Abstract
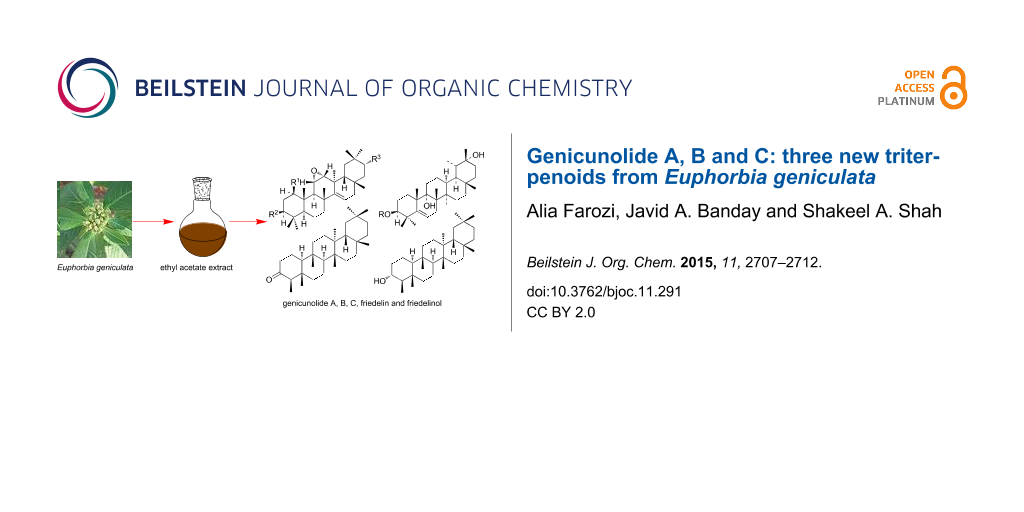
Three new triterpenoids, designated as genicunolide A (1), B (2) and C (3), along with friedelin (4) and friedelinol (5), were isolated from the aerial parts of Euphorbia geniculata. They were characterized as 1β-acetoxy-3β-hydroxy-11α,12α-oxidotaraxer-14-ene, 1β,3β-diacetoxy-21α-hydroxy-11α,12α-oxidotaraxer-14-ene and 3β,9α,20α-trihydroxy-Ψ-taraxast-5-ene, respectively, by spectral and chemical methods.
Graphical Abstract
Introduction
Euphorbia (Euphorbiaceae) is a very large and diverse genus of flowering plants comprising of about 2,000 members and is found all over the world, ranging from short annual plants to well developed tall trees [1].
The plants of the family Euphorbiaceae contain well-known skin irritating and tumor-promoting diterpenoids with tigliane, ingenane and daphnane skeletons [2]. Some of the species are used in folk medicine to cure skin diseases, gonorrhea, migraines, intestinal parasites, and warts [3] and as a purgative [4-6]. Several macrocyclic diterpenoids with antibacterial, anticancer, anti-multidrug-resistant, antifeedant, anti-HIV and analgesic activity have been isolated from different Euphorbia species. They include jatrophane, ingol and myrsinane diterpenoids [7-13].
Triterpenoids which have been reported from various species of Euphorbia include β-amyrin [1], β-amyrin acetate [14,18], cycloeucalenol, obtusifoliol, 24-methylenecycloartan-3-β-ol, β-sitosterol, betulin, erythrodiol, oleanolic acid, β-sitosterol glucoside[15], 29-norcycloart-5-ene-5,8-lanostadiene-3β-ol, 3β,24S,25-trihydroxycycloartane, 3β,24(R),25-trihydroxy-cycloartane, 24-methylenecycloartan-3β-ol [16], cycloart-23-ene-3,5-diol [17], lupeol, lupeol acetate, ginnone, ambrein, lupeone [18], 24-methylenecycloartanol [19], cycloart-25-en-3β,24-diol [20] and cycloart-22-ene-3β,25-diol [21]. In addition, nor-isoprenoids and coumarins have also been reported from few species of Euphorbia [22-24].
Euphorbia geniculata Orteg. [25,26], is a wild weed found in the Jammu region of India [27]. The plant is locally used for the treatment of bacterial infections and inflammations. Previous phytochemical investigations have demonstrated that this plant contains flavonoids: kaempferol, quercetin and 3-rhamnosyl quercetin [28] and triterpenes β-amyrin acetate [29] and geniculatin [30].
Reinvestigation of chemistry of the plant led to isolation of three new triterpenoids, designated as genicunolide A (1), B (2) and C (3), together with friedelin (4) [31] and friedelinol (5) [32], from the ethyl acetate extract of the aerial parts of the plant. Herein, we report the characterization of the three compounds by spectral and chemical methods.
Results and Discussion
The compounds 1–3 (Figure 1) responded positively to the characteristic Liebermann–Burchard [33], TCA [34,35] and TNM tests [35] for unsaturated triterpenoids.
Figure 1: Structures of compounds 1, 2, 3, 1a, 2a, 1b, 2b, 3a, 4 and 5.
Figure 1: Structures of compounds 1, 2, 3, 1a, 2a, 1b, 2b, 3a, 4 and 5.
The compound 1, M+ at m/z 498.0695 (calculated for C32H50O4, 498.0700), possessed eight tertiary methyl groups, an acetoxy functionality [νmax 1736 cm−1, δ 2.03 (s, 3H), δC 170.2, 21.3], a trisubstituted double bond [νmax 1630, 1042, 880 cm−1, δ 5.56 (d, J = 5.2 Hz, 1H, H-15)] [36] and a secondary equatorial hydroxy group [νmax 3500 cm−1] whose carbinylic proton resonated at δ 3.16 (dd, J = 8.1 Hz, 1H, H-3). On acetylation with Ac2O–C5H5N, at room temperature, compound 1 afforded the diacetate 1a, δ 2.06 (s, 6H), and on oxidation with CrO3–C5H5N yielded a ketoacetate 1b, νmax 1738, 1680 cm–1, δC 216.7, 170.8, which responded positively to the characteristic Zimmermann test for 3-keto function [37], thereby placing the hydroxy group in compound 1 at 3β position, δC 77.2 [38].
The mass spectrum of compound 1 displayed the characteristic features of the taraxer-14-ene skeleton [39] by exhibiting RDA fragment ion peaks at m/z 374 (rings A/B/C) and 124 (ring E) and the vinylic carbon resonance signals at δC 118.8 (C-15) and 157.0 (C-14) [40].
The presence of a cis-oxido functionality in compound 1 was evident from a pair of AB doublets at δ 2.82 and 3.01 (Jae = 4.7 Hz, 1H each, He-12 and Ha-11), in its 1H NMR spectrum, two methine carbon resonance signals at δC 53.4 (C-11) and 58.3 (C-12) [41] and loss of CO and H2O, via rearrangement of hydrogen [42] from the RDA fragment ion at m/z 374 to give abundant ion peaks at m/z 346 and 354, respectively.
The acetoxy functionality in compound 1 was placed at C-1 β-position on the basis of the chemical shift, multiplicity and coupling constant of the carbinylic proton [δ 4.53 (dd, Jae = 7.4 Hz, Jaa = 8.5 Hz, 1H, H-1)] together with the identical chemical shift of H-1 and H-3, in the 1H NMR spectrum of 1a, and the comparable chemical shifts of C-1 and C-3 of 1a (δC 80.7 and 80.6, respectively) with that of 1β,3β-diacetoxylupenes [43].
The structure of compound 1 was further confirmed by 1H,1H and 1H,13C COSY, HMBC and HSQC experiments which allowed unambiguous fixation of protons to appropriate carbons and also 13C-chemical shifts (Table 1). The long range correlations between the protons at δ 1.96 (Ha-2) and 1.98 (He-2) and carbonyl signal at δC 170.8 confirmed the presence of acetoxy carbonyl at C-1. This was further substantiated by the long range mutual coupling of the carbinylic proton at δ 4.53 with the proton at δ 3.16 as also with the carbon at δC 41.1 ppm (C-4). The correlation cross peaks between H-23 and H-25 and H-2 in the NOESY experiment confirmed that the acetoxy function at C-1 was β-oriented. The proton at δ 2.82 was correlated to the olefinic carbon at δC 157 ppm (C-14), three bonds away and allowed joining of spin systems separated by a methyl-bearing quaternary carbon on one side. The chemical shifts, multiplicity and coupling constants of the A-ring carbinylic protons and an inspection of the molecular models suggested that 1,3-cis-diequatorial functions in ring A caused flattening of this ring.
Table 1: 13C NMR data of 1, 2, 3 and their acetates in CDCl3 (δ in ppm, 125 MHz).
| C | 1 | 1a | 2 | 2a | 3 | 3a |
|---|---|---|---|---|---|---|
| 1 | 80.6 | 80.7 | 80.9 | 80.9 | 21.5 | 21.5 |
| 2 | 27.8 | 29.8 | 29.9 | 29.7 | 28.6 | 29.0 |
| 3 | 77.2 | 80.6 | 80.6 | 80.8 | 71.8 | 80.5 |
| 4 | 41.1 | 41.5 | 41.1 | 41.3 | 41.9 | 42.0 |
| 5 | 54.5 | 54.6 | 54.6 | 54.6 | 140.7 | 139.0 |
| 6 | 18.4 | 19.5 | 19.5 | 20.1 | 121.7 | 122.0 |
| 7 | 33.3 | 30.2 | 30.2 | 30.2 | 23.5 | 23.4 |
| 8 | 39.6 | 39.9 | 39.6 | 40.1 | 42.7 | 42.9 |
| 9 | 52.6 | 52.7 | 52.6 | 52.8 | 89.5 | 89.5 |
| 10 | 37.6 | 38.1 | 37.6 | 38.1 | 42.7 | 42.8 |
| 11 | 53.4 | 53.4 | 53.4 | 53.3 | 23.4 | 23.5 |
| 12 | 58.2 | 58.3 | 58.2 | 58.4 | 26.4 | 26.4 |
| 13 | 36.5 | 36.4 | 36.4 | 36.4 | 39.7 | 39.8 |
| 14 | 157.1 | 157.1 | 157.2 | 157.1 | 36.9 | 37.0 |
| 15 | 118.9 | 118.9 | 118.9 | 118.8 | 33.9 | 34.0 |
| 16 | 35.7 | 35.9 | 35.7 | 35.8 | 31.9 | 31.8 |
| 17 | 35.4 | 35.8 | 35.8 | 35.8 | 32.3 | 32.5 |
| 18 | 48.1 | 48.5 | 48.1 | 48.6 | 56.1 | 56.3 |
| 19 | 40.2 | 42.1 | 41.5 | 42.1 | 46.2 | 46.1 |
| 20 | 28.7 | 28.8 | 31.9 | 27.5 | 77.2 | 77.3 |
| 21 | 35.7 | 35.9 | 77.2 | 80.1 | 31.9 | 42.5 |
| 22 | 35.6 | 35.7 | 39.6 | 35.1 | 42.7 | 42.5 |
| 23 | 27.0 | 27.1 | 27.0 | 27.2 | 31.9 | 31.8 |
| 24 | 17.0 | 16.5 | 16.7 | 16.7 | 29.3 | 29.5 |
| 25 | 16.6 | 16.5 | 16.5 | 27.5 | 20.3 | 20.3 |
| 26 | 27.0 | 27.5 | 27.8 | 30.1 | 19.8 | 19.9 |
| 27 | 30.2 | 30.2 | 30.2 | 29.8 | 12.1 | 12.3 |
| 28 | 29.9 | 29.8 | 29.9 | 29.9 | 23.1 | 23.2 |
| 29 | 33.6 | 33.1 | 33.3 | 33.3 | 20.0 | 20.1 |
| 30 | 19.5 | 19.5 | 19.5 | 19.5 | 36.1 | 36.2 |
| 1-OAc | 170.8, 21.3 | 170.7, 21.2 | 170.8, 21.1 | 170.8, 21.1 | – | – |
| 3-OAc | – | 170.9, 21.4 | 170.9, 21.2 | 170.9, 21.2 | – | 170.5, 21.4 |
| 21-OAc | – | – | – | 170.1, 21.3 | – | – |
The spectral patterns of compound 2, M+ at m/z 556.0739, C34H52O6, resembled closely with those of 1a, except that it was shown to possess an extra secondary hydroxy group [νmax 3350 cm–1, δ 3.17 (dd, J = 11.1 Hz, 1H)]. Its presence was confirmed by acetylation of 2 to 2a [δ 2.05 (s, 9H), δC 170.1, 170.7 and 170.8] and oxidation to diacetoxy ketone 2b [νmax 1736, 1730, 1680 cm–1, δC 170.5, 170.6, 215.2 (C-21)]. The mass spectrum of compound 2 exhibited RDA fragment ions at m/z 416 and 140, placing the hydroxy group in ring E. The hydroxy group was placed at C-21 α-position (δC 77.2) [38] on the basis of coupling constant of carbinylic proton in the 1H NMR spectrum of 2, the downfield chemical shift of C-30 methyl protons (δ 1.09) in the spectrum of 2b, and 1H,1H, 1H,13C COSY, HMBC and HSQC spectra of 2 which showed long range correlations of the carbinylic proton at δ 3.17 (H-21) and methyl protons at δ 0.87 (H-29) as also carbons at δC 35.7 (C-16) and 48.1 (C-18).
The 1H NMR, 13C NMR (Table 1) and DEPT (135°) spectra of compound 3, M+ at m/z 458.1495, C30H50O3, revealed that the compound possesses seven tertiary methyls, one of which resonated downfield at δ 1.53; a secondary methyl [δ 0.85 (d, J = 4.2 Hz, 3H)], a trisubstituted double bond [νmax 1640, 1040, 890 cm−1, δ 5.36 (d, J = 4.7 Hz, 1H)], and a secondary hydroxy group [νmax 3465 cm−1, δ 3.52 (dd, Jaa = 7.6 Hz, H-3, 1H)]. On acetylation with Ac2O–C5H5N, at room temperature, it afforded monoacetate 3a [1735, δ 2.05 (s, 3H), δC 170.5] and on oxidation with CrO3–C5H5N, it yielded a ketone which gave a positive Zimmermann test for 3-keto group [37] confirming the presence of the C-3 equatorial secondary hydroxy group [δC 71.8] in 3. The mass spectrum of compound 3 revealed that the double bond triggered the typical RDA fragmentation of ring B [39] to give densely populated ion peaks at m/z 166 (ring A) and 292 (rings C/D/E) placing the double bond at C-5 [δC 122.0 (C-6), 139.9 (C-5)] [38] and two hydroxy groups in rings C/D/E. Since the monoacetate 3a still retained a hydroxy group and its mass spectrum also showed a RDA fragment ion at m/z 292, compound 3, therefore, carried two tertiary hydroxy groups on rings C/D/E. The presence of a secondary methyl group together with a pair of doublets at δ 1.56 and 1.85 (d br, J = 10.0 Hz, 1H each, H-18 and H-19) showed that the compound belonged to the Ψ-taraxastane [35] series. The downfield shift of C-30 methyl singlet (δ 1.53) suggested that one of the tertiary hydroxy groups was at C-20 (δC 77.3). Had it been on C-19, the 13C signal would have been observed upfield at δC 73.0–73.2 [44]. The densely populated ion peaks at m/z 221 (rings A/B) and 203 (221 − H2O.+), arising from the fission of 9, 11 and 8, 14 bonds in ring C, together with the downfield carbon signal at δC 89.5 placed the second tertiary hydroxy groups at C-9. The structure of compound 3 was further confirmed by 1H,1H, 1H,13C COSY and long range 1H,13C COSY experiments. The presence of the C-9 hydroxy group was proved by linking the carbon signal at δC 89.5 to proton signals at δ 1.01 (C-25) and 0.93 (C-26) in the 1H,13C long-range coupled spectrum. Other data for 1D and 2D NMR spectra of 3 were in agreement with the assigned structure.
Comparison of physical characteristics and spectral data of compounds 4 and 5, with those reported in literature [31,32], confirmed them to be friedelin and friedelinol, respectively.
Conclusion
The compounds 1–5 were, thus, characterized as 1β-acetoxy-3β-hydroxy-11α,12α-oxido-taraxer-14-ene (1); 1β,3β-diacetoxy-21α-hydroxy-11α,12α-oxido-taraxer-14-ene (2); 3β,9α,20α-trihydroxy-Ψ-taraxast-5-ene (3); friedelin (4) and friedelinol (5); respectively. Compounds 1–3 are new triterpenoids while 4 and 5 appear to have been isolated for the first time from the genus Euphorbia.
Experimental
General procedures
Melting points were determined in centigrade scale in one end open capillaries on a Büchi 570 melting point apparatus and are uncorrected. IR spectra were recorded on a Perkin-Elmer Paragon-1000 spectrophotometer or an Esquire 3000 spectrometer. 1H and 13C NMR spectra were recorded by a Bruker 500 and 125 MHz instrument using TMS as internal standard and CDCl3 as solvent. High-resolution mass spectra were recorded on a Bruker 400 mass spectrometer. Column chromatography was carried out with Merk silica gel (60–120 mesh). Optical rotation was measured on a Perkin-Elmer polarimeter.
Plant material
The aerial parts of Euphorbia geniculata (Orteg) were collected from Jammu, (J&K, India) in July 2013. The specimen was identified by Akhtar H. Malik, Curator, Centre for Biodiversity & Taxanomy, University of Kashmir (Specimen deposited under accession No. 1850 – KASH Herbarium).
Extraction and isolation
The shade dried aerial parts of Euphorbia geniculata (3.0 kg) were extracted sequentially with petroleum ether (60–80 °C), ethyl acetate and methanol in a soxhlet apparatus to afford respective extracts which were concentrated under reduced pressure. The ethyl acetate extract (40 g) was subjected to chromatography on silica gel (60–120 mesh, B.D.H.) column using graded solvent systems of petroleum ether–ethyl acetate. The fractions collected with petroleum ether–ethyl acetate (9:1), F-1; (8:2), F-2; (7:3), F-3 and ethyl acetate, F-4, whose components gave green, pink and violet colouration on TLC (silica gel G) plates, after development with cerric ammonium sulfate–H2SO4, were subjected to re-chromatography. The fraction F-1 on re-chromatography and elution with petroleum ether–dichloromethane (8:2) gave 4 (300 mg) and 5 (410 mg). The fraction F-2 on further chromatography and elution with petroleum ether–dichloromethane (7:3) and (8:4) gave two mixtures. The mixture obtained with petroleum ether–dichloromethane (8:4) was subjected to preparative TLC using petroleum ether–chloroform (19:3) as solvent system to get compound 1 (48 mg). The fraction F-3 on further chromatography and elution with petroleum ether–dichloromethane (8:2) gave compound 2 (45 mg). The fraction F-4 on repeated chromatography using the same sequence of graded solvent systems, as for crude extract, gave compound 3 (38 mg) with petroleum ether–dichloromethane (3:7) and a mixture containing 3 and 2, which was again resolved by preparative TLC using benzene–ethyl acetate (9:1) as solvent system.
Genicunolide A (1): Colourless crystals (CHCl3–Me2CO), mp 150 °C; [α]D25 +20.5° (c 0.50, CHCl3); HRMS: m/z (rel. int.) 498.0695 (18) (M+) (calcd for C32H50O4, 498.0700), 483 (36.2), 480 (21.4), 456 (28.6), 441 (47.1), 374 (71.3) (RDA, rings A/B/C), 346 (57.2), 314 (42.7), 124 (65.8) (RDA, ring E), 108 (100); IR: νmax 3500 (OH), 3030, 2850, 1736 (OAc), 1630, 1456, 1042, 880 cm−1; 1H NMR (500 MHz, CDCl3) δ 0.80 (s, 3H, H-28), 0.86 (s, 6H, H-24, H-29), 0.89 (s, 3H, H-30), 0.90 (s, 3H, H-23), 1.01 (s, 3H, H-25), 1.09 (s, 3H, H-26), 1.25 (s, 3H, H-27), 2.06 (s, 3H, OCOCH3), 2.31 (d, J = 6.9 Hz, 2H, H-16), 2.82 (d, J = 4.7 Hz, 1H, H-12), 3.01 (d, J = 4.7 Hz, 1H, H-11), 3.16 (dd, J = 5.5, 8.1 Hz, 1H, H-3), 4.53 (dd, J = 7.4, 8.5 Hz, 1H, H- 1), 5.56 (d, J = 5.2 Hz, 1H, H-15); 13C NMR: Table 1.
Genicunolide B (2): Colourless crystals (CHCl3–Me2CO), mp 160 °C, [α]D25 + 34.2° (c 0.40, CHCl3); HRMS: m/z 556.0739 (M+) (calcd for C34H52O6, 556.0744), 541 (M+ − .CH3), 514 (M+ − CH2CO), 472 (514 − CH2CO), 454 (472 − H2O), 416 (RDA, rings A/B/C), 356 (416 − HOAc), 286 (356 − CO − CH2CO), 140, 124 (RDA, ring E), 108 (124 – H2O)(100); IR: νmax 3550, 3025, 2863, 1736 (OAc), 1730 (OAc), 1625, 1450, 1045, 890 cm−1; 1H NMR (500 MHz, CDCl3) δ 0.80 (s, 3H, H-28), 0.87 (s, 6H, H-24, H-29), 0.97 (s, 3H, H-30), 0.98 (s, 3H, H-23), 1.01 (s, 3H, H-25), 1.09 (s, 3H, H-26), 1.25 (s, 3H, H-27), 2.06 (s, 6H, 2 x OAc), 2.31 (s, 2H, H-16), 2.80 (d, J = 4.7 Hz, 1H, H-12), 3.01 (d, J = 4.8 Hz, 1H, H-11), 3.17 (dd, J = 5.5, 11.1 Hz, 1H, H-21), 4.53 (dd, J = 7.6, 8.5 Hz, 2H, Ha-1, Ha-3), 5.56 (d, J = 5.2 Hz, 1H, H-15); 13C NMR: Table 1.
Genicunolide C (3): Colourless needles, mp 210–211 °C, [α]D25 +30.3° (c 0.3, CHCl3); HRMS: m/z 458.1495 (M.+) (calcd for C30H50O3, 458.1500) (M.+), 443 (M+ − CH3), 440 (M+ − H2O), 425 (M+ − CH3 − H2O), 413 (M+ − CH3CH=+OH), 292 (RDA, rings C/D/E), 237 (RDA, rings D/E), 221, 203, 166 (RDA, ring A), 163, 107, 83, 45 (100); IR: νmax 3580, 3465, 1640, 1445, 1040, 1025, 890 cm−1; 1H NMR (500 MHz, CDCl3) δ 0.68 (s, 3H, H-28), 0.79 (s, 3H, H-27), 0.81 (s, 3H, H-23), 0.85 (d, J = 4.2 Hz, 3H, H-29), 0.90 (s, 3H, H-24), 0.93 (s, 3H, H-26), 1.01 (s, 3H, H-25), 1.53 (s, 3H, H-30), 1.56 (d br, J = 10.0 Hz, 1H, H-18), 1.85 (d br, J = 10.0 Hz, 1H, H-19), 2.28 (s, 2H, H-7), 3.52 (dd, J = 4.8, 7.6 Hz, 1H, H-3), 5.36 (d, J = 4.7 Hz, 1H, H-6); 13C NMR: Table 1.
Acetylation of 1, 2 and 3: Compounds 1, 2 and 3 (20 mg each) were dissolved separately in C5H5N (2 mL) and Ac2O (2 mL) was added. The reaction mixtures were left overnight, diluted with water and extracted with chloroform. The chloroform solutions were washed with 5% HCl–H2O solution (10 mL each time) and dried over anhydrous K2CO3. After removal of the solvent, the crude acetates were purified by column chromatography on silica gel using petroleum ether–benzene (9:1, 7:3 and 1:1 v/v) when 1a, 2a and 3a (18 mg, 17 mg and 19 mg, respectively) were recovered.
Oxidation of 1 and 2: Compound 1 (12 mg) and compound 2 (15 mg) were dissolved separately in C5H5N (1 mL) and treated with freshly prepared CrO3–C5H5N complex. The reaction mixtures were left overnight, diluted with water (10 mL) and extracted with chloroform (3 × 20 mL). The chloroform layer was washed with water, 0.1 N HCl, water and dried over anhydrous MgSO4. After removal of the solvent, the residues were purified by column chromatography over silica gel using a petroleum ether–benzene (1:1 v/v) solvent system, and crystallized from CHCl3–Me2CO.
Supporting Information
| Supporting Information File 1: Spectral data of genicunolide A acetate (1a), genicunolide B acetate (2a), genicunolide C acetate (3a), oxogenicunolide A (1b), oxogenicunolide B (2b), friedelin (4) and friedelinol (5). | ||
| Format: PDF | Size: 212.9 KB | Download |
Acknowledgements
The authors are highly thankful to the Director of NIT Srinagar for providing all types of facilities during the work. One of the authors, Alia Farozi, would like to thank the Ministry of Human Resource Development (MHRD) New Delhi for providing the Institute Fellowship to carry out this work.
References
-
Stebbins, G. L.; Hoogland, R. D. Plant Syst. Evol. 1976, 125, 139–154.
Return to citation in text: [1] [2] -
Evans, F. J.; Taylor, S. E. Progress in the Chemistry of Organic Natural Products; Springer-Verlag: New York, 1983; Vol. 44.
Return to citation in text: [1] -
Singla, A. K.; Pathak, K. Fitoterapia 1990, 61, 483–516.
Return to citation in text: [1] -
Upadhyay, R. R.; Zarintan, M. H.; Ansarin, M. Planta Med. 1976, 30, 32–34. doi:10.1055/s-0028-1097689
Return to citation in text: [1] -
Upadhyay, R. R.; Zarintan, M. H.; Ansarin, M. Planta Med. 1976, 30, 196–197. doi:10.1055/s-0028-1097717
Return to citation in text: [1] -
Upadhyay, R. R.; Mohaddes, G. Curr. Sci. 1987, 56, 1058–1059.
Return to citation in text: [1] -
Abdelgaleil, S. A. M.; Kassem, S. M. I.; Doe, M.; Baba, M.; Nakatani, M. Phytochemistry 2001, 58, 1135–1139. doi:10.1016/S0031-9422(01)00393-4
Return to citation in text: [1] -
Hohmann, J.; Rédei, D.; Evanics, F.; Kálmán, A.; Argay, G.; Bartók, T. Tetrahedron 2000, 56, 3619–3623. doi:10.1016/S0040-4020(00)00278-7
Return to citation in text: [1] -
Hohmann, J.; Molnár, J.; Rédei, D.; Evanics, F.; Forgo, P.; Kálmán, A.; Argay, G.; Szabó, P. J. Med. Chem. 2002, 45, 2425–2431. doi:10.1021/jm0111301
Return to citation in text: [1] -
Hohmann, J.; Rédei, D.; Forgo, P.; Molnár, J.; Dombi, G.; Zorig, T. J. Nat. Prod. 2003, 66, 976–979. doi:10.1021/np030036f
Return to citation in text: [1] -
Ravikanth, V.; Reddy, V. L. N.; Rao, T. P.; Diwan, P. V.; Ramakrishna, S.; Venkateswarlu, Y. Phytochemistry 2002, 59, 331–335. doi:10.1016/S0031-9422(01)00461-7
Return to citation in text: [1] -
Wang, L.-Y.; Wang, N.-L.; Yao, X.-S.; Miyata, S.; Kitanaka, S. J. Nat. Prod. 2002, 65, 1246–1251. doi:10.1021/np0200921
Return to citation in text: [1] -
Öksüz, S.; Gürek, F.; Gil, R. R.; Pengsuparp, T.; Pezzuto, J. M.; Cordell, G. A. Phytochemistry 1995, 38, 1457–1462. doi:10.1016/0031-9422(94)00806-5
Return to citation in text: [1] -
Ahmad, V. U.; Hussain, H.; Hussain, J.; Jassbi, A. R.; Bukhari, I. A.; Yasin, A.; Choudhary, M. I.; Dar, A. Z. Naturforsch., B: J. Chem. Sci. 2002, 57, 1066–1071.
Return to citation in text: [1] -
Jassbi, A. R. Phytochemical Investigations on Some Medicinal Plants from Families Euphorbiaceae and Lamiaceae. Ph.D. Thesis, HEJ Research Institute of Chemistry, Karachi University, Pakistan, 2000.
Return to citation in text: [1] -
Jassbi, A. R.; Zamanizadehnajari, S.; Tahara, S. Z. Naturforsch., C: J. Biosci. 2004, 59, 15–18.
Return to citation in text: [1] -
Ahmad, V. U.; Zahid, M.; Khan, T.; Asim, M.; Ahmad, A. Proc. Pak. Acad. Sci. 2002, 39, 201–205.
Return to citation in text: [1] -
Ulubelen, A.; Aynehchi, Y.; Halfon, B. Doga: Tip Eczacilik 1986, 10, 211–213.
Chem. Abstr. 1986, 105, 168929v.
Return to citation in text: [1] [2] -
De, P. T.; Urones, J. G.; Marcos, I. S.; Basabe, P.; Cuadrado, M. J. S.; Moro, R. F. Phytochemistry 1987, 26, 1767–1776. doi:10.1016/S0031-9422(00)82286-4
Return to citation in text: [1] -
Anjaneyulu, V.; Rao, G. S.; Connolly, J. D. Phytochemistry 1985, 24, 1610–1612. doi:10.1016/S0031-9422(00)81079-1
Return to citation in text: [1] -
Öksüz, S.; Shieh, H.-L.; Pezzuto, J. M.; Özhatay, N.; Cordell, G. A. Planta Med. 1993, 59, 472–473. doi:10.1055/s-2006-959736
Return to citation in text: [1] -
Pousset, J.-L.; Poisson, J. Tetrahedron Lett. 1969, 10, 1173–1174. doi:10.1016/S0040-4039(01)87834-5
Return to citation in text: [1] -
Bhakuni, D. S.; Joshi, P. P.; Uprety, H.; Kapil, R. S. Phytochemistry 1974, 13, 2541–2543. doi:10.1016/S0031-9422(00)86933-2
Return to citation in text: [1] -
Bindra, R. S.; Satti, N. K.; Suri, O. P. Phytochemistry 1988, 27, 2313–2315. doi:10.1016/0031-9422(88)80150-X
Return to citation in text: [1] -
Nadkarni, A. K. Indian Materia Medica; Popular Prakashan: Bombay, India, 1976.
Return to citation in text: [1] -
Jafri, S. M. H. Flora of Karachi; The Book Corp.: Karachi, 1966.
Return to citation in text: [1] -
Sharma, B. M.; Kachroo, P. Flora of Jammu and Plants of Neighbourhood; Narosa Publications: India, 1981.
Return to citation in text: [1] -
Ismail, S. I.; el-Missiry, M. M.; Hammoida, F. M.; Rizk, A. M. Pharmazie 1977, 32, 538–542.
Return to citation in text: [1] -
Rizk, A. M.; Hammouda, F. M.; el-Missiry, M. M.; Radwan, H. M.; Evans, F. J. Phytochemistry 1985, 24, 1605–1606. doi:10.1016/S0031-9422(00)81076-6
Return to citation in text: [1] -
Tripathi, R. D.; Tiwari, K. P. Phytochemistry 1980, 19, 2163–2166. doi:10.1016/S0031-9422(00)82215-3
Return to citation in text: [1] -
Klass, J.; Tinto, W. F.; McLean, S.; Reynolds, W. F. J. Nat. Prod. 1992, 55, 1626–1630. doi:10.1021/np50089a010
Return to citation in text: [1] [2] -
Ho, L.-K.; Chang, C.-R.; Chang, Y.-S. J. Chin. Chem. Soc. 1995, 42, 93–95. doi:10.1002/jccs.199500016
Return to citation in text: [1] [2] -
Brieskorn, C. H.; Capuano, L. Chem. Ber. 1953, 86, 866–873. doi:10.1002/cber.19530860709
Return to citation in text: [1] -
Hashimoto, Y. An. Acad. Bras. Cien. 1970, 42 (suppl.). Chem. Abstr. 1971, 75, 58443.
Return to citation in text: [1] -
Razdan, T. K.; Kachroo, V.; Harkar, S.; Koul, G. L. Tetrahedron 1982, 38, 991–992. doi:10.1016/0040-4020(82)85077-1
Return to citation in text: [1] [2] [3] -
Williams, D. H.; Bhacca, N. S.; Djerassi, C. J. Am. Chem. Soc. 1963, 85, 2810–2813. doi:10.1021/ja00901a031
Return to citation in text: [1] -
Fried, J.; Edwards, J. A. Organic reactions in steroid chemistry; van Nostrand Reinhold: New York, 1972.
Return to citation in text: [1] [2] -
Mahato, S. B.; Kundu, A. P. Phytochemistry 1994, 37, 1517–1575. doi:10.1016/S0031-9422(00)89569-2
Return to citation in text: [1] [2] [3] -
Budzikiewicz, H.; Wilson, J. M.; Djerassi, C. J. Am. Chem. Soc. 1963, 85, 3688–3699. doi:10.1021/ja00905a036
Return to citation in text: [1] [2] -
Tanaka, R.; Matsunaga, S. Phytochemistry 1988, 27, 3579–3584. doi:10.1016/0031-9422(88)80772-6
Return to citation in text: [1] -
Ito, K.; Lai, J. Yakugaku Zasshi 1978, 98, 1285–1287.
Return to citation in text: [1] -
Matsunaga, S.; Tanaka, R.; Akagi, M. Phytochemistry 1988, 27, 535–537. doi:10.1016/0031-9422(88)83136-4
Return to citation in text: [1] -
Savona, G.; Bruno, M.; Rodriguez, B.; Marko, J. L. Phytochemistry 1987, 26, 3305–3308. doi:10.1016/S0031-9422(00)82493-0
Return to citation in text: [1] -
Rai, N.; Singh, J. Indian J. Chem., Sect. B 2001, 40, 320–323.
Return to citation in text: [1]
| 38. | Mahato, S. B.; Kundu, A. P. Phytochemistry 1994, 37, 1517–1575. doi:10.1016/S0031-9422(00)89569-2 |
| 39. | Budzikiewicz, H.; Wilson, J. M.; Djerassi, C. J. Am. Chem. Soc. 1963, 85, 3688–3699. doi:10.1021/ja00905a036 |
| 40. | Tanaka, R.; Matsunaga, S. Phytochemistry 1988, 27, 3579–3584. doi:10.1016/0031-9422(88)80772-6 |
| 7. | Abdelgaleil, S. A. M.; Kassem, S. M. I.; Doe, M.; Baba, M.; Nakatani, M. Phytochemistry 2001, 58, 1135–1139. doi:10.1016/S0031-9422(01)00393-4 |
| 8. | Hohmann, J.; Rédei, D.; Evanics, F.; Kálmán, A.; Argay, G.; Bartók, T. Tetrahedron 2000, 56, 3619–3623. doi:10.1016/S0040-4020(00)00278-7 |
| 9. | Hohmann, J.; Molnár, J.; Rédei, D.; Evanics, F.; Forgo, P.; Kálmán, A.; Argay, G.; Szabó, P. J. Med. Chem. 2002, 45, 2425–2431. doi:10.1021/jm0111301 |
| 10. | Hohmann, J.; Rédei, D.; Forgo, P.; Molnár, J.; Dombi, G.; Zorig, T. J. Nat. Prod. 2003, 66, 976–979. doi:10.1021/np030036f |
| 11. | Ravikanth, V.; Reddy, V. L. N.; Rao, T. P.; Diwan, P. V.; Ramakrishna, S.; Venkateswarlu, Y. Phytochemistry 2002, 59, 331–335. doi:10.1016/S0031-9422(01)00461-7 |
| 12. | Wang, L.-Y.; Wang, N.-L.; Yao, X.-S.; Miyata, S.; Kitanaka, S. J. Nat. Prod. 2002, 65, 1246–1251. doi:10.1021/np0200921 |
| 13. | Öksüz, S.; Gürek, F.; Gil, R. R.; Pengsuparp, T.; Pezzuto, J. M.; Cordell, G. A. Phytochemistry 1995, 38, 1457–1462. doi:10.1016/0031-9422(94)00806-5 |
| 22. | Pousset, J.-L.; Poisson, J. Tetrahedron Lett. 1969, 10, 1173–1174. doi:10.1016/S0040-4039(01)87834-5 |
| 23. | Bhakuni, D. S.; Joshi, P. P.; Uprety, H.; Kapil, R. S. Phytochemistry 1974, 13, 2541–2543. doi:10.1016/S0031-9422(00)86933-2 |
| 24. | Bindra, R. S.; Satti, N. K.; Suri, O. P. Phytochemistry 1988, 27, 2313–2315. doi:10.1016/0031-9422(88)80150-X |
| 38. | Mahato, S. B.; Kundu, A. P. Phytochemistry 1994, 37, 1517–1575. doi:10.1016/S0031-9422(00)89569-2 |
| 4. | Upadhyay, R. R.; Zarintan, M. H.; Ansarin, M. Planta Med. 1976, 30, 32–34. doi:10.1055/s-0028-1097689 |
| 5. | Upadhyay, R. R.; Zarintan, M. H.; Ansarin, M. Planta Med. 1976, 30, 196–197. doi:10.1055/s-0028-1097717 |
| 6. | Upadhyay, R. R.; Mohaddes, G. Curr. Sci. 1987, 56, 1058–1059. |
| 25. | Nadkarni, A. K. Indian Materia Medica; Popular Prakashan: Bombay, India, 1976. |
| 26. | Jafri, S. M. H. Flora of Karachi; The Book Corp.: Karachi, 1966. |
| 35. | Razdan, T. K.; Kachroo, V.; Harkar, S.; Koul, G. L. Tetrahedron 1982, 38, 991–992. doi:10.1016/0040-4020(82)85077-1 |
| 20. | Anjaneyulu, V.; Rao, G. S.; Connolly, J. D. Phytochemistry 1985, 24, 1610–1612. doi:10.1016/S0031-9422(00)81079-1 |
| 37. | Fried, J.; Edwards, J. A. Organic reactions in steroid chemistry; van Nostrand Reinhold: New York, 1972. |
| 2. | Evans, F. J.; Taylor, S. E. Progress in the Chemistry of Organic Natural Products; Springer-Verlag: New York, 1983; Vol. 44. |
| 21. | Öksüz, S.; Shieh, H.-L.; Pezzuto, J. M.; Özhatay, N.; Cordell, G. A. Planta Med. 1993, 59, 472–473. doi:10.1055/s-2006-959736 |
| 39. | Budzikiewicz, H.; Wilson, J. M.; Djerassi, C. J. Am. Chem. Soc. 1963, 85, 3688–3699. doi:10.1021/ja00905a036 |
| 16. | Jassbi, A. R.; Zamanizadehnajari, S.; Tahara, S. Z. Naturforsch., C: J. Biosci. 2004, 59, 15–18. |
| 18. |
Ulubelen, A.; Aynehchi, Y.; Halfon, B. Doga: Tip Eczacilik 1986, 10, 211–213.
Chem. Abstr. 1986, 105, 168929v. |
| 43. | Savona, G.; Bruno, M.; Rodriguez, B.; Marko, J. L. Phytochemistry 1987, 26, 3305–3308. doi:10.1016/S0031-9422(00)82493-0 |
| 15. | Jassbi, A. R. Phytochemical Investigations on Some Medicinal Plants from Families Euphorbiaceae and Lamiaceae. Ph.D. Thesis, HEJ Research Institute of Chemistry, Karachi University, Pakistan, 2000. |
| 19. | De, P. T.; Urones, J. G.; Marcos, I. S.; Basabe, P.; Cuadrado, M. J. S.; Moro, R. F. Phytochemistry 1987, 26, 1767–1776. doi:10.1016/S0031-9422(00)82286-4 |
| 38. | Mahato, S. B.; Kundu, A. P. Phytochemistry 1994, 37, 1517–1575. doi:10.1016/S0031-9422(00)89569-2 |
| 14. | Ahmad, V. U.; Hussain, H.; Hussain, J.; Jassbi, A. R.; Bukhari, I. A.; Yasin, A.; Choudhary, M. I.; Dar, A. Z. Naturforsch., B: J. Chem. Sci. 2002, 57, 1066–1071. |
| 18. |
Ulubelen, A.; Aynehchi, Y.; Halfon, B. Doga: Tip Eczacilik 1986, 10, 211–213.
Chem. Abstr. 1986, 105, 168929v. |
| 17. | Ahmad, V. U.; Zahid, M.; Khan, T.; Asim, M.; Ahmad, A. Proc. Pak. Acad. Sci. 2002, 39, 201–205. |
| 42. | Matsunaga, S.; Tanaka, R.; Akagi, M. Phytochemistry 1988, 27, 535–537. doi:10.1016/0031-9422(88)83136-4 |
| 29. | Rizk, A. M.; Hammouda, F. M.; el-Missiry, M. M.; Radwan, H. M.; Evans, F. J. Phytochemistry 1985, 24, 1605–1606. doi:10.1016/S0031-9422(00)81076-6 |
| 27. | Sharma, B. M.; Kachroo, P. Flora of Jammu and Plants of Neighbourhood; Narosa Publications: India, 1981. |
| 28. | Ismail, S. I.; el-Missiry, M. M.; Hammoida, F. M.; Rizk, A. M. Pharmazie 1977, 32, 538–542. |
| 31. | Klass, J.; Tinto, W. F.; McLean, S.; Reynolds, W. F. J. Nat. Prod. 1992, 55, 1626–1630. doi:10.1021/np50089a010 |
| 32. | Ho, L.-K.; Chang, C.-R.; Chang, Y.-S. J. Chin. Chem. Soc. 1995, 42, 93–95. doi:10.1002/jccs.199500016 |
| 36. | Williams, D. H.; Bhacca, N. S.; Djerassi, C. J. Am. Chem. Soc. 1963, 85, 2810–2813. doi:10.1021/ja00901a031 |
| 37. | Fried, J.; Edwards, J. A. Organic reactions in steroid chemistry; van Nostrand Reinhold: New York, 1972. |
| 34. | Hashimoto, Y. An. Acad. Bras. Cien. 1970, 42 (suppl.). Chem. Abstr. 1971, 75, 58443. |
| 35. | Razdan, T. K.; Kachroo, V.; Harkar, S.; Koul, G. L. Tetrahedron 1982, 38, 991–992. doi:10.1016/0040-4020(82)85077-1 |
| 35. | Razdan, T. K.; Kachroo, V.; Harkar, S.; Koul, G. L. Tetrahedron 1982, 38, 991–992. doi:10.1016/0040-4020(82)85077-1 |
| 32. | Ho, L.-K.; Chang, C.-R.; Chang, Y.-S. J. Chin. Chem. Soc. 1995, 42, 93–95. doi:10.1002/jccs.199500016 |
| 33. | Brieskorn, C. H.; Capuano, L. Chem. Ber. 1953, 86, 866–873. doi:10.1002/cber.19530860709 |
| 30. | Tripathi, R. D.; Tiwari, K. P. Phytochemistry 1980, 19, 2163–2166. doi:10.1016/S0031-9422(00)82215-3 |
| 31. | Klass, J.; Tinto, W. F.; McLean, S.; Reynolds, W. F. J. Nat. Prod. 1992, 55, 1626–1630. doi:10.1021/np50089a010 |
© 2015 Farozi et al; licensee Beilstein-Institut.
This is an Open Access article under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
The license is subject to the Beilstein Journal of Organic Chemistry terms and conditions: (http://www.beilstein-journals.org/bjoc)