem. 2007, 16, 217–226. doi:10.1016/S1003-9953(07)60052-8 |
| 12. | Kato, H.; Kobayashi, H.; Kudo, A. J. Phys. Chem. B 2002, 106, 12441–12447. doi:10.1021/jp025974n |
| 15. | Teramura, K.; Okuoka, S.-i.; Tsuneoka, H.; Shishido, T.; Tanaka, T. Appl. Catal., B 2010, 96, 565–568. doi:10.1016/j.apcatb.2010.03.021 |
| 11. | Kato, H.; Kudo, A. Chem. Phys. Lett. 1998, 295, 487–492. doi:10.1016/S0009-2614(98)01001-X |
| 43. | Habisreutinger, S. N.; Schmidt-Mende, L.; Stolarczyk, J. K. Angew. Chem., Int. Ed. 2013, 52, 7372–7408. doi:10.1002/anie.201207199 |
| 10. | Kato, H.; Kudo, A. Catal. Today 2003, 78, 561–569. doi:10.1016/S0920-5861(02)00355-3 |
| 44. | Markiewicz, M. E. P.; Hebert, D. M.; Bergens, S. H. J. Power Sources 2006, 161, 761–767. doi:10.1016/j.jpowsour.2006.05.002 |
| 45. | Mitoraj, D.; Kisch, H. J. Phys. Chem. C 2009, 113, 20890–20895. doi:10.1021/jp903893w |
| 28. | Kaneco, S.; Shimizu, Y.; Ohta, K.; Mizuno, T. J. Photochem. Photobiol., A: Chem. 1998, 115, 223–226. doi:10.1016/S1010-6030(98)00274-3 |
| 29. | Dey, G. R.; Belapurkar, A. D.; Kishore, K. J. Photochem. Photobiol., A: Chem. 2004, 163, 503–508. doi:10.1016/j.jphotochem.2004.01.022 |
| 30. | Dey, G. R.; Pushpa, K. K. Res. Chem. Intermed. 2007, 33, 631–644. doi:10.1163/156856707781749883 |
| 20. | Wang, S.; Ding, Z.; Yao, W.; Lin, J.; Wang, X. Angew. Chem., Int. Ed. 2014, 53, 1034–1038. doi:10.1002/anie.201309426 |
| 21. | Wang, S.; Ding, Z.; Wang, X. Chem. Commun. 2015, 51, 1517–1519. doi:10.1039/C4CC07225A |
| 22. | Liu, D.; Fernández, Y.; Ola, O.; Mackintosh, S.; Maroto-Valer, M.; Parlett, C. M. A.; Lee, A. F.; Wu, J. C. S. Catal. Commun. 2012, 25, 78–82. doi:10.1016/j.catcom.2012.03.025 |
| 23. | Núñez, J.; de la Peña O’Shea, V. A.; Jana, P.; Coronado, J. M.; Serrano, D. P. Catal. Today 2013, 209, 21–27. doi:10.1016/j.cattod.2012.12.022 |
| 24. | Wu, J.; Saito, M.; Takeuchi, M.; Watanabe, T. Appl. Catal., A 2001, 218, 235–240. doi:10.1016/S0926-860X(01)00650-0 |
| 25. | Slamet; Nasution, H. W.; Purnama, E.; Riyani, K.; Gunlazuardi, J. World Appl. Sci. J. 2009, 6, 112–122. |
| 26. | Fujitani, T.; Nakamura, J. Appl. Catal., A 2000, 191, 111–129. doi:10.1016/S0926-860X(99)00313-0 |
| 27. | Wang, S.; Hou, Y.; Wang, X. ACS Appl. Mater. Interfaces 2015, 7, 4327–4335. doi:10.1021/am508766s |
| 40. | Liu, B.-J.; Torimoto, T.; Yoneyama, H. J. Photochem. Photobiol., A 1998, 115, 227–230. doi:10.1016/S1010-6030(98)00272-X |
| 35. | Qin, S.; Xin, F.; Liu, Y.; Yin, X.; Ma, W. J. Colloid Interface Sci. 2011, 356, 257–261. doi:10.1016/j.jcis.2010.12.034 |
| 36. | Liu, L.; Gao, F.; Zhao, H.; Li, Y. Appl. Catal., B 2013, 134–135, 349–358. doi:10.1016/j.apcatb.2013.01.040 |
| 37. | Huang, L.; Peng, F.; Ohuchi, F. S. Surf. Sci. 2009, 603, 2825–2834. doi:10.1016/j.susc.2009.07.030 |
| 38. | Li, G.; Dimitrijevic, N. M.; Chen, L.; Rajh, T.; Gray, K. A. J. Phys. Chem. C 2008, 112, 19040–19044. doi:10.1021/jp8068392 |
| 39. | Colón, G.; Maicu, M.; Hidalgo, M. C.; Navío, J. A. Appl. Catal., B 2006, 67, 41–51. doi:10.1016/j.apcatb.2006.03.019 |
| 34. | He, Y.; Zhu, Y.; Wu, N. J. Solid State Chem. 2004, 177, 3868–3872. doi:10.1016/j.jssc.2004.07.011 |
| 35. | Qin, S.; Xin, F.; Liu, Y.; Yin, X.; Ma, W. J. Colloid Interface Sci. 2011, 356, 257–261. doi:10.1016/j.jcis.2010.12.034 |
| 31. | Li, X.; Zang, J. J. Phys. Chem. C 2009, 113, 19411–19418. doi:10.1021/jp907334z |
| 32. | Xiong, P.; Tan, G.; Zhang, W.; Xia, A.; Ren, H. J. Cluster Sci. 2013, 24, 515–522. doi:10.1007/s10876-013-0557-4 |
| 33. | Shi, J.; Liu, G.; Wang, N.; Li, C. J. Mater. Chem. 2012, 22, 18808–18813. doi:10.1039/c2jm33470d |
© 2016 Xiang et al; licensee Beilstein-Institut.
This is an Open Access article under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
The license is subject to the Beilstein Journal of Nanotechnology terms and conditions: (http://www.beilstein-journals.org/bjnano)




![[2190-4286-7-69-7]](/bjnano/content/figures/2190-4286-7-69-7.png?scale=2.0&max-width=1024&background=FFFFFF)