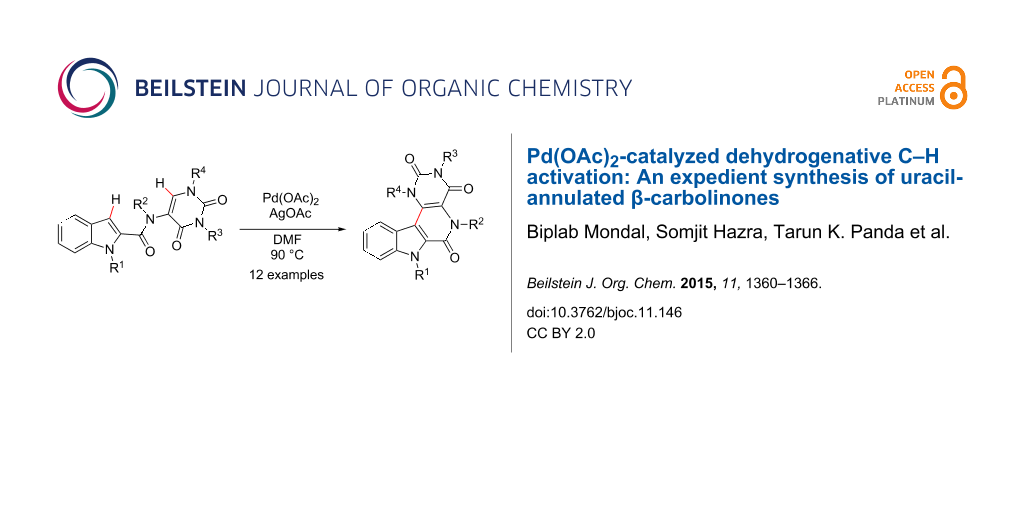
Abstract
An intramolecular dehydrogenative C–H activation enabled an efficient synthesis of an uracil-annulated β-carbolinone ring system. The reaction is simple, efficient and high yielding (85–92%).
Graphical Abstract
Introduction
The presence of a β-carbolinone skeleton in various natural products, such as secofascaplysin A (I), SL651498 (II) (Figure 1), and their remarkable biological and pharmacological properties make them stand out amongst all the carboline class of compounds [1-12]. For example, SL651498 was documented as a potential drug development candidate in a research program designed to discover subtype-selective GABAA receptor agonists for the treatment of muscle spasms and generalized anxiety disorder [13]. β-Carbolinones such as strychnocarpine, the alkaloid from Alstonia venenata, and substituted 1-oxo-13-carbolines were also shown to have serotonin-receptor-binding activity (5-HT receptor) [14]. Moreover the natural and synthetic β-carbolines are also known to show anticancer activity against colon and lung cancers, and some β-carbolinones act as biological control agents for receptor research on bioenzyme inhibitors, such as the inhibition of HLE (human leukocyte elastase) [15-18].
Figure 1: Naturally occurring β-carbolinones.
Figure 1: Naturally occurring β-carbolinones.
Uracil, on the other hand, is one of the four nucleobases of RNA. It holds immense importance from a pharmaceutical and biological point of view [19]. For example, 5-fluorouracil [20] and other uracil-based molecules [21-23] such as 3’-azido-3’-deoxythymidine (AZT), 2’,3’-dideoxycytidine (DDC), (E)-5-[2-(bromovinyl)-2’-deoxyuridine] (BVDU), are active against cancer and the HI virus [24-31].
Diverse approaches towards the synthesis of β-carbolinones have been developed [32-36], such as an intramolecular Heck reaction strategy of 2- and 3-iodoindoles for the synthesis of β- or γ-carbolinones by Beccalli et al. [32], AuCl3 and Pd-catalyzed cycloisomerization of indole-2-carboxamides to β-carbolinones [33-35], Pd-catalyzed dehydrogenative annulation of indole-carboxamides with alkynes etc [36]. The development of metal-catalyzed C–H activation reaction has revolutionized the way a synthetic chemist now approaches a traditional C–C bond disconnection [32-44]. Dehydrogenative C–H activation [45-52] is the most elegant alternative in this endeavor as it avoids pre-functionalization of any C–H bond beforehand. But regioselectivity is the main problem in this type of reaction due to the ubiquitous presence of various C–H bonds in a simple organic molecule. However, sometimes the issue of regioselectivity can be resolved by the electronic property of the substrate itself. Pioneering work published by Fagnou et al. shows how a catalyst inverts its selectivity and reactivity between the coupling partners to achieve indole C3-arylation in a cross coupling reaction of an unactivated arene and N-acetylindole [53]. Driven by the same logic and guided by our previous work we envisioned that an intramolecular dehydrogenative cross coupling reaction could be achievable between the electron deficient uracil C6–H bond adjacent to the nitogen atom and the electron rich indole C3–H bond for the synthesis of uracil annulated β-carbolinones [54-56]. Herein, we report our novel approach towards the synthesis of uracil annulated β-carbolinones via an intramolecular dehydrogenative coupling reaction of indole-2-carboxamides.
Results and Discussion
We started our investigation with the preparation of amide precursors 4 from N-substituted indole-2-carboxylic acids 1 (Scheme 1). The acids were treated with oxalyl chloride at room temperature [33] and the resulting acid chloride obtained was transformed to the amide by the reaction with amine 3 in dry THF using NaH as a base.
Scheme 1: Preparation of starting substrate.
Scheme 1: Preparation of starting substrate.
The amide precursor was then subjected to a series of reactions in pursuit of the best reaction conditions for the dehydrogenative cross-coupling process. Assuming that the reaction goes through an electrophilic metallation pathway, it was projected that Pd(OAc)2 would be an excellent starting point for catalyst screening. The amide 4a (R1 = R2 = R3 = R4 = Me) was used as a model substrate for this dehydrogenative coupling reaction. The reaction was set up in the presence of Pd(OAc)2 (10 mol %), Cu(OAc)2 (2 equiv) in DMF under open air at 70 °C (Table 1, entry 1). After 8 h we obtained 35% yield of 5a with 52% conversion of starting material. Increasing the temperature to 90 °C (Table 1, entry 2) afforded 63% yield of 5a with 80% conversion of 4a. Then different oxidants [Cu(OTf)2, PhI(OAc)2, K2S2O8, (NH4)2S2O8, p-benzoquinone (BQ), oxone, AgOAc, molecular oxygen] were examined under these reaction conditions. With Cu(OTf)2 (Table 1, entry 3) and oxone (Table 1, entry 8), total recovery of starting material was observed while PhI(OAc)2 (Table 1, entry 4), (NH4)2S2O8 (Table 1, entry 6) showed total decomposition of starting material. With BQ the yield was almost the same (65%) as with Cu(OAc)2 (Table 1, entry 7). The use of K2S2O8 increased the yield of 5a to 83% (Table 1, entry 5) with complete conversion of starting material; AgOAc further increased the yield to 91% (Table 1, entry 9). Molecular oxygen also used as an oxidant resulted in a low 30% yield of 5a (Table 1, entry 10) with 51% conversion of 4a. Examination of different solvents led to determining that the polar solvents (DMF/DMSO (9:1), DMSO, DMAc, Table 1, entries 11, 12, 13) were far superior compared to non-polar solvent toluene (Table 1, entry 14). But the optimal result was obtained with the use of DMF (Table 1, entry 9). Testing the efficiency of other Pd catalysts for this reaction revealed that Pd2dba3, i.e., Pd(0) did not show any catalytic activity (Table 1, entry 17). The yield decreased sharply for other Pd catalysts such as PdCl2, Pd(CH3CN)2Cl2 and Pd(PPh3)2Cl2 presumably be due to the less electrophilic nature of these catalysts (Table 1, entries 15, 16, 18).
Table 1: Optimization of intramolecular dehydrogenative coupling.
|
|
||||||
| Entry | Catalyst | Oxidant | Solvent | Temp (°C) | Conversion (%)a | Yield (%)b |
|---|---|---|---|---|---|---|
| 1 | Pd(OAc)2 | Cu(OAc)2 | DMF | 70 | 52 | 35 |
| 2 | Pd(OAc)2 | Cu(OAc)2 | DMF | 90 | 80 | 63 |
| 3 | Pd(OAc)2 | Cu(OTf)2 | DMF | 90 | 0 | 0 |
| 4 | Pd(OAc)2 | PhI(OAc)2 | DMF | 90 | 0 | 0 |
| 5 | Pd(OAc)2 | K2S2O8 | DMF | 90 | 100 | 83 |
| 6 | Pd(OAc)2 | (NH4)2S2O8 | DMF | 90 | 0 | 0 |
| 7 | Pd(OAc)2 | BQ | DMF | 90 | 78 | 65 |
| 8 | Pd(OAc)2 | oxone | DMF | 90 | 0 | 0 |
| 9 | Pd(OAc)2 | AgOAc | DMF | 90 | 100 | 91 |
| 10 | Pd(OAc)2 | O2 | DMF | 90 | 51 | 30 |
| 11 | Pd(OAc)2 | AgOAc | DMF/DMSO (9:1) | 90 | 100 | 82 |
| 12 | Pd(OAc)2 | AgOAc | DMSO | 90 | 100 | 78 |
| 13 | Pd(OAc)2 | AgOAc | DMAc | 90 | 100 | 80 |
| 14 | Pd(OAc)2 | AgOAc | toluene | 90 | 40 | 30 |
| 15 | PdCl2 | AgOAc | DMF | 90 | 83 | 52 |
| 16 | Pd(CH3CN)2Cl2 | AgOAc | DMF | 90 | 70 | 35 |
| 17 | Pd2(dba)3 | AgOAc | DMF | 90 | 0 | 0 |
| 18 | Pd(PPh3)2Cl2 | AgOAc | DMF | 90 | 40 | 32 |
All reactions were performed with 1 equiv starting material (4), 10 mol % Pd-catalyst, 2 equiv oxidant in 5 mL solvent at mentioned tempetature, 8 h under open air. aCalculation on the basis of isolation of starting material. bIsolated yield.
With the optimized reaction conditions in hand (10 mol % Pd(OAc)2, 2 equiv AgOAc in DMF at 90 °C for 8 h), we further explored the substrate scope of the reaction (Scheme 2).
Scheme 2: Synthesis of various β-carbolinone derivatives.
Scheme 2: Synthesis of various β-carbolinone derivatives.
All the reactions went very smoothly giving excellent yields in the range of (85–92%). No distinct steric influence was noticed when the indole-N-methyl group was replaced by ethyl, butyl or benzyl groups. However, the reaction did not proceed at all with the unsubstituted indole precursor (R1 = H, 4m), this result may be explained with potential coordination of the Pd catalyst between the indole nitrogen and amide carbonyl oxygen. A representative X-ray crystal structure of β-carbolinone derivative 5h was obtained [57] (Figure 2).
The inactivity of Pd(0) (Table 1, entry 17), and inferior reactivity of other less electrophilic Pd catalysts indicates a mechanistic pathway that commence with electrophilic metalation at the indole C3 postion. The nucleophilicity of indole at C3 is well known [58] and a similar kind of electrophilic reaction leads to the intermediate A (Scheme 3). We believe this intermediate then undergoes a σ-bond metathesis reaction to form a seven membered palladacycle B which in turn produces the product β-carbolinones after reductive elimination from the 7-membered palladacycle B. The catalytic cycle is completed by AgOAc.
Conclusion
In conclusion we have developed an elegant method for the preparation of uracil annulated β-carbolinones via a high yielding dehydrogenative C–H activation process. The key to the success of this reaction is the complementary electronic properties of the indole C3–H bond and the uracil C6–H bond. It is anticipated this efficient and atom economic approach can be emulated for the preparation of other β-carbolinones as well, and further results in this regard will be reported in due course.
Experimental
Representative procedure for the preparation of uracil annulated β-carbolinones (5a–m):
In a flame-dried round bottomed flask equipped with a magnetic bar, a mixture of 1 equiv starting material 4, 5 mL dry DMF, 2 equiv of AgOAc and 10 mol % Pd(OAc)2 was taken and stirred at room temperature for 5 min. Then the reaction mixture was heated in an oil bath fixed at 90 °C for 8 h under air. Completion of the reaction was monitored by checking TLC. The reaction mixture was cooled to room temperature, diluted with water and 50 mL of EtOAc and passed through a pad of celite. The organic layer was washed with H2O (2 × 10 mL) and saturated NaCl (aq) (1 × 10 mL). The organic part was dried over Na2SO4, evaporated and purified by flash chromatography, using ethyl acetate/petroleum ether (2:8) as the eluent to afford product 5. For details see Supporting Information File 1.
Supporting Information
| Supporting Information File 1: Experimental and analytical data. | ||
| Format: PDF | Size: 4.9 MB | Download |
Acknowledgements
We thank DST (New Delhi, India) for financial assistance through the DST-PURSE programme, DST fast track scheme, UGC-SAP programme and University of Kalyani. B.M. & S.H. are thankful to University of Kalyani and CSIR (New Delhi, India) respectively for research fellowships. We also thank DST (New Delhi, India) for providing NMR (400 MHz) and mass spectrometer instruments.
References
-
Larsen, L. K.; Moore, R. E.; Patterson, G. M. L. J. Nat. Prod. 1994, 57, 419–421. doi:10.1021/np50105a018
Return to citation in text: [1] -
Mouaddib, A.; Joseph, B.; Hasnaoui, A.; Mérour, J.-Y. Synthesis 2000, 549–556. doi:10.1055/s-2000-6374
Return to citation in text: [1] -
Abramovitch, R. A.; Spenser, I. D. Adv. Heterocycl. Chem. 1964, 3, 79–207. doi:10.1016/S0065-2725(08)60542-5
Return to citation in text: [1] -
Stuart, K.; Woo-Ming, R. Heterocycles 1975, 3, 223–264. doi:10.3987/R-1975-03-0223
Return to citation in text: [1] -
Smith, T. A. Phytochemistry 1977, 16, 171–175. doi:10.1016/S0031-9422(00)86778-3
Return to citation in text: [1] -
Allen, J. R. F.; Holmstedt, B. R. Phytochemistry 1980, 19, 1573–1582. doi:10.1016/S0031-9422(00)83773-5
Return to citation in text: [1] -
Braestrup, C.; Nielsen, M.; Olsen, C. E. Proc. Natl. Acad. Sci. U. S. A. 1980, 77, 2288–2292.
Return to citation in text: [1] -
Schlecker, W.; Huth, A.; Ottow, E.; Mulzer, J. Synthesis 1995, 1225–1227. doi:10.1055/s-1995-4081
Return to citation in text: [1] -
Molina, P.; Fresneda, P. M. J. Chem. Soc., Perkin Trans. 1 1988, 7, 1819–1822. doi:10.1039/P19880001819
Return to citation in text: [1] -
Molina, P.; Fresneda, P. M.; García-Zafra, S.; Almendros, P. Tetrahedron Lett. 1994, 35, 8851–8854. doi:10.1016/S0040-4039(00)78515-7
Return to citation in text: [1] -
Dodd, R. H.; Ouannes, C.; Robert-Gero, M.; Potier, P. J. Med. Chem. 1989, 32, 1272–1276. doi:10.1021/jm00126a021
Return to citation in text: [1] -
Srivastava, S. K.; Agarwal, A.; Chauhan, P. M. S.; Agarwal, S. K.; Bhaduri, A. P.; Singh, S. N.; Fatima, N.; Chatterjee, R. K. J. Med. Chem. 1999, 42, 1667–1672. doi:10.1021/jm9800705
Return to citation in text: [1] -
Griebel, G.; Perrault, G.; Simiand, J.; Cohen, C.; Granger, P.; Depoortere, H.; Françon, D.; Avenet, P.; Schoemaker, H.; Evanno, Y.; Sevrin, M.; George, P.; Scatton, B. CNS Drug Rev. 2003, 9, 3–20. doi:10.1111/j.1527-3458.2003.tb00241.x
Return to citation in text: [1] -
Nelson, D. L.; Herbert, A.; Bourgoin, S.; Glowinski, J.; Hamon, M. Mol. Pharmacol. 1978, 14, 983–995.
Return to citation in text: [1] -
Veale, C. A.; Damewood, J. R., Jr.; Steelman, G. B.; Bryant, C.; Gomes, B.; Williams, J. J. Med. Chem. 1995, 38, 86–97. doi:10.1021/jm00001a014
Return to citation in text: [1] -
Ritzeler, O.; Castro, A.; Grenler, L.; Soucy, F. Substituted beta-carbolines as lkB kinase inhibitors. Eur. Pat. Appl. EP1134221 A1, Sept 19, 2001.
Return to citation in text: [1] -
Nielsch, U.; Sperzel, M.; Bethe, B.; Junge, B.; Lieb, F.; Velten, R. Treating tumor necrosis factor mediated inflammatory disease, e.g. arteriosclerosis, using new or known beta-carboline derivatives. Ger. Pat. Appl. DE19807993 A1, Sept 2, 1999.
Return to citation in text: [1] -
Menta, E.; Pescalli, N.; Spinelli, S. 1H-pirido[3, 4-b]indol-1-one derivavives. WO Patent WO2001/009129, Feb 8, 2001.
Return to citation in text: [1] -
Myers, R. L. The 100 Most Important Chemical Compounds: A Reference Guide; Greenwood Press: Westport, United States of America, 2007.
Return to citation in text: [1] -
Garrett, R. H.; Grisham, C. M. Principals of Biochemistry with a Human Focus; Brooks/Cole: Pacific Grove, CA, 1997.
Return to citation in text: [1] -
Macilwain, C. Nature 1993, 365, 378. doi:10.1038/365378a0
Return to citation in text: [1] -
Balzarini, J.; Pauwels, R.; Hardewijn, P.; De Clercq, E.; Cooney, D. A.; Kang, G.-J.; Dalal, M.; Johns, D. G.; Broder, S. Biochem. Biophys. Res. Commun. 1986, 140, 735–742. doi:10.1016/0006-291X(86)90793-X
Return to citation in text: [1] -
Jones, A. S.; Sayers, J. R.; Walker, R. T.; De Clercq, E. J. Med. Chem. 1988, 31, 268–271. doi:10.1021/jm00396a043
Return to citation in text: [1] -
Miyasaka, T.; Tanaka, H.; Baba, M.; Hayakawa, H.; Walker, R. T.; Balzarini, J.; De Clercq, E. J. Med. Chem. 1989, 32, 2507–2509. doi:10.1021/jm00132a002
Return to citation in text: [1] -
Baba, M.; Panwels, R.; Herdwewijn, P.; De Clercq, E.; Desmyster, J.; Vandeputte, M. Biochem. Biophys. Res. Commun. 1987, 142, 128–134. doi:10.1016/0006-291X(87)90460-8
Return to citation in text: [1] -
De Clercq, E. J. Med. Chem. 1986, 29, 1561–1569. doi:10.1021/jm00159a001
Return to citation in text: [1] -
Breault, G. A.; Ellston, R. P. A.; Green, S.; James, S. R.; Jewsbury, P. J.; Midgley, C. J.; Pauptit, R. A.; Minshull, C. A.; Tucker, J. A.; Pease, J. E. Bioorg. Med. Chem. Lett. 2003, 13, 2961–2966. doi:10.1016/S0960-894X(03)00203-8
Return to citation in text: [1] -
Chiang, C.-S.; Yu, C.-F.; Chaing, L.-W.; Chen, S.-W.; Lo, J.-M.; Yu, C.-S. Chem. Pharm. Bull. 2008, 56, 109–111. doi:10.1248/cpb.56.109
Return to citation in text: [1] -
Colin, D.; Gimazne, A.; Lizard, G.; Iizard, G.; Solary, E.; Latruffe, N.; Delmas, D. Int. J. Cancer 2009, 124, 2780–2788. doi:10.1002/ijc.24264
Return to citation in text: [1] -
Xie, F.; Zhao, H.; Zhao, L.; Lou, L.; Ho, Y. Bioorg. Med. Chem. Lett. 2009, 19, 275–278. doi:10.1016/j.bmcl.2008.09.067
Return to citation in text: [1] -
Evdokimov, N. M.; Van Slambrouck, S.; Heffeter, P.; Tu, L.; Le Calvé, B.; Lamoral-Theys, D.; Hooten, C. J.; Uglinskii, P. Y.; Rogelj, S.; Kiss, R.; Steelant, W. F. A.; Berger, W.; Yang, J. J.; Bologa, C. G.; Kornienko, A.; Magedov, I. V. J. Med. Chem. 2011, 54, 2012–2021. doi:10.1021/jm1009428
Return to citation in text: [1] -
Beccalli, E. M.; Broggini, G.; Marchesini, A.; Rossi, E. Tetrahedron 2002, 58, 6673–6678. doi:10.1016/S0040-4020(02)00688-9
Return to citation in text: [1] [2] [3] -
England, D. B.; Padwa, A. Org. Lett. 2008, 10, 3631–3634. doi:10.1021/ol801385h
Return to citation in text: [1] [2] [3] [4] -
Abbiati, G.; Beccalli, E. M.; Broggini, G.; Zoni, C. J. Org. Chem. 2003, 68, 7625–7628. doi:10.1021/jo034636v
Return to citation in text: [1] [2] [3] -
Beccalli, E. M.; Broggini, G. Tetrahedron Lett. 2003, 44, 1919–1921. doi:10.1016/S0040-4039(03)00116-3
Return to citation in text: [1] [2] [3] -
Shi, Z.; Cui, Y.; Jiao, N. Org. Lett. 2010, 12, 2908–2911. doi:10.1021/ol1007839
Return to citation in text: [1] [2] [3] -
Newhouse, T.; Baran, P. S.; Hoffmann, R. W. Chem. Soc. Rev. 2009, 38, 3010–3021. doi:10.1039/B821200G
Return to citation in text: [1] -
Gaich, T.; Baran, P. S. J. Org. Chem. 2010, 75, 4657–4673. doi:10.1021/jo1006812
Return to citation in text: [1] -
Hendrickson, J. B. J. Am. Chem. Soc. 1975, 97, 5784–5800. doi:10.1021/ja00853a023
Return to citation in text: [1] -
Chen, X.; Engle, K. M.; Wang, D.-H.; Yu, J.-Q. Angew. Chem., Int. Ed. 2009, 48, 5094–5115. doi:10.1002/anie.200806273
Return to citation in text: [1] -
Newhouse, T.; Baran, P. S. Angew. Chem., Int. Ed. 2011, 50, 3362–3374. doi:10.1002/anie.201006368
Return to citation in text: [1] -
Gutekunst, W. R.; Baran, P. S. Chem. Soc. Rev. 2011, 40, 1976–1991. doi:10.1039/c0cs00182a
Return to citation in text: [1] -
Brückl, T.; Baxter, R. D.; Ishihara, Y.; Baran, P. S. Acc. Chem. Res. 2012, 45, 826–839. doi:10.1021/ar200194b
Return to citation in text: [1] -
Li, B.; Dixneuf, P. H. Chem. Soc. Rev. 2013, 42, 5744–5767. doi:10.1039/C3CS60020C
Return to citation in text: [1] -
Li, C.-J. Acc. Chem. Res. 2009, 42, 335–344. doi:10.1021/ar800164n
Return to citation in text: [1] -
Li, Z.; Bohle, D. S.; Li, C.-J. Proc. Natl. Acad. Sci. U. S. A. 2006, 103, 8928–8933. doi:10.1073/pnas.0601687103
Return to citation in text: [1] -
Li, C.-J.; Li, Z. Pure Appl. Chem. 2006, 78, 935–945. doi:10.1351/pac200678050935
Return to citation in text: [1] -
Yoo, W.-J.; Li, C.-J. Top. Curr. Chem. 2010, 292, 281–302. doi:10.1007/128_2009_17
Return to citation in text: [1] -
Scheuermann, C. J. Chem. – Asian J. 2010, 5, 436–451. doi:10.1002/asia.200900487
Return to citation in text: [1] -
Yeung, C. S.; Dong, V. M. Chem. Rev. 2011, 111, 1215–1292. doi:10.1021/cr100280d
Return to citation in text: [1] -
Liu, C.; Zhang, H.; Shi, W.; Lei, A. Chem. Rev. 2011, 111, 1780–1824. doi:10.1021/cr100379j
Return to citation in text: [1] -
Lie, B.-J.; Shi, Z.-J. Chem. Soc. Rev. 2012, 41, 5588–5598. doi:10.1039/C2CS35096C
Return to citation in text: [1] -
Stuart, D. R.; Fagnou, K. Science 2007, 316, 1172–1175. doi:10.1126/science.1141956
Return to citation in text: [1] -
Mondal, B.; Hazra, S.; Roy, B. Tetrahedron Lett. 2014, 55, 1077–1081. doi:10.1016/j.tetlet.2013.12.092
Return to citation in text: [1] -
Roy, B.; Hazra, S.; Mondal, B.; Majumdar, K. C. Eur. J. Org. Chem. 2013, 4570–4577. doi:10.1002/ejoc.201300275
Return to citation in text: [1] -
Hazra, S.; Mondal, B.; De, R. N.; Roy, B. RSC Adv. 2015, 5, 22480–22489. doi:10.1039/C4RA16661B
Return to citation in text: [1] -
CCDC 1020159.
Return to citation in text: [1] -
Liégault, B.; Petrov, I.; Gorelsky, S. I.; Fagnou, K. J. Org. Chem. 2010, 75, 1047–1060. doi:10.1021/jo902515z
Return to citation in text: [1]
| 58. | Liégault, B.; Petrov, I.; Gorelsky, S. I.; Fagnou, K. J. Org. Chem. 2010, 75, 1047–1060. doi:10.1021/jo902515z |
| 33. | England, D. B.; Padwa, A. Org. Lett. 2008, 10, 3631–3634. doi:10.1021/ol801385h |
| 1. | Larsen, L. K.; Moore, R. E.; Patterson, G. M. L. J. Nat. Prod. 1994, 57, 419–421. doi:10.1021/np50105a018 |
| 2. | Mouaddib, A.; Joseph, B.; Hasnaoui, A.; Mérour, J.-Y. Synthesis 2000, 549–556. doi:10.1055/s-2000-6374 |
| 3. | Abramovitch, R. A.; Spenser, I. D. Adv. Heterocycl. Chem. 1964, 3, 79–207. doi:10.1016/S0065-2725(08)60542-5 |
| 4. | Stuart, K.; Woo-Ming, R. Heterocycles 1975, 3, 223–264. doi:10.3987/R-1975-03-0223 |
| 5. | Smith, T. A. Phytochemistry 1977, 16, 171–175. doi:10.1016/S0031-9422(00)86778-3 |
| 6. | Allen, J. R. F.; Holmstedt, B. R. Phytochemistry 1980, 19, 1573–1582. doi:10.1016/S0031-9422(00)83773-5 |
| 7. | Braestrup, C.; Nielsen, M.; Olsen, C. E. Proc. Natl. Acad. Sci. U. S. A. 1980, 77, 2288–2292. |
| 8. | Schlecker, W.; Huth, A.; Ottow, E.; Mulzer, J. Synthesis 1995, 1225–1227. doi:10.1055/s-1995-4081 |
| 9. | Molina, P.; Fresneda, P. M. J. Chem. Soc., Perkin Trans. 1 1988, 7, 1819–1822. doi:10.1039/P19880001819 |
| 10. | Molina, P.; Fresneda, P. M.; García-Zafra, S.; Almendros, P. Tetrahedron Lett. 1994, 35, 8851–8854. doi:10.1016/S0040-4039(00)78515-7 |
| 11. | Dodd, R. H.; Ouannes, C.; Robert-Gero, M.; Potier, P. J. Med. Chem. 1989, 32, 1272–1276. doi:10.1021/jm00126a021 |
| 12. | Srivastava, S. K.; Agarwal, A.; Chauhan, P. M. S.; Agarwal, S. K.; Bhaduri, A. P.; Singh, S. N.; Fatima, N.; Chatterjee, R. K. J. Med. Chem. 1999, 42, 1667–1672. doi:10.1021/jm9800705 |
| 19. | Myers, R. L. The 100 Most Important Chemical Compounds: A Reference Guide; Greenwood Press: Westport, United States of America, 2007. |
| 53. | Stuart, D. R.; Fagnou, K. Science 2007, 316, 1172–1175. doi:10.1126/science.1141956 |
| 15. | Veale, C. A.; Damewood, J. R., Jr.; Steelman, G. B.; Bryant, C.; Gomes, B.; Williams, J. J. Med. Chem. 1995, 38, 86–97. doi:10.1021/jm00001a014 |
| 16. | Ritzeler, O.; Castro, A.; Grenler, L.; Soucy, F. Substituted beta-carbolines as lkB kinase inhibitors. Eur. Pat. Appl. EP1134221 A1, Sept 19, 2001. |
| 17. | Nielsch, U.; Sperzel, M.; Bethe, B.; Junge, B.; Lieb, F.; Velten, R. Treating tumor necrosis factor mediated inflammatory disease, e.g. arteriosclerosis, using new or known beta-carboline derivatives. Ger. Pat. Appl. DE19807993 A1, Sept 2, 1999. |
| 18. | Menta, E.; Pescalli, N.; Spinelli, S. 1H-pirido[3, 4-b]indol-1-one derivavives. WO Patent WO2001/009129, Feb 8, 2001. |
| 54. | Mondal, B.; Hazra, S.; Roy, B. Tetrahedron Lett. 2014, 55, 1077–1081. doi:10.1016/j.tetlet.2013.12.092 |
| 55. | Roy, B.; Hazra, S.; Mondal, B.; Majumdar, K. C. Eur. J. Org. Chem. 2013, 4570–4577. doi:10.1002/ejoc.201300275 |
| 56. | Hazra, S.; Mondal, B.; De, R. N.; Roy, B. RSC Adv. 2015, 5, 22480–22489. doi:10.1039/C4RA16661B |
| 14. | Nelson, D. L.; Herbert, A.; Bourgoin, S.; Glowinski, J.; Hamon, M. Mol. Pharmacol. 1978, 14, 983–995. |
| 32. | Beccalli, E. M.; Broggini, G.; Marchesini, A.; Rossi, E. Tetrahedron 2002, 58, 6673–6678. doi:10.1016/S0040-4020(02)00688-9 |
| 33. | England, D. B.; Padwa, A. Org. Lett. 2008, 10, 3631–3634. doi:10.1021/ol801385h |
| 34. | Abbiati, G.; Beccalli, E. M.; Broggini, G.; Zoni, C. J. Org. Chem. 2003, 68, 7625–7628. doi:10.1021/jo034636v |
| 35. | Beccalli, E. M.; Broggini, G. Tetrahedron Lett. 2003, 44, 1919–1921. doi:10.1016/S0040-4039(03)00116-3 |
| 36. | Shi, Z.; Cui, Y.; Jiao, N. Org. Lett. 2010, 12, 2908–2911. doi:10.1021/ol1007839 |
| 37. | Newhouse, T.; Baran, P. S.; Hoffmann, R. W. Chem. Soc. Rev. 2009, 38, 3010–3021. doi:10.1039/B821200G |
| 38. | Gaich, T.; Baran, P. S. J. Org. Chem. 2010, 75, 4657–4673. doi:10.1021/jo1006812 |
| 39. | Hendrickson, J. B. J. Am. Chem. Soc. 1975, 97, 5784–5800. doi:10.1021/ja00853a023 |
| 40. | Chen, X.; Engle, K. M.; Wang, D.-H.; Yu, J.-Q. Angew. Chem., Int. Ed. 2009, 48, 5094–5115. doi:10.1002/anie.200806273 |
| 41. | Newhouse, T.; Baran, P. S. Angew. Chem., Int. Ed. 2011, 50, 3362–3374. doi:10.1002/anie.201006368 |
| 42. | Gutekunst, W. R.; Baran, P. S. Chem. Soc. Rev. 2011, 40, 1976–1991. doi:10.1039/c0cs00182a |
| 43. | Brückl, T.; Baxter, R. D.; Ishihara, Y.; Baran, P. S. Acc. Chem. Res. 2012, 45, 826–839. doi:10.1021/ar200194b |
| 44. | Li, B.; Dixneuf, P. H. Chem. Soc. Rev. 2013, 42, 5744–5767. doi:10.1039/C3CS60020C |
| 13. | Griebel, G.; Perrault, G.; Simiand, J.; Cohen, C.; Granger, P.; Depoortere, H.; Françon, D.; Avenet, P.; Schoemaker, H.; Evanno, Y.; Sevrin, M.; George, P.; Scatton, B. CNS Drug Rev. 2003, 9, 3–20. doi:10.1111/j.1527-3458.2003.tb00241.x |
| 45. | Li, C.-J. Acc. Chem. Res. 2009, 42, 335–344. doi:10.1021/ar800164n |
| 46. | Li, Z.; Bohle, D. S.; Li, C.-J. Proc. Natl. Acad. Sci. U. S. A. 2006, 103, 8928–8933. doi:10.1073/pnas.0601687103 |
| 47. | Li, C.-J.; Li, Z. Pure Appl. Chem. 2006, 78, 935–945. doi:10.1351/pac200678050935 |
| 48. | Yoo, W.-J.; Li, C.-J. Top. Curr. Chem. 2010, 292, 281–302. doi:10.1007/128_2009_17 |
| 49. | Scheuermann, C. J. Chem. – Asian J. 2010, 5, 436–451. doi:10.1002/asia.200900487 |
| 50. | Yeung, C. S.; Dong, V. M. Chem. Rev. 2011, 111, 1215–1292. doi:10.1021/cr100280d |
| 51. | Liu, C.; Zhang, H.; Shi, W.; Lei, A. Chem. Rev. 2011, 111, 1780–1824. doi:10.1021/cr100379j |
| 52. | Lie, B.-J.; Shi, Z.-J. Chem. Soc. Rev. 2012, 41, 5588–5598. doi:10.1039/C2CS35096C |
| 32. | Beccalli, E. M.; Broggini, G.; Marchesini, A.; Rossi, E. Tetrahedron 2002, 58, 6673–6678. doi:10.1016/S0040-4020(02)00688-9 |
| 33. | England, D. B.; Padwa, A. Org. Lett. 2008, 10, 3631–3634. doi:10.1021/ol801385h |
| 34. | Abbiati, G.; Beccalli, E. M.; Broggini, G.; Zoni, C. J. Org. Chem. 2003, 68, 7625–7628. doi:10.1021/jo034636v |
| 35. | Beccalli, E. M.; Broggini, G. Tetrahedron Lett. 2003, 44, 1919–1921. doi:10.1016/S0040-4039(03)00116-3 |
| 36. | Shi, Z.; Cui, Y.; Jiao, N. Org. Lett. 2010, 12, 2908–2911. doi:10.1021/ol1007839 |
| 33. | England, D. B.; Padwa, A. Org. Lett. 2008, 10, 3631–3634. doi:10.1021/ol801385h |
| 34. | Abbiati, G.; Beccalli, E. M.; Broggini, G.; Zoni, C. J. Org. Chem. 2003, 68, 7625–7628. doi:10.1021/jo034636v |
| 35. | Beccalli, E. M.; Broggini, G. Tetrahedron Lett. 2003, 44, 1919–1921. doi:10.1016/S0040-4039(03)00116-3 |
| 24. | Miyasaka, T.; Tanaka, H.; Baba, M.; Hayakawa, H.; Walker, R. T.; Balzarini, J.; De Clercq, E. J. Med. Chem. 1989, 32, 2507–2509. doi:10.1021/jm00132a002 |
| 25. | Baba, M.; Panwels, R.; Herdwewijn, P.; De Clercq, E.; Desmyster, J.; Vandeputte, M. Biochem. Biophys. Res. Commun. 1987, 142, 128–134. doi:10.1016/0006-291X(87)90460-8 |
| 26. | De Clercq, E. J. Med. Chem. 1986, 29, 1561–1569. doi:10.1021/jm00159a001 |
| 27. | Breault, G. A.; Ellston, R. P. A.; Green, S.; James, S. R.; Jewsbury, P. J.; Midgley, C. J.; Pauptit, R. A.; Minshull, C. A.; Tucker, J. A.; Pease, J. E. Bioorg. Med. Chem. Lett. 2003, 13, 2961–2966. doi:10.1016/S0960-894X(03)00203-8 |
| 28. | Chiang, C.-S.; Yu, C.-F.; Chaing, L.-W.; Chen, S.-W.; Lo, J.-M.; Yu, C.-S. Chem. Pharm. Bull. 2008, 56, 109–111. doi:10.1248/cpb.56.109 |
| 29. | Colin, D.; Gimazne, A.; Lizard, G.; Iizard, G.; Solary, E.; Latruffe, N.; Delmas, D. Int. J. Cancer 2009, 124, 2780–2788. doi:10.1002/ijc.24264 |
| 30. | Xie, F.; Zhao, H.; Zhao, L.; Lou, L.; Ho, Y. Bioorg. Med. Chem. Lett. 2009, 19, 275–278. doi:10.1016/j.bmcl.2008.09.067 |
| 31. | Evdokimov, N. M.; Van Slambrouck, S.; Heffeter, P.; Tu, L.; Le Calvé, B.; Lamoral-Theys, D.; Hooten, C. J.; Uglinskii, P. Y.; Rogelj, S.; Kiss, R.; Steelant, W. F. A.; Berger, W.; Yang, J. J.; Bologa, C. G.; Kornienko, A.; Magedov, I. V. J. Med. Chem. 2011, 54, 2012–2021. doi:10.1021/jm1009428 |
| 36. | Shi, Z.; Cui, Y.; Jiao, N. Org. Lett. 2010, 12, 2908–2911. doi:10.1021/ol1007839 |
| 21. | Macilwain, C. Nature 1993, 365, 378. doi:10.1038/365378a0 |
| 22. | Balzarini, J.; Pauwels, R.; Hardewijn, P.; De Clercq, E.; Cooney, D. A.; Kang, G.-J.; Dalal, M.; Johns, D. G.; Broder, S. Biochem. Biophys. Res. Commun. 1986, 140, 735–742. doi:10.1016/0006-291X(86)90793-X |
| 23. | Jones, A. S.; Sayers, J. R.; Walker, R. T.; De Clercq, E. J. Med. Chem. 1988, 31, 268–271. doi:10.1021/jm00396a043 |
| 20. | Garrett, R. H.; Grisham, C. M. Principals of Biochemistry with a Human Focus; Brooks/Cole: Pacific Grove, CA, 1997. |
| 32. | Beccalli, E. M.; Broggini, G.; Marchesini, A.; Rossi, E. Tetrahedron 2002, 58, 6673–6678. doi:10.1016/S0040-4020(02)00688-9 |
© 2015 Mondal et al; licensee Beilstein-Institut.
This is an Open Access article under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
The license is subject to the Beilstein Journal of Organic Chemistry terms and conditions: (http://www.beilstein-journals.org/bjoc)




![[1860-5397-11-146-2]](/bjoc/content/figures/1860-5397-11-146-2.png?scale=2.0&max-width=1024&background=FFFFFF)