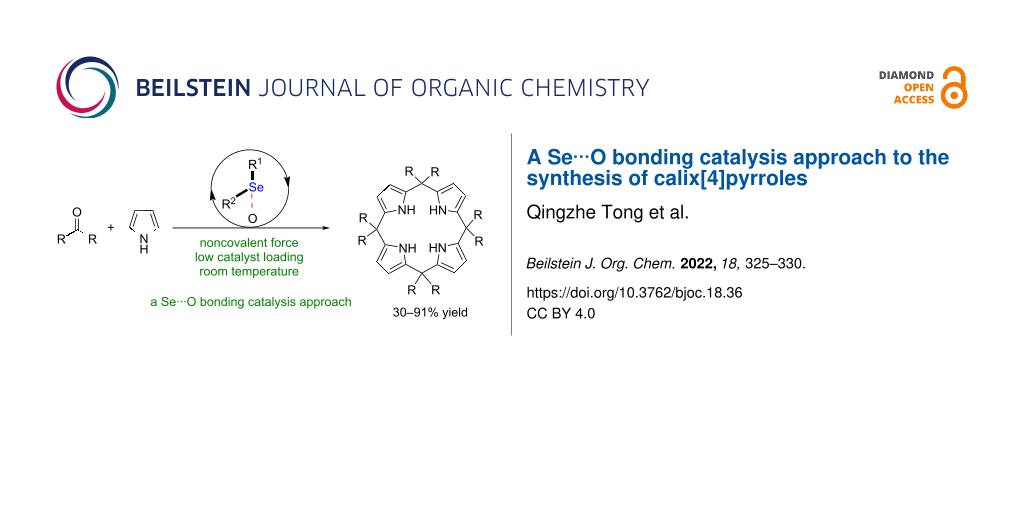
Abstract
Described herein is a chalcogen bonding catalysis approach to the synthesis of calix[4]pyrrole derivatives. The Se···O bonding interactions between selenide catalysts and ketones gave rise to the catalytic activity in the condensation reactions between pyrrole and ketones, leading to the generation of calix[4]pyrrole derivatives in moderate to high yields. This chalcogen bonding catalysis approach was efficient since only 5 mol % catalyst loading was used to promote the consecutive condensation processes while the reactions could be carried out at room temperature, thus highlighting the potential of this type of nonclassical interactions in catalyzing relative complex transformations.
Graphical Abstract
Introduction
Noncovalent catalysis has been established as one of the fundamental concepts in organic synthesis that enables achieving numerous chemical transformations [1]. Among these noncovalent forces, hydrogen bonding interactions play a central role in noncovalent catalysis [2] while halogen bonding interactions have lately been exploited as a new tool to catalyze a diverse array of reactions [3-5]. In addition, nonclassical interactions such as anion–π [6-11] as well as chalcogen [12-17] and pnictogen [18-23] bonds were established as emerging driving forces for the development of organic reactions. Very recently, catalysis with carbon bonding interactions was realized and this type of catalysis mode was capable of facilitating a range of typical reactions [24], thus providing a new platform for organic synthesis.
The phenomenon of chalcogen bonding was initially observed in the crystal structures of small organic molecules as well as proteins [25]. The application of this type of bonding interactions has achieved significant advances in the research fields of crystal engineering [26], medicinal chemistry [27], anion recognition [28-32] and transport [33-35]. In addition, intramolecular chalcogen bonding interactions have been suggested to stabilize reactive intermediates in a range of isothiourea-catalyzed transformations, which play a key factor to modulate the selectivity of these reactions [36-40]. In addition, few examples demonstrated that disubstituted chalcogens could be used as effective catalysts through intermolecular chalcogen bonding interactions [41-49]. Despite these significant advances, catalysis with chalcogen bonding interactions is still in its infancy and the development of new types of reactions is highly desirable.
Calix[4]pyrrole derivatives have been widely used as transition metal ligands and functional materials [50-53]. Thus far, several synthetic methods to access these compounds have been reported [54,55]. The classical approaches to synthesis of calix[4]pyrrole derivatives mainly involved a stepwise synthesis and Lewis acid as well as Brønsted acid catalysis [54,55]. Notably, a noncovalent catalysis approach to accessing calix[4]pyrrole derivatives remains underdeveloped. To provide a new strategy to synthesize calix[4]pyrrole derivatives, herein, we describe a Se···O bonding catalysis approach to accessing this type of compounds (Scheme 1).
Scheme 1: Proposal of a Se···O bonding catalysis approach.
Scheme 1: Proposal of a Se···O bonding catalysis approach.
Results and Discussion
Evaluation of catalysts
We developed a class of phosphonium selenide catalysts which showed catalytic activity in assembly reactions [41], Michael addition reactions [41], Rauhut–Currier reactions [42], cyanosilylation reactions [43], and cycloaddition of vinylindoles through chalcogen–π bonding catalysis [44]. Our previous works demonstrated that Se···O bonding interactions between phosphonium selenides and carbonyls can significantly activate carbonyl groups [41-43], thus providing a new opportunity to develop carbonyl chemistry. To expand the catalysis capability of chalcogen bonding interactions, we envisioned that consecutive condensations between ketones and pyrrole might take place to give calix[4]pyrrole derivatives under catalysis of a selenide catalyst. In the absence of a catalyst, no reaction took place. Indeed, even in presence of 5 mol % representative catalyst Ch1 [44], the condensation reaction between acetone and pyrrole worked efficiently at room temperature. We note that this reaction did not stop at a bis(pyrrole)methane stage, but consecutive condensations between four molecules of acetone and four molecules of pyrrole took place to give calix[4]pyrrole 2a in 91% yield after 4 h (Scheme 2). Further investigations revealed that the monodentate catalysts were less active and only a moderate yield was obtained regardless of whether Ch2 or Ch3 was used. In the presence of 10 mol % catalyst Ch3, 75% yield of 2a was obtained.
Scheme 2: Se···O bonding catalysis approach to the synthesis of calix[4]pyrrole 2a.
Scheme 2: Se···O bonding catalysis approach to the synthesis of calix[4]pyrrole 2a.
Reaction scope
Inspired by the good result obtained with catalyst Ch1, the scope of ketones was investigated (Scheme 3). Both linear and cyclic aliphatic ketones could be used to synthesize calix[4]pyrrole derivatives under catalysis of 5 mol % Ch1 at room temperature. It was found that this chalcogen bonding catalysis approach was susceptible to the variation of the steric environment of ketones. Upon changing acetone to pentan-3-one, the chemical yield decreased significantly and product 2b was obtained in 42% yield. Using cyclopentanone as a reactant, product 2c was obtained in 45% yield. Moreover, cyclohexanone and cycloheptanone could also be used as effective reactants, and products 2d and 2e were obtained in 58% and 42% yield, respectively. Further investigation revealed that cyclobutanone was reactive in this transformation to give product 2f, albeit with 30% yield. However, benzophenone and 2,4-dimethylpentan-3-one with high steric hindrance failed to give desirable products 2g and 2h. Further investigation on using an asymmetric ketone such as pentan-2-one as a reactant showed that the reaction gave an inseparable mixture of diastereomers. Meanwhile, upon using benzaldehyde as a reactant, the reaction system was complex and there was no major product.
Proposed activation mode
The chalcogen bonding interactions between catalysts Ch1, Ch2 and acetone were examined by 13C NMR experiments in CD2Cl2. The interaction between bidentate catalyst Ch1 or monodentate catalyst Ch2 and acetone could result in a variation of the 13C signal of the carbonyl group. Analysis of a 1:1 mixture of Ch1 and acetone in CD2Cl2 indicated a 1.07 ppm downfield shift of the 13C signal of the carbonyl group, while a 0.28 ppm downfield shift of the 13C signal of the carbonyl group was observed upon analysis of a 2:1 mixture of Ch2 and acetone (Scheme 4). Therefore, in line with the catalytic results as depicted in Scheme 2, both monodentate and bidentate catalysts could activate ketones. Accordingly, either a single activation or a double activation mode could be an effective driving force to promote this transformation, albeit with distinct catalytic activity.
Conclusion
In summary, we developed a Se···O bonding catalysis approach to the synthesis of calix[4]pyrroles. In the presence of 5 mol % selenide catalyst, calix[4]pyrrole products were obtained in moderate to good yields at room temperature. The experimental results showed that both bidentate and monodentate catalysts were catalytically active in the condensation reactions between pyrrole and ketones. In addition, both cyclic and linear aliphatic ketones were effective reactants in this transformation. This work provides a new strategy to access calix[4]pyrrole derivatives and makes an important complementation to the research topic of chalcogen bonding catalysis.
Supporting Information
| Supporting Information File 1: Full experimental procedures and compound characterization. | ||
| Format: PDF | Size: 934.3 KB | Download |
References
-
van Leeuwen, P. W. N. M.; Raynal, M., Eds. Supramolecular Catalysis: New Directions and Developments; Wiley-VCH: Weinheim, Germany, 2022. doi:10.1002/9783527832033
Return to citation in text: [1] -
Doyle, A. G.; Jacobsen, E. N. Chem. Rev. 2007, 107, 5713–5743. doi:10.1021/cr068373r
Return to citation in text: [1] -
Alkorta, I.; Elguero, J.; Frontera, A. Crystals 2020, 10, 180. doi:10.3390/cryst10030180
Return to citation in text: [1] -
Bulfield, D.; Huber, S. M. Chem. – Eur. J. 2016, 22, 14434–14450. doi:10.1002/chem.201601844
Return to citation in text: [1] -
Sutar, R. L.; Huber, S. M. ACS Catal. 2019, 9, 9622–9639. doi:10.1021/acscatal.9b02894
Return to citation in text: [1] -
Zhao, Y.; Domoto, Y.; Orentas, E.; Beuchat, C.; Emery, D.; Mareda, J.; Sakai, N.; Matile, S. Angew. Chem., Int. Ed. 2013, 52, 9940–9943. doi:10.1002/anie.201305356
Return to citation in text: [1] -
Zhao, Y.; Beuchat, C.; Domoto, Y.; Gajewy, J.; Wilson, A.; Mareda, J.; Sakai, N.; Matile, S. J. Am. Chem. Soc. 2014, 136, 2101–2111. doi:10.1021/ja412290r
Return to citation in text: [1] -
Zhao, Y.; Cotelle, Y.; Liu, L.; López-Andarias, J.; Bornhof, A.-B.; Akamatsu, M.; Sakai, N.; Matile, S. Acc. Chem. Res. 2018, 51, 2255–2263. doi:10.1021/acs.accounts.8b00223
Return to citation in text: [1] -
Paraja, M.; Matile, S. Angew. Chem., Int. Ed. 2020, 59, 6273–6277. doi:10.1002/anie.202000579
Return to citation in text: [1] -
Luo, N.; Ao, Y.-F.; Wang, D.-X.; Wang, Q.-Q. Angew. Chem., Int. Ed. 2021, 60, 20650–20655. doi:10.1002/anie.202106509
Return to citation in text: [1] -
Luo, N.; Ao, Y.-F.; Wang, D.-X.; Wang, Q.-Q. Chem. – Eur. J. 2022, 28, e202103303. doi:10.1002/chem.202103303
Return to citation in text: [1] -
Aakeroy, C. B.; Bryce, D. L.; Desiraju, G. R.; Frontera, A.; Legon, A. C.; Nicotra, F.; Rissanen, K.; Scheiner, S.; Terraneo, G.; Metrangolo, P.; Resnati, G. Pure Appl. Chem. 2019, 91, 1889–1892. doi:10.1515/pac-2018-0713
Return to citation in text: [1] -
Yan, W.; Zheng, M.; Xu, C.; Chen, F.-E. Green Synth. Catal. 2021, 2, 329–336. doi:10.1016/j.gresc.2021.08.002
Return to citation in text: [1] -
Vogel, L.; Wonner, P.; Huber, S. M. Angew. Chem., Int. Ed. 2019, 58, 1880–1891. doi:10.1002/anie.201809432
Return to citation in text: [1] -
Breugst, M.; Koenig, J. J. Eur. J. Org. Chem. 2020, 5473–5487. doi:10.1002/ejoc.202000660
Return to citation in text: [1] -
Bamberger, J.; Ostler, F.; Mancheño, O. G. ChemCatChem 2019, 11, 5198–5211. doi:10.1002/cctc.201901215
Return to citation in text: [1] -
Frontera, A.; Bauza, A. Int. J. Mol. Sci. 2021, 22, 12550. doi:10.3390/ijms222212550
Return to citation in text: [1] -
Benz, S.; Poblador-Bahamonde, A. I.; Low-Ders, N.; Matile, S. Angew. Chem., Int. Ed. 2018, 57, 5408–5412. doi:10.1002/anie.201801452
Return to citation in text: [1] -
Yang, M.; Tofan, D.; Chen, C.-H.; Jack, K. M.; Gabbaï, F. P. Angew. Chem., Int. Ed. 2018, 57, 13868–13872. doi:10.1002/anie.201808551
Return to citation in text: [1] -
Gini, A.; Paraja, M.; Galmés, B.; Besnard, C.; Poblador-Bahamonde, A. I.; Sakai, N.; Frontera, A.; Matile, S. Chem. Sci. 2020, 11, 7086–7091. doi:10.1039/d0sc02551h
Return to citation in text: [1] -
Yang, M.; Hirai, M.; Gabbaï, F. P. Dalton Trans. 2019, 48, 6685–6689. doi:10.1039/c9dt01357a
Return to citation in text: [1] -
Paraja, M.; Gini, A.; Sakai, N.; Matile, S. Chem. – Eur. J. 2020, 26, 15471–15476. doi:10.1002/chem.202003426
Return to citation in text: [1] -
Zhang, J.; Wei, J.; Ding, W.-Y.; Li, S.; Xiang, S.-H.; Tan, B. J. Am. Chem. Soc. 2021, 143, 6382–6387. doi:10.1021/jacs.1c02808
Return to citation in text: [1] -
Wang, W.; Li, X.; Zhou, P.-P.; Wang, Y. Angew. Chem., Int. Ed. 2021, 60, 22717–22721. doi:10.1002/anie.202108973
Return to citation in text: [1] -
Rosenfield, R. E., Jr.; Parthasarathy, R.; Dunitz, J. D. J. Am. Chem. Soc. 1977, 99, 4860–4862. doi:10.1021/ja00456a072
Return to citation in text: [1] -
Fourmigué, M.; Dhaka, A. Coord. Chem. Rev. 2020, 403, 213084. doi:10.1016/j.ccr.2019.213084
Return to citation in text: [1] -
Beno, B. R.; Yeung, K.-S.; Bartberger, M. D.; Pennington, L. D.; Meanwell, N. A. J. Med. Chem. 2015, 58, 4383–4438. doi:10.1021/jm501853m
Return to citation in text: [1] -
Zhao, H.; Gabbaï, F. P. Nat. Chem. 2010, 2, 984–990. doi:10.1038/nchem.838
Return to citation in text: [1] -
Garrett, G. E.; Gibson, G. L.; Straus, R. N.; Seferos, D. S.; Taylor, M. S. J. Am. Chem. Soc. 2015, 137, 4126–4133. doi:10.1021/ja512183e
Return to citation in text: [1] -
Garrett, G. E.; Carrera, E. I.; Seferos, D. S.; Taylor, M. S. Chem. Commun. 2016, 52, 9881–9884. doi:10.1039/c6cc04818h
Return to citation in text: [1] -
Lim, J. Y. C.; Marques, I.; Thompson, A. L.; Christensen, K. E.; Félix, V.; Beer, P. D. J. Am. Chem. Soc. 2017, 139, 3122–3133. doi:10.1021/jacs.6b12745
Return to citation in text: [1] -
Borissov, A.; Marques, I.; Lim, J. Y. C.; Félix, V.; Smith, M. D.; Beer, P. D. J. Am. Chem. Soc. 2019, 141, 4119–4129. doi:10.1021/jacs.9b00148
Return to citation in text: [1] -
Benz, S.; Macchione, M.; Verolet, Q.; Mareda, J.; Sakai, N.; Matile, S. J. Am. Chem. Soc. 2016, 138, 9093–9096. doi:10.1021/jacs.6b05779
Return to citation in text: [1] -
Macchione, M.; Tsemperouli, M.; Goujon, A.; Mallia, A. R.; Sakai, N.; Sugihara, K.; Matile, S. Helv. Chim. Acta 2018, 101, e1800014. doi:10.1002/hlca.201800014
Return to citation in text: [1] -
Lee, L. M.; Tsemperouli, M.; Poblador-Bahamonde, A. I.; Benz, S.; Sakai, N.; Sugihara, K.; Matile, S. J. Am. Chem. Soc. 2019, 141, 810–814. doi:10.1021/jacs.8b12554
Return to citation in text: [1] -
Birman, V. B.; Li, X.; Han, Z. Org. Lett. 2007, 9, 37–40. doi:10.1021/ol0623419
Return to citation in text: [1] -
Leverett, C. A.; Purohit, V. C.; Romo, D. Angew. Chem., Int. Ed. 2010, 49, 9479–9483. doi:10.1002/anie.201004671
Return to citation in text: [1] -
Robinson, E. R. T.; Fallan, C.; Simal, C.; Slawin, A. M. Z.; Smith, A. D. Chem. Sci. 2013, 4, 2193–2200. doi:10.1039/c3sc50199j
Return to citation in text: [1] -
Robinson, E. R. T.; Walden, D. M.; Fallan, C.; Greenhalgh, M. D.; Cheong, P. H.-Y.; Smith, A. D. Chem. Sci. 2016, 7, 6919–6927. doi:10.1039/c6sc00940a
Return to citation in text: [1] -
Young, C. M.; Elmi, A.; Pascoe, D. J.; Morris, R. K.; McLaughlin, C.; Woods, A. M.; Frost, A. B.; de la Houpliere, A.; Ling, K. B.; Smith, T. K.; Slawin, A. M. Z.; Willoughby, P. H.; Cockroft, S. L.; Smith, A. D. Angew. Chem., Int. Ed. 2020, 59, 3705–3710. doi:10.1002/anie.201914421
Return to citation in text: [1] -
Wang, W.; Zhu, H.; Liu, S.; Zhao, Z.; Zhang, L.; Hao, J.; Wang, Y. J. Am. Chem. Soc. 2019, 141, 9175–9179. doi:10.1021/jacs.9b03806
Return to citation in text: [1] [2] [3] [4] -
Wang, W.; Zhu, H.; Feng, L.; Yu, Q.; Hao, J.; Zhu, R.; Wang, Y. J. Am. Chem. Soc. 2020, 142, 3117–3124. doi:10.1021/jacs.9b12610
Return to citation in text: [1] [2] [3] -
Bao, L.; Kong, X.; Wang, Y. Asian J. Org. Chem. 2020, 9, 757–760. doi:10.1002/ajoc.202000127
Return to citation in text: [1] [2] [3] -
Kong, X.; Zhou, P.-P.; Wang, Y. Angew. Chem., Int. Ed. 2021, 60, 9395–9400. doi:10.1002/anie.202101140
Return to citation in text: [1] [2] [3] -
Benz, S.; López‐Andarias, J.; Mareda, J.; Sakai, N.; Matile, S. Angew. Chem., Int. Ed. 2017, 56, 812–815. doi:10.1002/anie.201611019
Return to citation in text: [1] -
Benz, S.; Mareda, J.; Besnard, C.; Sakai, N.; Matile, S. Chem. Sci. 2017, 8, 8164–8169. doi:10.1039/c7sc03866f
Return to citation in text: [1] -
Wonner, P.; Vogel, L.; Düser, M.; Gomes, L.; Kniep, F.; Mallick, B.; Werz, D. B.; Huber, S. M. Angew. Chem., Int. Ed. 2017, 56, 12009–12012. doi:10.1002/anie.201704816
Return to citation in text: [1] -
Wonner, P.; Dreger, A.; Vogel, L.; Engelage, E.; Huber, S. M. Angew. Chem., Int. Ed. 2019, 58, 16923–16927. doi:10.1002/anie.201910639
Return to citation in text: [1] -
Wonner, P.; Steinke, T.; Vogel, L.; Huber, S. M. Chem. – Eur. J. 2020, 26, 1258–1262. doi:10.1002/chem.201905057
Return to citation in text: [1] -
Kim, D. S.; Sessler, J. L. Chem. Soc. Rev. 2015, 44, 532–546. doi:10.1039/c4cs00157e
Return to citation in text: [1] -
Ding, Y.; Tang, Y.; Zhu, W.; Xie, Y. Chem. Soc. Rev. 2015, 44, 1101–1112. doi:10.1039/c4cs00436a
Return to citation in text: [1] -
Tanaka, T.; Osuka, A. Chem. Soc. Rev. 2015, 44, 943–969. doi:10.1039/c3cs60443h
Return to citation in text: [1] -
Saha, I.; Lee, J. T.; Lee, C.-H. Eur. J. Org. Chem. 2015, 3859–3885. doi:10.1002/ejoc.201403701
Return to citation in text: [1] -
Lindsey, J. S. Acc. Chem. Res. 2010, 43, 300–311. doi:10.1021/ar900212t
Return to citation in text: [1] [2] -
Rather, I. A.; Ali, R. Green Chem. 2021, 23, 5849–5855. doi:10.1039/d1gc01515j
Return to citation in text: [1] [2]
| 41. | Wang, W.; Zhu, H.; Liu, S.; Zhao, Z.; Zhang, L.; Hao, J.; Wang, Y. J. Am. Chem. Soc. 2019, 141, 9175–9179. doi:10.1021/jacs.9b03806 |
| 54. | Lindsey, J. S. Acc. Chem. Res. 2010, 43, 300–311. doi:10.1021/ar900212t |
| 55. | Rather, I. A.; Ali, R. Green Chem. 2021, 23, 5849–5855. doi:10.1039/d1gc01515j |
| 41. | Wang, W.; Zhu, H.; Liu, S.; Zhao, Z.; Zhang, L.; Hao, J.; Wang, Y. J. Am. Chem. Soc. 2019, 141, 9175–9179. doi:10.1021/jacs.9b03806 |
| 1. | van Leeuwen, P. W. N. M.; Raynal, M., Eds. Supramolecular Catalysis: New Directions and Developments; Wiley-VCH: Weinheim, Germany, 2022. doi:10.1002/9783527832033 |
| 12. | Aakeroy, C. B.; Bryce, D. L.; Desiraju, G. R.; Frontera, A.; Legon, A. C.; Nicotra, F.; Rissanen, K.; Scheiner, S.; Terraneo, G.; Metrangolo, P.; Resnati, G. Pure Appl. Chem. 2019, 91, 1889–1892. doi:10.1515/pac-2018-0713 |
| 13. | Yan, W.; Zheng, M.; Xu, C.; Chen, F.-E. Green Synth. Catal. 2021, 2, 329–336. doi:10.1016/j.gresc.2021.08.002 |
| 14. | Vogel, L.; Wonner, P.; Huber, S. M. Angew. Chem., Int. Ed. 2019, 58, 1880–1891. doi:10.1002/anie.201809432 |
| 15. | Breugst, M.; Koenig, J. J. Eur. J. Org. Chem. 2020, 5473–5487. doi:10.1002/ejoc.202000660 |
| 16. | Bamberger, J.; Ostler, F.; Mancheño, O. G. ChemCatChem 2019, 11, 5198–5211. doi:10.1002/cctc.201901215 |
| 17. | Frontera, A.; Bauza, A. Int. J. Mol. Sci. 2021, 22, 12550. doi:10.3390/ijms222212550 |
| 50. | Kim, D. S.; Sessler, J. L. Chem. Soc. Rev. 2015, 44, 532–546. doi:10.1039/c4cs00157e |
| 51. | Ding, Y.; Tang, Y.; Zhu, W.; Xie, Y. Chem. Soc. Rev. 2015, 44, 1101–1112. doi:10.1039/c4cs00436a |
| 52. | Tanaka, T.; Osuka, A. Chem. Soc. Rev. 2015, 44, 943–969. doi:10.1039/c3cs60443h |
| 53. | Saha, I.; Lee, J. T.; Lee, C.-H. Eur. J. Org. Chem. 2015, 3859–3885. doi:10.1002/ejoc.201403701 |
| 6. | Zhao, Y.; Domoto, Y.; Orentas, E.; Beuchat, C.; Emery, D.; Mareda, J.; Sakai, N.; Matile, S. Angew. Chem., Int. Ed. 2013, 52, 9940–9943. doi:10.1002/anie.201305356 |
| 7. | Zhao, Y.; Beuchat, C.; Domoto, Y.; Gajewy, J.; Wilson, A.; Mareda, J.; Sakai, N.; Matile, S. J. Am. Chem. Soc. 2014, 136, 2101–2111. doi:10.1021/ja412290r |
| 8. | Zhao, Y.; Cotelle, Y.; Liu, L.; López-Andarias, J.; Bornhof, A.-B.; Akamatsu, M.; Sakai, N.; Matile, S. Acc. Chem. Res. 2018, 51, 2255–2263. doi:10.1021/acs.accounts.8b00223 |
| 9. | Paraja, M.; Matile, S. Angew. Chem., Int. Ed. 2020, 59, 6273–6277. doi:10.1002/anie.202000579 |
| 10. | Luo, N.; Ao, Y.-F.; Wang, D.-X.; Wang, Q.-Q. Angew. Chem., Int. Ed. 2021, 60, 20650–20655. doi:10.1002/anie.202106509 |
| 11. | Luo, N.; Ao, Y.-F.; Wang, D.-X.; Wang, Q.-Q. Chem. – Eur. J. 2022, 28, e202103303. doi:10.1002/chem.202103303 |
| 54. | Lindsey, J. S. Acc. Chem. Res. 2010, 43, 300–311. doi:10.1021/ar900212t |
| 55. | Rather, I. A.; Ali, R. Green Chem. 2021, 23, 5849–5855. doi:10.1039/d1gc01515j |
| 3. | Alkorta, I.; Elguero, J.; Frontera, A. Crystals 2020, 10, 180. doi:10.3390/cryst10030180 |
| 4. | Bulfield, D.; Huber, S. M. Chem. – Eur. J. 2016, 22, 14434–14450. doi:10.1002/chem.201601844 |
| 5. | Sutar, R. L.; Huber, S. M. ACS Catal. 2019, 9, 9622–9639. doi:10.1021/acscatal.9b02894 |
| 36. | Birman, V. B.; Li, X.; Han, Z. Org. Lett. 2007, 9, 37–40. doi:10.1021/ol0623419 |
| 37. | Leverett, C. A.; Purohit, V. C.; Romo, D. Angew. Chem., Int. Ed. 2010, 49, 9479–9483. doi:10.1002/anie.201004671 |
| 38. | Robinson, E. R. T.; Fallan, C.; Simal, C.; Slawin, A. M. Z.; Smith, A. D. Chem. Sci. 2013, 4, 2193–2200. doi:10.1039/c3sc50199j |
| 39. | Robinson, E. R. T.; Walden, D. M.; Fallan, C.; Greenhalgh, M. D.; Cheong, P. H.-Y.; Smith, A. D. Chem. Sci. 2016, 7, 6919–6927. doi:10.1039/c6sc00940a |
| 40. | Young, C. M.; Elmi, A.; Pascoe, D. J.; Morris, R. K.; McLaughlin, C.; Woods, A. M.; Frost, A. B.; de la Houpliere, A.; Ling, K. B.; Smith, T. K.; Slawin, A. M. Z.; Willoughby, P. H.; Cockroft, S. L.; Smith, A. D. Angew. Chem., Int. Ed. 2020, 59, 3705–3710. doi:10.1002/anie.201914421 |
| 44. | Kong, X.; Zhou, P.-P.; Wang, Y. Angew. Chem., Int. Ed. 2021, 60, 9395–9400. doi:10.1002/anie.202101140 |
| 2. | Doyle, A. G.; Jacobsen, E. N. Chem. Rev. 2007, 107, 5713–5743. doi:10.1021/cr068373r |
| 41. | Wang, W.; Zhu, H.; Liu, S.; Zhao, Z.; Zhang, L.; Hao, J.; Wang, Y. J. Am. Chem. Soc. 2019, 141, 9175–9179. doi:10.1021/jacs.9b03806 |
| 42. | Wang, W.; Zhu, H.; Feng, L.; Yu, Q.; Hao, J.; Zhu, R.; Wang, Y. J. Am. Chem. Soc. 2020, 142, 3117–3124. doi:10.1021/jacs.9b12610 |
| 43. | Bao, L.; Kong, X.; Wang, Y. Asian J. Org. Chem. 2020, 9, 757–760. doi:10.1002/ajoc.202000127 |
| 44. | Kong, X.; Zhou, P.-P.; Wang, Y. Angew. Chem., Int. Ed. 2021, 60, 9395–9400. doi:10.1002/anie.202101140 |
| 45. | Benz, S.; López‐Andarias, J.; Mareda, J.; Sakai, N.; Matile, S. Angew. Chem., Int. Ed. 2017, 56, 812–815. doi:10.1002/anie.201611019 |
| 46. | Benz, S.; Mareda, J.; Besnard, C.; Sakai, N.; Matile, S. Chem. Sci. 2017, 8, 8164–8169. doi:10.1039/c7sc03866f |
| 47. | Wonner, P.; Vogel, L.; Düser, M.; Gomes, L.; Kniep, F.; Mallick, B.; Werz, D. B.; Huber, S. M. Angew. Chem., Int. Ed. 2017, 56, 12009–12012. doi:10.1002/anie.201704816 |
| 48. | Wonner, P.; Dreger, A.; Vogel, L.; Engelage, E.; Huber, S. M. Angew. Chem., Int. Ed. 2019, 58, 16923–16927. doi:10.1002/anie.201910639 |
| 49. | Wonner, P.; Steinke, T.; Vogel, L.; Huber, S. M. Chem. – Eur. J. 2020, 26, 1258–1262. doi:10.1002/chem.201905057 |
| 26. | Fourmigué, M.; Dhaka, A. Coord. Chem. Rev. 2020, 403, 213084. doi:10.1016/j.ccr.2019.213084 |
| 28. | Zhao, H.; Gabbaï, F. P. Nat. Chem. 2010, 2, 984–990. doi:10.1038/nchem.838 |
| 29. | Garrett, G. E.; Gibson, G. L.; Straus, R. N.; Seferos, D. S.; Taylor, M. S. J. Am. Chem. Soc. 2015, 137, 4126–4133. doi:10.1021/ja512183e |
| 30. | Garrett, G. E.; Carrera, E. I.; Seferos, D. S.; Taylor, M. S. Chem. Commun. 2016, 52, 9881–9884. doi:10.1039/c6cc04818h |
| 31. | Lim, J. Y. C.; Marques, I.; Thompson, A. L.; Christensen, K. E.; Félix, V.; Beer, P. D. J. Am. Chem. Soc. 2017, 139, 3122–3133. doi:10.1021/jacs.6b12745 |
| 32. | Borissov, A.; Marques, I.; Lim, J. Y. C.; Félix, V.; Smith, M. D.; Beer, P. D. J. Am. Chem. Soc. 2019, 141, 4119–4129. doi:10.1021/jacs.9b00148 |
| 44. | Kong, X.; Zhou, P.-P.; Wang, Y. Angew. Chem., Int. Ed. 2021, 60, 9395–9400. doi:10.1002/anie.202101140 |
| 25. | Rosenfield, R. E., Jr.; Parthasarathy, R.; Dunitz, J. D. J. Am. Chem. Soc. 1977, 99, 4860–4862. doi:10.1021/ja00456a072 |
| 33. | Benz, S.; Macchione, M.; Verolet, Q.; Mareda, J.; Sakai, N.; Matile, S. J. Am. Chem. Soc. 2016, 138, 9093–9096. doi:10.1021/jacs.6b05779 |
| 34. | Macchione, M.; Tsemperouli, M.; Goujon, A.; Mallia, A. R.; Sakai, N.; Sugihara, K.; Matile, S. Helv. Chim. Acta 2018, 101, e1800014. doi:10.1002/hlca.201800014 |
| 35. | Lee, L. M.; Tsemperouli, M.; Poblador-Bahamonde, A. I.; Benz, S.; Sakai, N.; Sugihara, K.; Matile, S. J. Am. Chem. Soc. 2019, 141, 810–814. doi:10.1021/jacs.8b12554 |
| 41. | Wang, W.; Zhu, H.; Liu, S.; Zhao, Z.; Zhang, L.; Hao, J.; Wang, Y. J. Am. Chem. Soc. 2019, 141, 9175–9179. doi:10.1021/jacs.9b03806 |
| 42. | Wang, W.; Zhu, H.; Feng, L.; Yu, Q.; Hao, J.; Zhu, R.; Wang, Y. J. Am. Chem. Soc. 2020, 142, 3117–3124. doi:10.1021/jacs.9b12610 |
| 43. | Bao, L.; Kong, X.; Wang, Y. Asian J. Org. Chem. 2020, 9, 757–760. doi:10.1002/ajoc.202000127 |
| 24. | Wang, W.; Li, X.; Zhou, P.-P.; Wang, Y. Angew. Chem., Int. Ed. 2021, 60, 22717–22721. doi:10.1002/anie.202108973 |
| 42. | Wang, W.; Zhu, H.; Feng, L.; Yu, Q.; Hao, J.; Zhu, R.; Wang, Y. J. Am. Chem. Soc. 2020, 142, 3117–3124. doi:10.1021/jacs.9b12610 |
| 18. | Benz, S.; Poblador-Bahamonde, A. I.; Low-Ders, N.; Matile, S. Angew. Chem., Int. Ed. 2018, 57, 5408–5412. doi:10.1002/anie.201801452 |
| 19. | Yang, M.; Tofan, D.; Chen, C.-H.; Jack, K. M.; Gabbaï, F. P. Angew. Chem., Int. Ed. 2018, 57, 13868–13872. doi:10.1002/anie.201808551 |
| 20. | Gini, A.; Paraja, M.; Galmés, B.; Besnard, C.; Poblador-Bahamonde, A. I.; Sakai, N.; Frontera, A.; Matile, S. Chem. Sci. 2020, 11, 7086–7091. doi:10.1039/d0sc02551h |
| 21. | Yang, M.; Hirai, M.; Gabbaï, F. P. Dalton Trans. 2019, 48, 6685–6689. doi:10.1039/c9dt01357a |
| 22. | Paraja, M.; Gini, A.; Sakai, N.; Matile, S. Chem. – Eur. J. 2020, 26, 15471–15476. doi:10.1002/chem.202003426 |
| 23. | Zhang, J.; Wei, J.; Ding, W.-Y.; Li, S.; Xiang, S.-H.; Tan, B. J. Am. Chem. Soc. 2021, 143, 6382–6387. doi:10.1021/jacs.1c02808 |
| 27. | Beno, B. R.; Yeung, K.-S.; Bartberger, M. D.; Pennington, L. D.; Meanwell, N. A. J. Med. Chem. 2015, 58, 4383–4438. doi:10.1021/jm501853m |
| 43. | Bao, L.; Kong, X.; Wang, Y. Asian J. Org. Chem. 2020, 9, 757–760. doi:10.1002/ajoc.202000127 |
© 2022 Tong et al.; licensee Beilstein-Institut.
This is an open access article licensed under the terms of the Beilstein-Institut Open Access License Agreement (https://www.beilstein-journals.org/bjoc/terms), which is identical to the Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0). The reuse of material under this license requires that the author(s), source and license are credited. Third-party material in this article could be subject to other licenses (typically indicated in the credit line), and in this case, users are required to obtain permission from the license holder to reuse the material.