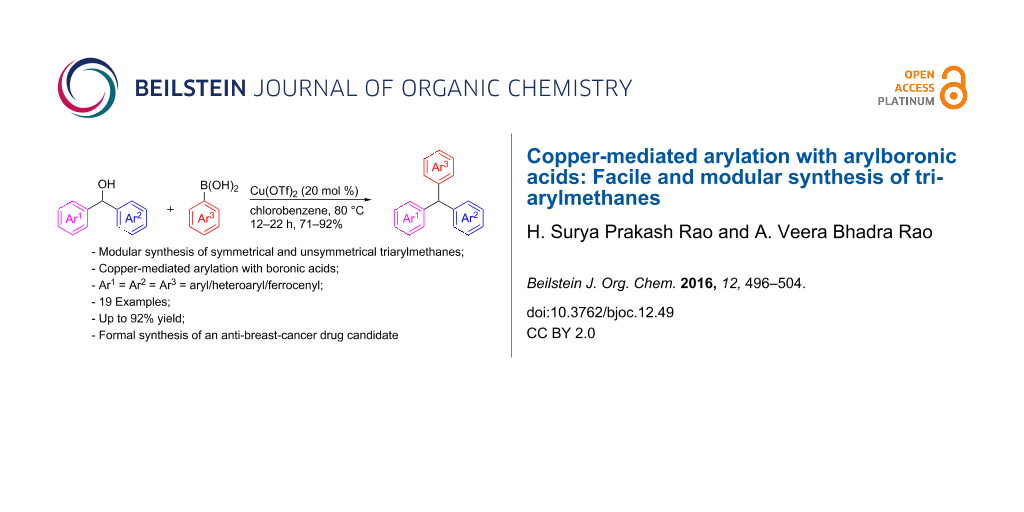
Abstract
A facile and modular synthesis of triarylmethanes was achieved in good yield via a two-step sequence in which the final step is the copper(II)-catalyzed arylation of diarylmethanols with arylboronic acids. By using this protocol a variety of symmetrical and unsymmetrical triarylmethanes were synthesized. As an application of the newly developed methodology, we demonstrate a high-yielding synthesis of the triarylmethane intermediate towards an anti-breast-cancer drug candidate.
Graphical Abstract
Introduction
The triarylmethanes form an exclusive group of organic molecules wherein three aryl groups are attached to the central sp3-hybridized carbon atom bearing a hydrogen atom [1-4]. Although the group can be restricted to such molecules, many closely related derivatives that have a triarylmethane motif (like those having a heteroatom attached to the central carbon atom or the central carbon is sp2 hybridized) have been included in this class [5]. Molecules with a triarylmethane motif are ubiquitous and found mainly in technologically and medicinally relevant molecules like dyes [6-9], pH indicators [10-12], fluorescent probes [13-18] and antibacterial drugs [19]. For example, malachite green (1) is a dye, cresol red (2) is a pH indicator and turbomycin (3) is an antibacterial medicinal drug (Figure 1). Genuine triarylmethane 4, having three different aryl groups on the central CH, is a proven anti-breast-cancer agent [20]. In addition to 4, several other triarylmethanes exhibit interesting biological activity, including oestrogen receptor binding affinity [21], inhibition of hepatic cholesterol [22], inhibition of aldose reductase [23], antiproliferative [24], antiviral, cytotoxic [25], antifungal [26], anti-HIV [27-29] and antibacterial activity [30]. Although rare, there are a few natural products, for example, melanervin (5), a flavanoid bearing the triarylmethane motif [31,32].
Figure 1: Representative examples of triarylmethanes.
Figure 1: Representative examples of triarylmethanes.
Triarylmethanes are typically synthesized by a Friedel–Crafts-type substitution of the three alkoxy groups in a trialkyl orthoformate (Scheme 1A, method 1) [33-35] or by sequential two-step addition of electron-rich aromatic nucleophiles to activated arene aldehydes followed by substitution of the resulting hydroxy group with another electron-rich aromatic compound (Scheme 1A, method 2) [36-39]. Both of the approaches are limited in scope and suffer from drawbacks such as (a) electron-rich aromatic systems that are required as nucleophiles and therefore, not amenable for the synthesis of triarylmethanes with electron-withdrawing groups, (b) the regioselectivity in the substitution at the aromatic ring that depends on the ortho- or para-directing nature of the substituent and also by the steric hindrance offered by the substitution, (c) the methods are rarely modular and not suitable for the preparation of triarylmethanes with three different aryl groups, and finaly, (d) Lewis [40,41] or protic acids [42] are required to catalyze the reactions. To overcome the above-mentioned difficulties, many efforts have recently been directed towards transition metal-catalyzed cross-couplings [43-48] or CH arylation followed by an arylative desulfonation [49,50]. The coupling reactions provide an opportunity to install an unactivated aryl group on a carbon bearing two more aryl groups to synthesize the triarylmethane motif. Recently, we reported a copper-catalyzed C–C bond formation by substitution of the labile C(4)SMe group in 4H-chromenes or C(3)–OH in isoindolinones with aryl/alkenyl groups by employing the corresponding boronic acids [51,52]. Continuing these efforts, we designed a copper-catalyzed synthesis of a variety of triarylmethanes through substitution of C(sp3)–OH in diarylmethanols with arylboronic acids (Scheme 1B). We reasoned that since diarylmethanols with two different aromatic rings can be made by a wide variety of methods [53,54] (e.g., addition of an aryl carbanion to an aryl aldehyde and a further step with a variety of aryl boronic acids), it should be possible to provide a unique opportunity for the modular synthesis of unsymmetrical triarylmethanes. If successful, the method could provide an opportunity for the synthesis of a combinatorial library of the coveted molecules. Herein, we report a copper(II) triflate-catalyzed modular synthesis of triarylmethanes by employing diarylmethanols 9 and arylboronic acids 10. It is advantageous to employ a base metal catalyst such as copper(II) triflate instead of palladium [55,56] or nickel (Ni) [57] catalysts and to avoid the use of phosphine ligands as it is less expensive and more readily facilitates purification.
Scheme 1: General methods and proposed method for the synthesis of triarylmethanes.
Scheme 1: General methods and proposed method for the synthesis of triarylmethanes.
Results and Discussion
We selected the copper-mediated cross-coupling reaction of diphenylmethanol (9a) with phenylboronic acid (10a) for the synthesis of triphenylmethane (11a) to optimize the reaction conditions and catalyst loading. Based on our accrued experience [52], in a first attempt, we employed Cu(OTf)2 (10 mol %) in refluxing 1,2-dichlorethane (DCE) to effect C–C coupling, but the reaction provided (phenoxymethylene)dibenzene (12) as the only product formed through the C–O coupling (Chan–Lam–Evans coupling product) [58-60] in 64% yield.
To obtain the desired triphenylmethane (11a) as the sole product, we screened various alternative solvents (Scheme 2). Of the solvents investigated, toluene gave a mixture of triphenylmethane (11a) and the toluene-incorporated product (p-tolylmethylene)dibenzene (14) in 36% and 52% yield, respectively (Scheme 2). Solvents like acetonitrile (polar, aprotic) and dioxane (oxygenated, aprotic) did not provide the triphenylmethane (11a). On the other hand, the higher-boiling, nonpolar chlorobenzene (Scheme 2) at 80 °C provided the coupled product triphenylmethane (11a) in 64% yield. When the reaction was conducted under oxygen atmosphere, the yield of 11a fell to 46%. Under these aerobic conditions, we isolated biphenyl (13) generated through homocoupling of phenylboronic acid. Attempts to improve the yield by the use of bases such as Na2CO3 (10 mol %) and K2CO3 (10 mol %) were not successful. The yield of triphenylmethane (11a) was further reduced in these cases. The base was employed to trap the boric acid, which is likely to be the side product of the reaction. From the above experiments, we concluded that chlorobenzene was the most suitable solvent for the synthesis of triphenylmethane (11a).
Scheme 2: Role of solvent and reaction conditions in the Cu(OTf)2-mediated coupling of diphenylmethanol (9a) with phenylboronic acid (10a) for the preparation of triphenylmethane (11a).
Scheme 2: Role of solvent and reaction conditions in the Cu(OTf)2-mediated coupling of diphenylmethanol (9a) ...
Next, we turned our attention to evaluate different copper salts to optimize the yield of triphenylmethane (11a); these efforts have been summarized in Table 1. We screened various Cu(II) catalysts such as Cu(OAc)2 (64%, Table 1, entry 1), Cu(CF3COO)2 (46%, Table 1, entry 2) Cu(acac)2 (36%, Table 1, entry 3), CuSO4·5H2O (36%, Table 1, entry 4), CuBr2 (14%, Table 1, entry 5), CuCl2·2H2O (24%, Table 1, entry 6) and CuO (no reaction, Table 1, entry 8), which did not provide the desired triphenylmethane (11a) in better yield. However, 20 mol % of Cu(OTf)2 (Table 1, entry 17) delivered the desired triphenylmethane (11a) in good yield (78%) after chromatographic purification. We screened other borderline Lewis acids such as Sc(OTf)3, Yb(OTf)3, Zn(OTf)2 and Fe(OTf)3, but the reaction did not afford triphenylmethane (11a) at all, which indicated that Cu(II) and not TfOH is responsible for the transformation. Thus, the optimal conditions for the copper-mediated coupling involve heating equimolar amounts of diphenylmethanol (9a) and phenylboronic acid (10a) in chlorobenzene at 80 °C in the presence of 20 mol % of Cu(OTf)2 under a blanket of oxygen-free nitrogen.
Table 1: Screening of metal catalysts for the arylation reaction.
|
|
||||
| Entry | Catalyst | Time (h) | Catalyst (mol %) | Yield (%)a |
|---|---|---|---|---|
| 1 | Cu(OAc)2·H2O | 21 | 10 | 64 |
| 2 | Cu(OOCCF3)2 | 18 | 10 | 46 |
| 3 | Cu(acac)2 | 21 | 10 | 36 |
| 4 | CuSO4·5H2O | 19 | 10 | 36 |
| 5 | CuBr2 | 16 | 10 | 14 |
| 6 | CuCl2·H2O | 16 | 10 | 24 |
| 7 | Cu(OTf)2 | 21 | 10 | 68 |
| 8 | CuO | 12 | 10 | n.r. |
| 9 | CuI | 21 | 10 | 42 |
| 10 | CuBr | 21 | 10 | 14 |
| 11 | CuCl | 19 | 10 | 28 |
| 12 | Cu2O | 12 | 10 | n.r. |
| 13 | Cu(I)BrSMe2 | 12 | 10 | n.r. |
| 14 | Cu(PPh3)2Br | 12 | 10 | n.r. |
| 15 | CuMeSal | 21 | 10 | 42 |
| 16 | CuTc | 21 | 10 | 27 |
| 17 | Cu(OTf)2 | 18 | 20 | 78 |
| 18 | Sc(OTf)3 | 12 | 10 | n.r. |
| 19 | Fe(OTf)3 | 12 | 10 | n.r. |
| 20 | Zn(OTf)2 | 12 | 10 | n.r. |
| 21 | Yb(OTf)3 | 12 | 10 | n.r. |
an.r. = no reaction.
Based on the above observations, we propose a mechanism for the copper-mediated coupling of phenylboronic acid with diphenylmethanol, leading to triphenylmethane and boric acid (Scheme 3). At the start of the cascade, the first step is the transmetallation of the copper(II) into phenylboronic acid to form reactive PhCu(OTf) (15) and B(OH)2(OTf) [61]. The intermediate 15 then reacts with diphenylmethanol 9 to provide the intermediate 16. Formation of the intermediate 16 can be attributed to Lewis acidic characteristics of 15 and Lewis basic characteristics of diphenylmethanol (9a). The crucial C–C bond formation with simultaneous C–O bond cleavage subsequently occurs in 16 to give the triarylmethane 11 and copper(OH)(OTf) (17). The reaction of 17 with arylboronic acid 10 regenerates 15 and results in the formation of stable boric acid. The driving force for the triarylmethane formation is the generation of a stable C–C bond in 11, a Cu–O bond in 17, and boric acid at the cost of weak Ar–Cu and C–OH bonds in 16 and 9, respectively [52].
Scheme 3: A plausible mechanism for the formation of triarylmethanes 11.
Scheme 3: A plausible mechanism for the formation of triarylmethanes 11.
With the optimized reaction conditions in hand, we examined the scope of the cross-coupling reaction for the synthesis of a variety of triarylmethanes from diphenylmethanol (9a). Ten more arylboronic acids were employed in the coupling reaction and good yields (77–92%) of the corresponding triarylmethanes 11b–k were realized (Table 2). The arylboronic acids 10b–k were selected considering their structural diversity and electron density in the aryl ring. Efficient cross-coupling could be noted irrespective of the presence of strongly electron-withdrawing (10b,c to 11b,c; Table 2, entries 1 and 2), mildly electron-withdrawing (10d,e to 11d,e; Table 2, entries 3 and 4), strongly electron-donating (10f to 11f; Table 2, entry 5) or mildly electron-donating (10g to 11g; Table 2, entry 6) groups at the C(4) position of the phenyl ring. The robust nature of the protocol was demonstrated by reacting ortho-methoxyphenylboronic acid (10h) to efficiently generate the desired triarylmethane 11h (Table 2, entry 7). The transformation showed that apart from the insensitivity towards electronic effects, the copper-mediated cross-coupling reaction is not very sensitive to steric crowding in the neighborhood of the reaction center. Next, we employed heteroaromatic boronic acids, such as furan-2-ylboronic acid (10i; Table 2, entry 8), thiophen-2-ylboronic acid (10j; Table 2, entry 9) and benzo[b]thiophen-2-ylboronic acid (10k; Table 2, entry 10) in the coupling reaction and the reactions furnished the corresponding triarylmethanes 11a–k in excellent yield. The transformations showed that heteroaromatic groups, including those bearing a sulfur atom, react efficiently to provide triarylmethanes.
Table 2: Scope of the Cu-catalyzed arylation with various arylboronic acids.
|
|
||||
| Entry | Ar in arylboronic acid | Triarylmethane | Time (h) | Yield (%) |
|---|---|---|---|---|
| 1 | 10b: 4-CF3C6H4 | 11b | 4 | 92 |
| 2 | 10c: 4-FC6H4 | 11c | 10 | 77 |
| 3 | 10d: 4-ClC6H4 | 11d | 6 | 79 |
| 4 | 10e: 4-BrC6H4 | 11e | 4 | 91 |
| 5 | 10f: 4-MeOC6H4 | 11f | 5 | 81 |
| 6 | 10g: 4-MeC6H4 | 11g | 6 | 81 |
| 7 | 10h: 2,5-(OMe)2C6H3 | 11h | 6 | 88 |
| 8 | 10i: 2-furyl | 11i | 8 | 85 |
| 9 | 10j: 2-thiophenyl | 11j | 8 | 89 |
| 10 | 10k: 2-benzothiophenyl | 11k | 10 | 86 |
However, when we employed 2,6-dimethoxyphenylboronic acid 10l, surprisingly, we isolated the triarylmethane 11l, in which the C–C bond formation took place on the C(3) carbon of the 2,6-dimethoxyphenylboronic acid instead of the C(1) carbon, as illustrated in 11m (Scheme 4). Structure of 11l was readily confirmed on the basis of 13C NMR and DEPT-135 spectra. We surmise that the initially formed, transmetallated product 18 rearranged to the more stable 18a before it could react with diphenylmethanol (9a, Scheme 4).
Scheme 4: Copper-catalyzed C–C bond formation synthesis of triarylmethane 10l.
Scheme 4: Copper-catalyzed C–C bond formation synthesis of triarylmethane 10l.
The scope of the copper-catalyzed coupling reaction of diarylmethanols 9b–d with phenylboronic acid (10a) was explored by changing one or both of the aryl rings in the diarylmethanol (Table 3) [62]. The copper-catalyzed reaction of phenyl(pyren-1-yl)methanol (9b) with phenylboronic acid (10a) was very facile and the product triarylmethane 11n was obtained in 72% yield (Table 3, entry 1). Similarly, the reaction of anthracen-9-yl(phenyl)methanol (9c) with phenylboronic acid (10a) provided the corresponding triarylmethane 11o in 82% yield (Table 3, entry 2). The last example in the genre is interesting, as one of the aryl rings is ferrocene in 11p. The reaction of ferrocene-1-yl(phenyl)methanol (9d) with phenyboronic acid (10a) was facile and it provided diphenylmethylferrocene (11p) without any difficulty in 71% yield.
Table 3: Scope of diarylmethanol 9b–d in the copper-catalyzed coupling reaction.
| Entry | Diarylmethanol | Arylboronic acid | Triarylmethanea |
|---|---|---|---|
| 1 |
9b |
10a |
11n (14 h, 72%) |
| 2 |
9c |
10a |
11o (16 h, 83%) |
| 3 |
9d |
10a |
11p (16 h, 71%) |
| 4 |
9b |
10f |
11q (16 h, 68%) |
| 5 |
9b |
10k |
11r (12 h, 72%) |
| 6 |
9d |
10m |
11s (4 h, 64%) |
aTime required for completion of the reaction and yield of the isolated and purified triarylmethanes are given in the parenthesis.
Modular synthesis of triarylmethanes
The synthetic method that we developed, through which three different aromatic rings on the central carbon can be assembled in a two-step protocol, is modular in nature. The first step is the synthesis of diarylmethanol and the second step is the replacement of the hydroxy group in the resulting diarylmethanol by a third aryl group by employing arylboronic acid under copper catalysis. As a proof of principle, we present the synthesis of three examples of triarylmethanes 11q–s that bear three different aromatic rings (Table 3). The copper-catalyzed coupling reaction of phenyl(pyren-1-yl)methanol (9b) with 4-methoxyphenylboronic acid (10f) and benzo[b]thiophen-2-ylboronic acid (10k) provided the respective pyrene-containing unsymmetrical triarylmethanes 11q–r in good yields (Table 3). Next, the coupling reaction of phenyl(ferrocenyl)methanol (9d) with thiophen-3-ylboronic acid (10m) provided triarylmethane 11s, which has ferrocene, thiophene and phenyl rings installed on the central carbon. The triarylmethane 11s was found to be unstable when kept as a solution in hexane. However, the compound was stable as a solid for at least two months when refrigerated (+5 °C).
To demonstrate an application of our newly developed Cu(OTf)2-catalyzed C–C coupling reaction for the synthesis of triarylmethanes, we designed a synthesis of the precursor 22 (Scheme 5) for the anti-breast-cancer agent 4 (Figure 1). Any method for the synthesis of 4 needs to take into account that it has two phenyl rings with different alkoxy groups at the respective C(4) position. We reasoned that one of the aryl groups could be a part of diarylmethanol and the other of the arylboronic acid. We designed the protection of the C(4) hydroxy group in the arylboronic aicd with the photolabile 2-nitrobenzyl (NB) group, so that it can be removed without affecting the rest of the molecule. The synthesis of triarylmethane 22 began with the preparation of the starting diarylmethanol 20, which was accomplished by the addition the anion from 9-bromophenanthrene [63] 19 to 4-methoxybenzaldehdye. The resulting diarylmethanol 20 was treated with bis(pinacolato)diboron [64] 10n, which has an 2-nitrobenzyl protecting group on the phenolic hydroxy group [52]. The reaction was conducted in the presence of 20 mol % Cu(OTf)2 under optimized conditions, providing triarylmethane 21 in 76% yield. Deprotection of the phenolic hydroxy group in 21 was facile under photocatalytic conditions by using UV LED lamps in wet acetonitrile. The reaction furnished the synthetic intermediate 22 in 86% yield [52]. Since the intermediate 22 has been previously converted into the drug candidate 4 [49], our efforts constitute a formal, alternate synthesis.
Scheme 5: Synthesis of anti-breast-cancer agent intermediate 22.
Scheme 5: Synthesis of anti-breast-cancer agent intermediate 22.
Conclusion
In conclusion, we have demonstrated a facile Cu(OTf)2-catalyzed synthesis of a variety of triarylmethanes from readily available diarylmethanols and arylboronic acids. This method is a novel synthetic approach for the preparation of multisubstituted triarylmethanes starting from easily preparable diarylmethanols and commercially available arylboronic acids. Structurally diverse, unsymmetrical triarylmethanes were prepared by employing this methodology. As an application to the newly developed methodology, we achieved a facile synthesis of the penultimate intermediate of an anti-breast-cancer agent. Hopefully the work described here will stimulate further work for the synthesis of a wide variety of triarylmethanes with tailor-made properties.
Supporting Information
| Supporting Information File 1: Experimental procedures, characterization data, details of the NMR structural determination of all new compounds and copies of 1H, 13C NMR and DEPT-135 spectra for all compounds prepared. | ||
| Format: PDF | Size: 2.4 MB | Download |
References
-
Mondal, S.; Panda, G. RSC Adv. 2014, 4, 28317–28358. doi:10.1039/c4ra01341g
Return to citation in text: [1] -
Nair, V.; Thomas, S.; Mathew, S. C.; Abhilash, K. G. Tetrahedron 2006, 62, 6731–6747. doi:10.1016/j.tet.2006.04.081
Return to citation in text: [1] -
Shchepinov, M. S.; Korshun, V. A. Chem. Soc. Rev. 2003, 32, 170–180. doi:10.1039/b008900l
Return to citation in text: [1] -
Duxbury, D. F. Chem. Rev. 1993, 93, 381–433. doi:10.1021/cr00017a018
Return to citation in text: [1] -
Oda, M.; Kawase, T.; Wei, C. Pure Appl. Chem. 1996, 68, 267–274. doi:10.1351/pac199668020267
Return to citation in text: [1] -
Mason, C. D.; Nord, F. F. J. Org. Chem. 1951, 16, 722–727. doi:10.1021/jo01145a011
Return to citation in text: [1] -
Ghaisas, V. V.; Kane, B. J.; Nord, F. F. J. Org. Chem. 1958, 23, 560–565. doi:10.1021/jo01098a016
Return to citation in text: [1] -
Irie, M. J. Am. Chem. Soc. 1983, 105, 2078–2079. doi:10.1021/ja00345a075
Return to citation in text: [1] -
Muthyala, R.; Katritzky, A. R.; Lan, X. Dyes Pigm. 1994, 25, 303–324. doi:10.1016/0143-7208(94)87017-9
Return to citation in text: [1] -
Rottman, C.; Grader, G.; De Hazan, Y.; Melchior, S.; Avnir, D. J. Am. Chem. Soc. 1999, 121, 8533–8543. doi:10.1021/ja991269p
Return to citation in text: [1] -
Yamaguchi, K.; Tamura, Z.; Maeda, M. Anal. Sci. 1997, 13, 521–522. doi:10.2116/analsci.13.521
Return to citation in text: [1] -
Heger, D.; Klánová, J.; Klán, P. J. Phys. Chem. B 2006, 110, 1277–1287. doi:10.1021/jp0553683
Return to citation in text: [1] -
Miura, T.; Urano, Y.; Tanaka, K.; Nagano, T.; Ohkubo, K.; Fukuzumi, S. J. Am. Chem. Soc. 2003, 125, 8666–8671. doi:10.1021/ja035282s
Return to citation in text: [1] -
Haugland, R. P. Molecular Probes. The Molecular Probes Handbook. A Guide to Fluorescent Probes and Labeling Technologies, 10th ed.; Life Technologies Corporation: Eugene, USA, 2005.
Return to citation in text: [1] -
Urano, Y.; Kamiya, M.; Kanada, K.; Ueno, T.; Hirose, K.; Nagano, T. J. Am. Chem. Soc. 2005, 127, 4888–4894. doi:10.1021/ja043919h
Return to citation in text: [1] -
Abe, H.; Wang, J.; Furukawa, K.; Oki, K.; Uda, M.; Tsuneda, S.; Ito, Y. Bioconjugate Chem. 2008, 19, 1219–1226. doi:10.1021/bc800014d
Return to citation in text: [1] -
Kim, H. N.; Lee, M. H.; Kim, H. J.; Kim, J. S.; Yoon, J. Chem. Soc. Rev. 2008, 37, 1465–1472. doi:10.1039/b802497a
Return to citation in text: [1] -
Beija, M.; Afonso, C. A. M.; Martinho, J. M. G. Chem. Soc. Rev. 2009, 38, 2410–2433. doi:10.1039/b901612k
Return to citation in text: [1] -
Parai, M. K.; Panda, G.; Chaturvedi, V.; Manju, Y. K.; Sinha, S. Bioorg. Med. Chem. Lett. 2008, 18, 289–292. doi:10.1016/j.bmcl.2007.10.083
Return to citation in text: [1] -
Shagufta; Srivastava, A. K.; Sharma, R.; Mishra, R.; Balapure, A. K.; Murthy, P. S. R.; Panda, G. Bioorg. Med. Chem. 2006, 14, 1497–1505. doi:10.1016/j.bmc.2005.10.002
Return to citation in text: [1] -
Bindal, R. D.; Golab, J. T.; Katzenellenbogen, J. A. J. Am. Chem. Soc. 1990, 112, 7861–7868. doi:10.1021/ja00178a003
Return to citation in text: [1] -
Jendralla, H.; Granzer, E.; von Kerekjarto, B.; Krause, R.; Schacht, U.; Baader, E.; Bartmann, W.; Beck, G.; Bergmann, A.; Kesseler, K.; Wess, G.; Chen, L.-J.; Granata, S.; Herchen, J.; Kleine, H.; Schüssler, H.; Wagner, K. J. Med. Chem. 1991, 34, 2962–2983. doi:10.1021/jm00114a004
Return to citation in text: [1] -
Costantino, L.; Ferrari, A. M.; Gamberini, M. C.; Rastelli, G. Bioorg. Med. Chem. 2002, 10, 3923–3931. doi:10.1016/S0968-0896(02)00318-8
Return to citation in text: [1] -
Al-Qawasmeh, R. A.; Lee, Y.; Cao, M.-Y.; Gu, X.; Vassilakos, A.; Wright, J. A.; Young, A. Bioorg. Med. Chem. Lett. 2004, 14, 347–350. doi:10.1016/j.bmcl.2003.11.004
Return to citation in text: [1] -
Mibu, N.; Yokomizo, K.; Uyeda, M.; Sumoto, K. Chem. Pharm. Bull. 2003, 51, 1325–1327. doi:10.1248/cpb.51.1325
Return to citation in text: [1] -
Schultz, T. W.; Sinks, G. D.; Cronin, M. T. D. Environ. Toxicol. 2002, 17, 14–23. doi:10.1002/tox.10027
Return to citation in text: [1] -
Wang, P.; Kozlowski, J.; Cushman, M. J. Org. Chem. 1992, 57, 3861–3866. doi:10.1021/jo00040a027
Return to citation in text: [1] -
Long, Y.-Q.; Jiang, X.-H.; Dayam, R.; Sanchez, T.; Shoemaker, R.; Sei, S.; Neamati, N. J. Med. Chem. 2004, 47, 2561–2573. doi:10.1021/jm030559k
Return to citation in text: [1] -
Cushman, M.; Kanamathareddy, S.; De Clerq, E.; Schols, D.; Goldman, M. E.; Bowen, J. A. J. Med. Chem. 1991, 34, 337–342. doi:10.1021/jm00105a053
Return to citation in text: [1] -
Parmar, V. S.; Bisht, K. S.; Jain, R.; Singh, S.; Sharma, S. K.; Gupta, S.; Malhotra, S.; Tyagi, O. D.; Vardhan, A.; Pati, H. N. Indian J. Chem., Sect. B 1996, 35, 220–232.
Return to citation in text: [1] -
Bindal, R. D.; Katzenellenbogen, J. A. J. Med. Chem. 1988, 31, 1978–1983. doi:10.1021/jm00118a020
Return to citation in text: [1] -
Seligmann, O.; Wagner, H. Tetrahedron 1981, 37, 2601–2606. doi:10.1016/S0040-4020(01)98963-X
Return to citation in text: [1] -
Pindur, U.; Müller, J. J. Heterocycl. Chem. 1987, 24, 289–290. doi:10.1002/jhet.5570240156
Return to citation in text: [1] -
Witzel, H.; Pindur, U. J. Heterocycl. Chem. 1988, 25, 907–910. doi:10.1002/jhet.5570250339
Return to citation in text: [1] -
Sato, T.; Higuchi, S.; Ito, K. Lett. Org. Chem. 2007, 4, 595–600. doi:10.2174/157017807782795574
Return to citation in text: [1] -
Podder, S.; Choudhury, J.; Roy, U. K.; Roy, S. J. Org. Chem. 2007, 72, 3100–3103. doi:10.1021/jo062633n
Return to citation in text: [1] -
Kodomari, M.; Nagamatsu, M.; Akaike, M.; Aoyama, T. Tetrahedron Lett. 2008, 49, 2537–2540. doi:10.1016/j.tetlet.2008.02.117
Return to citation in text: [1] -
Yamamoto, Y.; Itonaga, K. Chem. – Eur. J. 2008, 14, 10705–10715. doi:10.1002/chem.200801105
Return to citation in text: [1] -
Genovese, S.; Epifano, F.; Pelucchini, C.; Curini, M. Eur. J. Org. Chem. 2009, 1132–1135. doi:10.1002/ejoc.200801115
Return to citation in text: [1] -
Hikawa, H.; Suzuki, H.; Azumaya, I. J. Org. Chem. 2013, 78, 12128–12135. doi:10.1021/jo402151g
Return to citation in text: [1] -
Li, Z.; Duan, Z.; Kang, J.; Wang, H.; Yu, L.; Wu, Y. Tetrahedron 2008, 64, 1924–1930. doi:10.1016/j.tet.2007.11.080
Return to citation in text: [1] -
Shirakawa, S.; Kobayashi, S. Org. Lett. 2007, 9, 311–314. doi:10.1021/ol062813j
Return to citation in text: [1] -
Thirupathi, P.; Kim, S. S. Tetrahedron 2010, 66, 2995–3003. doi:10.1016/j.tet.2010.02.063
Return to citation in text: [1] -
Shacklady-McAtee, D. M.; Roberts, K. M.; Basch, C. H.; Song, Y.-G.; Watson, M. P. Tetrahedron 2014, 70, 4257–4263. doi:10.1016/j.tet.2014.03.039
Return to citation in text: [1] -
Harris, M. R.; Hanna, L. E.; Greene, M. A.; Moore, C. E.; Jarvo, E. R. J. Am. Chem. Soc. 2013, 135, 3303–3306. doi:10.1021/ja311783k
Return to citation in text: [1] -
Zhou, Q.; Srinivas, H. D.; Dasgupta, S.; Watson, M. P. J. Am. Chem. Soc. 2013, 135, 3307–3310. doi:10.1021/ja312087x
Return to citation in text: [1] -
Tabuchi, S.; Hirano, K.; Satoh, T.; Miura, M. J. Org. Chem. 2014, 79, 5401–5411. doi:10.1021/jo5010636
Return to citation in text: [1] -
Matthew, S. C.; Glasspoole, B. W.; Eisenberger, P.; Crudden, C. M. J. Am. Chem. Soc. 2014, 136, 5828–5831. doi:10.1021/ja412159g
Return to citation in text: [1] -
Nambo, M.; Crudden, C. M. Angew. Chem., Int. Ed. 2014, 53, 742–746. doi:10.1002/anie.201307019
Return to citation in text: [1] [2] -
Nambo, M.; Crudden, C. M. ACS Catal. 2015, 5, 4734–4742. doi:10.1021/acscatal.5b00909
Return to citation in text: [1] -
Rao, H. S. P.; Rao, A. V. B. Eur. J. Org. Chem. 2014, 3646–3655. doi:10.1002/ejoc.201402003
Return to citation in text: [1] -
Rao, H. S. P.; Rao, A. V. B. J. Org. Chem. 2015, 80, 1506–1516. doi:10.1021/jo502446k
Return to citation in text: [1] [2] [3] [4] [5] -
Boymond, L.; Rottländer, M.; Cahiez, G.; Knochel, P. Angew. Chem., Int. Ed. 1998, 37, 1701–1703. doi:10.1002/(SICI)1521-3773(19980703)37:12<1701::AID-ANIE1701>3.0.CO;2-U
Return to citation in text: [1] -
Majumdar, K. K.; Cheng, C.-H. Org. Lett. 2000, 2, 2295–2298. doi:10.1021/ol006064w
Return to citation in text: [1] -
Niwa, T.; Yorimitsu, H.; Oshima, K. Org. Lett. 2007, 9, 2373–2375. doi:10.1021/ol0708119
Return to citation in text: [1] -
Yu, J.-Y.; Kuwano, R. Org. Lett. 2008, 10, 973–976. doi:10.1021/ol800078j
Return to citation in text: [1] -
Cao, X.; Sha, S.-C.; Li, M.; Kim, B.-S.; Morgan, C.; Huang, R.; Yang, X.; Walsh, P. J. Chem. Sci. 2016, 7, 611–618. doi:10.1039/C5SC03704B
Return to citation in text: [1] -
Chan, D. M. T.; Monaco, K. L.; Wang, R.-P.; Winters, M. P. Tetrahedron Lett. 1998, 39, 2933–2936. doi:10.1016/S0040-4039(98)00503-6
Return to citation in text: [1] -
Lam, P. Y. S.; Clark, C. G.; Saubern, S.; Adams, J.; Winters, M. P.; Chan, D. M. T.; Combs, A. Tetrahedron Lett. 1998, 39, 2941–2944. doi:10.1016/S0040-4039(98)00504-8
Return to citation in text: [1] -
Evans, D. A.; Katz, J. L.; West, T. R. Tetrahedron Lett. 1998, 39, 2937–2940. doi:10.1016/S0040-4039(98)00502-4
Return to citation in text: [1] -
Itoh, T.; Shimizu, Y.; Kanai, M. Org. Lett. 2014, 16, 2736–2739. doi:10.1021/ol501022d
Return to citation in text: [1] -
Furniss, B. S.; Hannaford, A. J.; Smith, P. W. G.; Tatchell, A. R. VOGEL’s Textbook of Practical Organic Chemistry, 5th ed.; John Wiley & Sons: New York, 2077; pp 523 ff.
Return to citation in text: [1] -
Kodomari, M.; Satoh, H.; Yoshitomi, S. J. Org. Chem. 1988, 53, 2093–2094. doi:10.1021/jo00244a046
Return to citation in text: [1] -
Lu, J.; Guan, Z.-Z.; Gao, J.-W.; Zhang, Z.-H. Appl. Organomet. Chem. 2011, 25, 537–541. doi:10.1002/aoc.1799
Return to citation in text: [1]
| 58. | Chan, D. M. T.; Monaco, K. L.; Wang, R.-P.; Winters, M. P. Tetrahedron Lett. 1998, 39, 2933–2936. doi:10.1016/S0040-4039(98)00503-6 |
| 59. | Lam, P. Y. S.; Clark, C. G.; Saubern, S.; Adams, J.; Winters, M. P.; Chan, D. M. T.; Combs, A. Tetrahedron Lett. 1998, 39, 2941–2944. doi:10.1016/S0040-4039(98)00504-8 |
| 60. | Evans, D. A.; Katz, J. L.; West, T. R. Tetrahedron Lett. 1998, 39, 2937–2940. doi:10.1016/S0040-4039(98)00502-4 |
| 61. | Itoh, T.; Shimizu, Y.; Kanai, M. Org. Lett. 2014, 16, 2736–2739. doi:10.1021/ol501022d |
| 52. | Rao, H. S. P.; Rao, A. V. B. J. Org. Chem. 2015, 80, 1506–1516. doi:10.1021/jo502446k |
| 1. | Mondal, S.; Panda, G. RSC Adv. 2014, 4, 28317–28358. doi:10.1039/c4ra01341g |
| 2. | Nair, V.; Thomas, S.; Mathew, S. C.; Abhilash, K. G. Tetrahedron 2006, 62, 6731–6747. doi:10.1016/j.tet.2006.04.081 |
| 3. | Shchepinov, M. S.; Korshun, V. A. Chem. Soc. Rev. 2003, 32, 170–180. doi:10.1039/b008900l |
| 4. | Duxbury, D. F. Chem. Rev. 1993, 93, 381–433. doi:10.1021/cr00017a018 |
| 13. | Miura, T.; Urano, Y.; Tanaka, K.; Nagano, T.; Ohkubo, K.; Fukuzumi, S. J. Am. Chem. Soc. 2003, 125, 8666–8671. doi:10.1021/ja035282s |
| 14. | Haugland, R. P. Molecular Probes. The Molecular Probes Handbook. A Guide to Fluorescent Probes and Labeling Technologies, 10th ed.; Life Technologies Corporation: Eugene, USA, 2005. |
| 15. | Urano, Y.; Kamiya, M.; Kanada, K.; Ueno, T.; Hirose, K.; Nagano, T. J. Am. Chem. Soc. 2005, 127, 4888–4894. doi:10.1021/ja043919h |
| 16. | Abe, H.; Wang, J.; Furukawa, K.; Oki, K.; Uda, M.; Tsuneda, S.; Ito, Y. Bioconjugate Chem. 2008, 19, 1219–1226. doi:10.1021/bc800014d |
| 17. | Kim, H. N.; Lee, M. H.; Kim, H. J.; Kim, J. S.; Yoon, J. Chem. Soc. Rev. 2008, 37, 1465–1472. doi:10.1039/b802497a |
| 18. | Beija, M.; Afonso, C. A. M.; Martinho, J. M. G. Chem. Soc. Rev. 2009, 38, 2410–2433. doi:10.1039/b901612k |
| 30. | Parmar, V. S.; Bisht, K. S.; Jain, R.; Singh, S.; Sharma, S. K.; Gupta, S.; Malhotra, S.; Tyagi, O. D.; Vardhan, A.; Pati, H. N. Indian J. Chem., Sect. B 1996, 35, 220–232. |
| 10. | Rottman, C.; Grader, G.; De Hazan, Y.; Melchior, S.; Avnir, D. J. Am. Chem. Soc. 1999, 121, 8533–8543. doi:10.1021/ja991269p |
| 11. | Yamaguchi, K.; Tamura, Z.; Maeda, M. Anal. Sci. 1997, 13, 521–522. doi:10.2116/analsci.13.521 |
| 12. | Heger, D.; Klánová, J.; Klán, P. J. Phys. Chem. B 2006, 110, 1277–1287. doi:10.1021/jp0553683 |
| 31. | Bindal, R. D.; Katzenellenbogen, J. A. J. Med. Chem. 1988, 31, 1978–1983. doi:10.1021/jm00118a020 |
| 32. | Seligmann, O.; Wagner, H. Tetrahedron 1981, 37, 2601–2606. doi:10.1016/S0040-4020(01)98963-X |
| 6. | Mason, C. D.; Nord, F. F. J. Org. Chem. 1951, 16, 722–727. doi:10.1021/jo01145a011 |
| 7. | Ghaisas, V. V.; Kane, B. J.; Nord, F. F. J. Org. Chem. 1958, 23, 560–565. doi:10.1021/jo01098a016 |
| 8. | Irie, M. J. Am. Chem. Soc. 1983, 105, 2078–2079. doi:10.1021/ja00345a075 |
| 9. | Muthyala, R.; Katritzky, A. R.; Lan, X. Dyes Pigm. 1994, 25, 303–324. doi:10.1016/0143-7208(94)87017-9 |
| 26. | Schultz, T. W.; Sinks, G. D.; Cronin, M. T. D. Environ. Toxicol. 2002, 17, 14–23. doi:10.1002/tox.10027 |
| 52. | Rao, H. S. P.; Rao, A. V. B. J. Org. Chem. 2015, 80, 1506–1516. doi:10.1021/jo502446k |
| 5. | Oda, M.; Kawase, T.; Wei, C. Pure Appl. Chem. 1996, 68, 267–274. doi:10.1351/pac199668020267 |
| 27. | Wang, P.; Kozlowski, J.; Cushman, M. J. Org. Chem. 1992, 57, 3861–3866. doi:10.1021/jo00040a027 |
| 28. | Long, Y.-Q.; Jiang, X.-H.; Dayam, R.; Sanchez, T.; Shoemaker, R.; Sei, S.; Neamati, N. J. Med. Chem. 2004, 47, 2561–2573. doi:10.1021/jm030559k |
| 29. | Cushman, M.; Kanamathareddy, S.; De Clerq, E.; Schols, D.; Goldman, M. E.; Bowen, J. A. J. Med. Chem. 1991, 34, 337–342. doi:10.1021/jm00105a053 |
| 49. | Nambo, M.; Crudden, C. M. Angew. Chem., Int. Ed. 2014, 53, 742–746. doi:10.1002/anie.201307019 |
| 22. | Jendralla, H.; Granzer, E.; von Kerekjarto, B.; Krause, R.; Schacht, U.; Baader, E.; Bartmann, W.; Beck, G.; Bergmann, A.; Kesseler, K.; Wess, G.; Chen, L.-J.; Granata, S.; Herchen, J.; Kleine, H.; Schüssler, H.; Wagner, K. J. Med. Chem. 1991, 34, 2962–2983. doi:10.1021/jm00114a004 |
| 24. | Al-Qawasmeh, R. A.; Lee, Y.; Cao, M.-Y.; Gu, X.; Vassilakos, A.; Wright, J. A.; Young, A. Bioorg. Med. Chem. Lett. 2004, 14, 347–350. doi:10.1016/j.bmcl.2003.11.004 |
| 64. | Lu, J.; Guan, Z.-Z.; Gao, J.-W.; Zhang, Z.-H. Appl. Organomet. Chem. 2011, 25, 537–541. doi:10.1002/aoc.1799 |
| 21. | Bindal, R. D.; Golab, J. T.; Katzenellenbogen, J. A. J. Am. Chem. Soc. 1990, 112, 7861–7868. doi:10.1021/ja00178a003 |
| 25. | Mibu, N.; Yokomizo, K.; Uyeda, M.; Sumoto, K. Chem. Pharm. Bull. 2003, 51, 1325–1327. doi:10.1248/cpb.51.1325 |
| 52. | Rao, H. S. P.; Rao, A. V. B. J. Org. Chem. 2015, 80, 1506–1516. doi:10.1021/jo502446k |
| 20. | Shagufta; Srivastava, A. K.; Sharma, R.; Mishra, R.; Balapure, A. K.; Murthy, P. S. R.; Panda, G. Bioorg. Med. Chem. 2006, 14, 1497–1505. doi:10.1016/j.bmc.2005.10.002 |
| 62. | Furniss, B. S.; Hannaford, A. J.; Smith, P. W. G.; Tatchell, A. R. VOGEL’s Textbook of Practical Organic Chemistry, 5th ed.; John Wiley & Sons: New York, 2077; pp 523 ff. |
| 19. | Parai, M. K.; Panda, G.; Chaturvedi, V.; Manju, Y. K.; Sinha, S. Bioorg. Med. Chem. Lett. 2008, 18, 289–292. doi:10.1016/j.bmcl.2007.10.083 |
| 23. | Costantino, L.; Ferrari, A. M.; Gamberini, M. C.; Rastelli, G. Bioorg. Med. Chem. 2002, 10, 3923–3931. doi:10.1016/S0968-0896(02)00318-8 |
| 63. | Kodomari, M.; Satoh, H.; Yoshitomi, S. J. Org. Chem. 1988, 53, 2093–2094. doi:10.1021/jo00244a046 |
| 40. | Hikawa, H.; Suzuki, H.; Azumaya, I. J. Org. Chem. 2013, 78, 12128–12135. doi:10.1021/jo402151g |
| 41. | Li, Z.; Duan, Z.; Kang, J.; Wang, H.; Yu, L.; Wu, Y. Tetrahedron 2008, 64, 1924–1930. doi:10.1016/j.tet.2007.11.080 |
| 33. | Pindur, U.; Müller, J. J. Heterocycl. Chem. 1987, 24, 289–290. doi:10.1002/jhet.5570240156 |
| 34. | Witzel, H.; Pindur, U. J. Heterocycl. Chem. 1988, 25, 907–910. doi:10.1002/jhet.5570250339 |
| 35. | Sato, T.; Higuchi, S.; Ito, K. Lett. Org. Chem. 2007, 4, 595–600. doi:10.2174/157017807782795574 |
| 36. | Podder, S.; Choudhury, J.; Roy, U. K.; Roy, S. J. Org. Chem. 2007, 72, 3100–3103. doi:10.1021/jo062633n |
| 37. | Kodomari, M.; Nagamatsu, M.; Akaike, M.; Aoyama, T. Tetrahedron Lett. 2008, 49, 2537–2540. doi:10.1016/j.tetlet.2008.02.117 |
| 38. | Yamamoto, Y.; Itonaga, K. Chem. – Eur. J. 2008, 14, 10705–10715. doi:10.1002/chem.200801105 |
| 39. | Genovese, S.; Epifano, F.; Pelucchini, C.; Curini, M. Eur. J. Org. Chem. 2009, 1132–1135. doi:10.1002/ejoc.200801115 |
| 57. | Cao, X.; Sha, S.-C.; Li, M.; Kim, B.-S.; Morgan, C.; Huang, R.; Yang, X.; Walsh, P. J. Chem. Sci. 2016, 7, 611–618. doi:10.1039/C5SC03704B |
| 52. | Rao, H. S. P.; Rao, A. V. B. J. Org. Chem. 2015, 80, 1506–1516. doi:10.1021/jo502446k |
| 53. | Boymond, L.; Rottländer, M.; Cahiez, G.; Knochel, P. Angew. Chem., Int. Ed. 1998, 37, 1701–1703. doi:10.1002/(SICI)1521-3773(19980703)37:12<1701::AID-ANIE1701>3.0.CO;2-U |
| 54. | Majumdar, K. K.; Cheng, C.-H. Org. Lett. 2000, 2, 2295–2298. doi:10.1021/ol006064w |
| 55. | Niwa, T.; Yorimitsu, H.; Oshima, K. Org. Lett. 2007, 9, 2373–2375. doi:10.1021/ol0708119 |
| 56. | Yu, J.-Y.; Kuwano, R. Org. Lett. 2008, 10, 973–976. doi:10.1021/ol800078j |
| 49. | Nambo, M.; Crudden, C. M. Angew. Chem., Int. Ed. 2014, 53, 742–746. doi:10.1002/anie.201307019 |
| 50. | Nambo, M.; Crudden, C. M. ACS Catal. 2015, 5, 4734–4742. doi:10.1021/acscatal.5b00909 |
| 51. | Rao, H. S. P.; Rao, A. V. B. Eur. J. Org. Chem. 2014, 3646–3655. doi:10.1002/ejoc.201402003 |
| 52. | Rao, H. S. P.; Rao, A. V. B. J. Org. Chem. 2015, 80, 1506–1516. doi:10.1021/jo502446k |
| 42. | Shirakawa, S.; Kobayashi, S. Org. Lett. 2007, 9, 311–314. doi:10.1021/ol062813j |
| 43. | Thirupathi, P.; Kim, S. S. Tetrahedron 2010, 66, 2995–3003. doi:10.1016/j.tet.2010.02.063 |
| 44. | Shacklady-McAtee, D. M.; Roberts, K. M.; Basch, C. H.; Song, Y.-G.; Watson, M. P. Tetrahedron 2014, 70, 4257–4263. doi:10.1016/j.tet.2014.03.039 |
| 45. | Harris, M. R.; Hanna, L. E.; Greene, M. A.; Moore, C. E.; Jarvo, E. R. J. Am. Chem. Soc. 2013, 135, 3303–3306. doi:10.1021/ja311783k |
| 46. | Zhou, Q.; Srinivas, H. D.; Dasgupta, S.; Watson, M. P. J. Am. Chem. Soc. 2013, 135, 3307–3310. doi:10.1021/ja312087x |
| 47. | Tabuchi, S.; Hirano, K.; Satoh, T.; Miura, M. J. Org. Chem. 2014, 79, 5401–5411. doi:10.1021/jo5010636 |
| 48. | Matthew, S. C.; Glasspoole, B. W.; Eisenberger, P.; Crudden, C. M. J. Am. Chem. Soc. 2014, 136, 5828–5831. doi:10.1021/ja412159g |
© 2016 Rao and Rao; licensee Beilstein-Institut.
This is an Open Access article under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
The license is subject to the Beilstein Journal of Organic Chemistry terms and conditions: (http://www.beilstein-journals.org/bjoc)