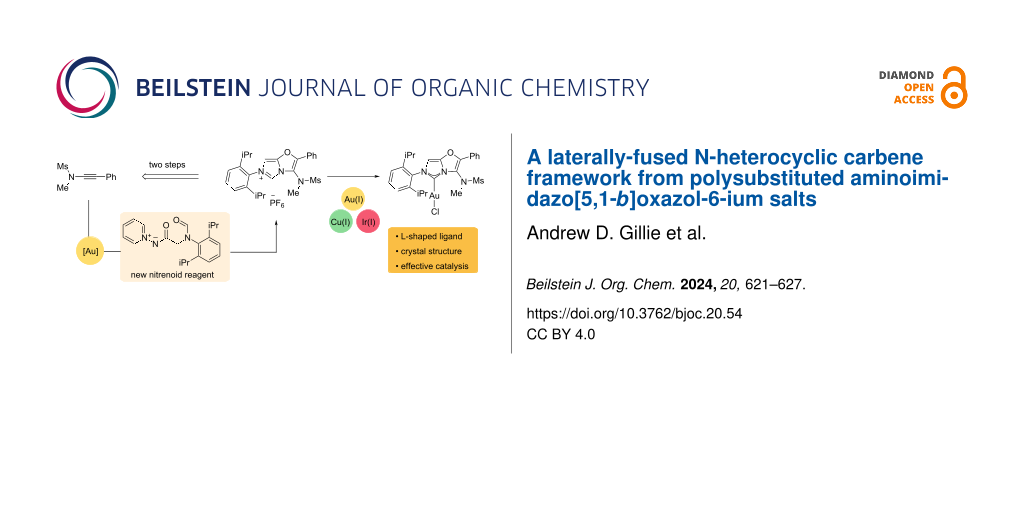
Abstract
A polysubstituted 3-aminoimidazo[5,1-b]oxazol-6-ium framework has been accessed from a new nitrenoid reagent by a two-step ynamide annulation and imidazolium ring-formation sequence. Metalation with Au(I), Cu(I) and Ir(I) at the C2 position provides an L-shaped NHC ligand scaffold that has been validated in gold-catalysed alkyne hydration and arylative cyclisation reactions.
Graphical Abstract
Introduction
Imidazolium-derived nucleophilic heterocyclic carbenes (NHCs) have had a sustained impact across the fields of organometallic and main group chemistry, transition-metal catalysis, materials synthesis and organocatalysis [1]. Laterally annellated polycyclic NHCs offer a useful contrast to the most widely used ‘umbrella-like’ NHCs (Figure 1) [2,3]. An extended π-system influences the donor and acceptor properties of the carbene whilst substitution on the polycycle can position groups adjacent to the active centre.
The imidazo[1,5-a]pyridin-3-ylidene motif (ImPy), independently introduced by the groups of Lassaletta [4] and Glorius [5], is the most widely explored framework for L-shaped ligands (Figure 1a). Even when only considering gold catalysis [6], the ImPy framework has been used to great effect [7]. The motif has been used to introduce sterically demanding NHCs with secondary gold-ligand interactions [8-10], chiral environments [11-13] including those enabling secondary interactions with substrates for asymmetric catalysis [14], cooperative and bimetallic catalysis [7,15], and redox-enabling function for Au(I)/(III) cycles [16,17].
Such L-shaped ligands provide scope to influence the reactivity profile of their resulting metal complexes through steric shielding, direct stabilising interactions with the metal, or by proximal effects to reactive species. Given the sensitivity of metal catalysis to even subtle steric and electronic changes in the ligand sphere, accessing more diverse fused imidazolium frameworks and different peripheral functionality offers significant scope to influence catalytic properties. Few studies into L-shaped imidazolylidines have explored core motifs beyond ImPy, with NHCs derived from two π-rich rings fused together particularly underinvestigated [2,18,19].
In this work we report the preparation of a new L-shaped NHC motif, the 3-aminoimidazo[5,1-b]oxazol-5-ylidene A (shortened hereafter to AImOx), which fuses two π-rich rings and positions a sulfonamide group alongside the metal centre (Figure 1b). We envisaged that the potential NHC precursor to A, a polysubstituted 3-aminoimidazo[5,1-b]oxazol-6-ium motif B, might be rapidly accessed from an ynamide by sequential oxazole-forming annulation and imidazolium formation steps. The basis of this approach was a gold-catalysed oxazole formation developed in our group [20,21] that should facilitate access to different groups at the oxazole C-2 position allowing a range of imidazolium-forming cyclisation strategies to be explored. Glorius and co-workers reported the formation of symmetrical NHCs by imidazolium ring formation from bisoxazoline motifs [22] but incorporating the unsaturated oxazole counterparts has not been explored.
Results and Discussion
Reaction of ynamide 1a with the N-acylpyridinium-N-aminide reagent 2 proceeded in good yield to afford oxazole 3 bearing a C-2 methyleneamino moiety as the first example of a free secondary amine in this annulation type (Scheme 1a, path a). However, attempts to form the desired imidazolium ring from 3 using triethyl orthoformate and different additives were unsuccessful. Similarly, an imine precursor 6, prepared in high yields by synthesising the known acetal-bearing oxazole 5 [21] and reacting it with 2,6-diisopropylphenylamine, could not be converted into the desired imidazolium salt (Scheme 1a, path b). Applying a range of conditions, including those successful on other annulated systems, led to unreacted starting material or hydrolysis products after work-up (see Supporting Information File 1) [5,18,19,23-27]. The unique Schiff base 6 can however be stored without precautions for several months without degradation and is prepared with minimal processing in 75% yield by telescoping the annulation and condensation steps.
Scheme 1: Synthetic studies into the formation of a 3-aminoimdazo[5,1-b]oxazol-6-ium motif based on a gold-catalysed oxazole formation. DIPP = 2,6-diisopropylphenylamine; Pic = picolinate; PMP = p-methoxyphenyl.
Scheme 1: Synthetic studies into the formation of a 3-aminoimdazo[5,1-b]oxazol-6-ium motif based on a gold-ca...
As the 4-aminooxazole motif appeared to be a poor nucleophile, we sought to introduce a formamide motif in place of the amine or imine to allow the use of more forcing cyclisation conditions (Scheme 1a, path c). Oxazole 8a was obtained in good yield from 1a using only a slight excess of nitrenoid 7 and 2 mol % catalyst loading. Heating 8a in the presence of POCl3 afforded the 3-aminoimidazo[5,1-b]oxazol-6-ium motif, followed by salt metathesis using KPF6 leading to the clean hexafluorophosphate salt 9a in 67% yield after recrystallisation [4].
This two-step assembly of the 3-aminoimidazo[5,1-b]oxazol-6-ium motif was also applied to ynamide 1b affording the PMP-substituted salt 9b in good yield.
The new nitrenoid reagent 7 is readily prepared from 2,6-diisopropylphenylamine in three steps. Alkylation with methyl bromoacetate is followed by formylation of 11 and then substitution [21] of 12 with N-aminopyridinium iodide to yield the bench-stable and crystalline N-acylpyridinium aminide 7 in good yield on a gram scale (Scheme 1b).
With the novel 3-aminoimidazo[5,1-b]oxazol-6-ium salt in hand, we examined its use as an NHC precursor for the preparation of late transition metal complexes. Treating compound 9a with triethylamine and either dimethyl sulfide gold(I) chloride or copper(I) chloride in acetone led to the formation of the desired AImOxAuCl and AImOxCuCl metal chloride complexes 13 and 14, respectively (Scheme 2) [7].
Scheme 2: The synthesis of AImOxAu(I)Cl, AImOxCu(I)Cl, and AImOxIr(CO)2Cl complexes from 6a. The single crystal X-ray diffraction structures of 13 and 14 have ellipsoids drawn at 50% probability, with hydrogens and solvent omitted for clarity. Selected bond angles and distances: 13: C1–Au: 1.98 Å, Au–Cl: 2.28 Å, N2–Au: 3.65 Å. N1–C1–N3: 102.7°, N3–C1–Au: 129.5°, N1–C1–Au: 127.1°, C1–Au–Cl: 175.8°. 14: C1–Au: 1.97 Å, Au–Cl: 2.28 Å, N2–Au: 3.66 Å. N1–C1–N3: 102.6°, N3–C1–Au: 129.4°, N1–C1–Au: 127.9°, C1–Au–Cl: 177.7°. Topographic steric maps of AImOxAuCl 13. Au–carbene bond selected as z-axis, nitrogen’s flanking carbene define xz plane. Bondi radii scaled by 1.17, sphere radius 3.5 Å, mesh spacing 0.10, H atoms removed for calculations. Colour coding represents positioning of steric bulk relative to the centre of the sphere, scale in Å.
Scheme 2: The synthesis of AImOxAu(I)Cl, AImOxCu(I)Cl, and AImOxIr(CO)2Cl complexes from 6a. The single cryst...
The 1H NMR spectra of the resulting AImOx metal complexes show a loss of symmetry for the diisopropyl substituents, indicating restricted rotation about the C(oxazole)–N(sulfonamide) bond. No coalescence is observed at up to 110 °C indicating that these motifs might be useful as a robust atropisomeric system. The molecular structure of 13 and 14 have been unambiguously determined by single crystal X-ray diffraction (Scheme 2) [28]. The N–metal interatomic distances are between 3.53 and 3.66 Å leaving insufficient space for bond rotation about the C–N axis with the sulfonamide substituents being approximately perpendicular to the fused aromatic unit. A percentage buried volume of 44.6% was calculated from the crystal structure of 13 using Cavallo’s method and Sambvca V.2.0 software (Scheme 2) [29]. Although a similar value to that reported for IPrAuCl (%Vbur = 45.4%) [30] the steric map shows a very different steric environment on either side of the ligand.
The AImOxIr(CO)2Cl complex 15 was targeted in order to assess the electronic effects of the fused imidazolium core (Scheme 2). No reaction was observed between 6a and [Ir(cod)Cl]2 in the presence of NEt3. A solution of the free carbene was prepared from 6 and reacted with [Ir(cod)Cl]2 and then CO to afford the AImOxIr(CO)Cl complex 15. A minor side-product with a strong red colour was formed which could not be fully purified or characterised but has a characteristic AQ quartet of two protons replacing the singlet for the N-methyl group in the 1H NMR spectra consistent with a cyclometallated complex from C–H insertion [31,32].
Three distinct sets of N-methyl and N-methylsulfonyl signals, with a major one accounting for approximately 80% of the total, were observed in the 1H NMR spectra of 15 likely due to restricted rotation around the metal carbene bond combining with the locked rotation around the oxazole C4–N bond. Elemental analysis was consistent with the proposed structure and only two sharp CO stretching frequencies were observed in the IR (Scheme 2) and so a value for Tolman’s electronic parameter (TEP) could be estimated. [33] At TEP[Ir] = 2053.1 cm−1 and 2052.8 cm−1 for 15a and 15b, respectively, the values for these AImOx ligands are towards the electron-deficient end seen with imidazolidines (cf. for IPr TEP[Ir] = 2050.2 cm−1) [34].
A benchmarking exercise was then performed looking at the reactivity of 13 compared against reaction of symmetrical IPrAuCl across a range of known gold-mediated transformations of alkynes featuring intermolecular attack [35], intramolecular cyclisation [36] or a mixture of both [8,37-39]. The new ligand system proved to deliver competent catalysis. Conversion was seen in all cases at 1 mol % catalyst loading (Scheme 3). Use of 13 resulted in a slight increase of the anti-Markovnikov hydration product 17 over 18 when compared to IPrAuCl [35]. In arylative cyclisations incomplete reaction was seen with enyne 19 [8,37] but ynone 22 [39] afforded high yield of 24. A quantitative conversion was seen in the intramolecular arylative cyclisation of 25 where 13 outperformed IPrAuCl [36].
Scheme 3: Use of AImOxAuCl 13 in catalysis. aYields are calculated from the 1H NMR spectra against an internal standard unless otherwise stated. Isolated yield of 17:18 with ratios determined from the 1H NMR spectra.
Scheme 3: Use of AImOxAuCl 13 in catalysis. aYields are calculated from the 1H NMR spectra against an interna...
Conclusion
An L-shaped NHC ligand motif, AImOx, has been developed and used to access monoligated Au(I), Cu(I) and Ir(I) complexes. The NHC precursors, polysubstituted 3-aminoimidazo[5,1-b]oxazol-6-ium salts are readily prepared in an efficient two-step sequence from ynamides using a newly developed nitrenoid reagent 4. The resulting AImOxAu(I) complex is catalytically competent across several transformations with excellent conversions at 1 mol % loading and with broadly comparable reactivity to IPrAuCl. Having validated the AImOx motif as a viable ligand platform for development, further elaboration and applications will be reported in due course.
Supporting Information
| Supporting Information File 1: Experimental procedures and characterisation data, additional cyclisation studies, XRD data and NMR spectra of compounds. | ||
| Format: PDF | Size: 3.2 MB | Download |
Acknowledgements
The authors gratefully acknowledge support from the Centre for Chemical and Materials Analysis in the School of Chemistry (UoB). We thank the EPSRC UK National Crystallography Service at the University of Southampton for the collection of the crystallographic data for compound 14. [40] We thank Dr Richard Mudd (UoB) for the preparation of literature substrates for catalysis studies. This work is based on Andrew D. Gillie’s doctoral thesis (“Synthesis and Applications of 4N-Substituted Oxazoles”, University of Birmingham, 2015).
Data Availability Statement
The data generated and analyzed during this study is openly available in the University of Birmingham eData Repository (UBIRA) at https://doi.org/10.25500/edata.bham.00001041.
References
-
Hopkinson, M. N.; Richter, C.; Schedler, M.; Glorius, F. Nature 2014, 510, 485–496. doi:10.1038/nature13384
Return to citation in text: [1] -
Reshi, N. U. D.; Bera, J. K. Coord. Chem. Rev. 2020, 422, 213334. doi:10.1016/j.ccr.2020.213334
Return to citation in text: [1] [2] -
Iglesias-Sigüenza, J.; Izquierdo, C.; Díez, E.; Fernández, R.; Lassaletta, J. M. Dalton Trans. 2016, 45, 10113–10117. doi:10.1039/c6dt01700b
Return to citation in text: [1] -
Alcarazo, M.; Roseblade, S. J.; Cowley, A. R.; Fernández, R.; Brown, J. M.; Lassaletta, J. M. J. Am. Chem. Soc. 2005, 127, 3290–3291. doi:10.1021/ja0423769
Return to citation in text: [1] [2] -
Burstein, C.; Lehmann, C. W.; Glorius, F. Tetrahedron 2005, 61, 6207–6217. doi:10.1016/j.tet.2005.03.115
Return to citation in text: [1] [2] -
Fürstner, A.; Davies, P. W. Angew. Chem., Int. Ed. 2007, 46, 3410–3449. doi:10.1002/anie.200604335
Return to citation in text: [1] -
Teixeira, P.; Bastin, S.; César, V. Isr. J. Chem. 2023, 63, e202200051. doi:10.1002/ijch.202200051
Return to citation in text: [1] [2] [3] -
Tang, Y.; Benaissa, I.; Huynh, M.; Vendier, L.; Lugan, N.; Bastin, S.; Belmont, P.; César, V.; Michelet, V. Angew. Chem., Int. Ed. 2019, 58, 7977–7981. doi:10.1002/anie.201901090
Return to citation in text: [1] [2] [3] -
Pedrazzani, R.; Pintus, A.; De Ventura, R.; Marchini, M.; Ceroni, P.; Silva López, C.; Monari, M.; Bandini, M. ACS Org. Inorg. Au 2022, 2, 229–235. doi:10.1021/acsorginorgau.1c00052
Return to citation in text: [1] -
Kim, Y.; Kim, Y.; Hur, M. Y.; Lee, E. J. Organomet. Chem. 2016, 820, 1–7. doi:10.1016/j.jorganchem.2016.07.023
Return to citation in text: [1] -
Varela, I.; Faustino, H.; Díez, E.; Iglesias-Sigüenza, J.; Grande-Carmona, F.; Fernández, R.; Lassaletta, J. M.; Mascareñas, J. L.; López, F. ACS Catal. 2017, 7, 2397–2402. doi:10.1021/acscatal.6b03651
Return to citation in text: [1] -
Pallova, L.; Abella, L.; Jean, M.; Vanthuyne, N.; Barthes, C.; Vendier, L.; Autschbach, J.; Crassous, J.; Bastin, S.; César, V. Chem. – Eur. J. 2022, 28, e202200166. doi:10.1002/chem.202200166
Return to citation in text: [1] -
Francos, J.; Grande-Carmona, F.; Faustino, H.; Iglesias-Sigüenza, J.; Díez, E.; Alonso, I.; Fernández, R.; Lassaletta, J. M.; López, F.; Mascareñas, J. L. J. Am. Chem. Soc. 2012, 134, 14322–14325. doi:10.1021/ja3065446
Return to citation in text: [1] -
Zhang, J.-Q.; Liu, Y.; Wang, X.-W.; Zhang, L. Organometallics 2019, 38, 3931–3938. doi:10.1021/acs.organomet.9b00400
Return to citation in text: [1] -
Rawat, V. K.; Higashida, K.; Sawamura, M. ACS Catal. 2022, 12, 8325–8330. doi:10.1021/acscatal.2c01701
Return to citation in text: [1] -
Gao, P.; Xu, J.; Zhou, T.; Liu, Y.; Bisz, E.; Dziuk, B.; Lalancette, R.; Szostak, R.; Zhang, D.; Szostak, M. Angew. Chem., Int. Ed. 2023, 62, e202218427. doi:10.1002/anie.202218427
Return to citation in text: [1] -
Scott, S. C.; Cadge, J. A.; Boden, G. K.; Bower, J. F.; Russell, C. A. Angew. Chem., Int. Ed. 2023, 62, e202301526. doi:10.1002/anie.202301526
Return to citation in text: [1] -
Kriechbaum, M.; List, M.; Berger, R. J. F.; Patzschke, M.; Monkowius, U. Chem. – Eur. J. 2012, 18, 5506–5509. doi:10.1002/chem.201200465
Return to citation in text: [1] [2] -
Lohre, C.; Fröhlich, R.; Glorius, F. Synthesis 2008, 2221–2228. doi:10.1055/s-2008-1067147
Return to citation in text: [1] [2] -
Davies, P. W.; Cremonesi, A.; Dumitrescu, L. Angew. Chem., Int. Ed. 2011, 50, 8931–8935. doi:10.1002/anie.201103563
Return to citation in text: [1] -
Gillie, A. D.; Jannapu Reddy, R.; Davies, P. W. Adv. Synth. Catal. 2016, 358, 226–239. doi:10.1002/adsc.201500905
Return to citation in text: [1] [2] [3] -
Glorius, F.; Altenhoff, G.; Goddard, R.; Lehmann, C. Chem. Commun. 2002, 2704–2705. doi:10.1039/b208045a
Return to citation in text: [1] -
Hintermann, L. Beilstein J. Org. Chem. 2007, 3, No. 22. doi:10.1186/1860-5397-3-22
Return to citation in text: [1] -
Calder, I. C.; Spotswood, T. M.; Sasse, W. H. P. Tetrahedron Lett. 1963, 4, 95–100. doi:10.1016/s0040-4039(01)90585-4
Return to citation in text: [1] -
Chien, C.-H.; Fujita, S.; Yamoto, S.; Hara, T.; Yamagata, T.; Watanabe, M.; Mashima, K. Dalton Trans. 2008, 916–923. doi:10.1039/b712901g
Return to citation in text: [1] -
Samanta, T.; Kumar Rana, B.; Roymahapatra, G.; Giri, S.; Mitra, P.; Pallepogu, R.; Kumar Chattaraj, P.; Dinda, J. Inorg. Chim. Acta 2011, 375, 271–279. doi:10.1016/j.ica.2011.05.017
Return to citation in text: [1] -
Zhang, J.-L.; Chen, L.-A.; Xu, R.-B.; Wang, C.-F.; Ruan, Y.-P.; Wang, A.-E.; Huang, P.-Q. Tetrahedron: Asymmetry 2013, 24, 492–498. doi:10.1016/j.tetasy.2013.03.004
Return to citation in text: [1] -
CCDC 2310256–2310257 contain the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via https://www.ccdc.cam.ac.uk/data_request/cif.
Return to citation in text: [1] -
Falivene, L.; Credendino, R.; Poater, A.; Petta, A.; Serra, L.; Oliva, R.; Scarano, V.; Cavallo, L. Organometallics 2016, 35, 2286–2293. doi:10.1021/acs.organomet.6b00371
Return to citation in text: [1] -
Gómez-Suárez, A.; Nelson, D. J.; Nolan, S. P. Chem. Commun. 2017, 53, 2650–2660. doi:10.1039/c7cc00255f
Return to citation in text: [1] -
Hanasaka, F.; Tanabe, Y.; Fujita, K.-i.; Yamaguchi, R. Organometallics 2006, 25, 826–831. doi:10.1021/om050723x
Return to citation in text: [1] -
Corberán, R.; Sanaú, M.; Peris, E. Organometallics 2006, 25, 4002–4008. doi:10.1021/om060343r
Return to citation in text: [1] -
Chianese, A. R.; Li, X.; Janzen, M. C.; Faller, J. W.; Crabtree, R. H. Organometallics 2003, 22, 1663–1667. doi:10.1021/om021029+
Return to citation in text: [1] -
Nelson, D. J.; Nolan, S. P. Chem. Soc. Rev. 2013, 42, 6723–6753. doi:10.1039/c3cs60146c
Return to citation in text: [1] -
Marion, N.; Ramón, R. S.; Nolan, S. P. J. Am. Chem. Soc. 2009, 131, 448–449. doi:10.1021/ja809403e
Return to citation in text: [1] [2] -
Nieto-Oberhuber, C.; López, S.; Echavarren, A. M. J. Am. Chem. Soc. 2005, 127, 6178–6179. doi:10.1021/ja042257t
Return to citation in text: [1] [2] -
Toullec, P. Y.; Genin, E.; Leseurre, L.; Genêt, J.-P.; Michelet, V. Angew. Chem., Int. Ed. 2006, 45, 7427–7430. doi:10.1002/anie.200601980
Return to citation in text: [1] [2] -
Yao, T.; Zhang, X.; Larock, R. C. J. Am. Chem. Soc. 2004, 126, 11164–11165. doi:10.1021/ja0466964
Return to citation in text: [1] -
Martí, À.; Montesinos-Magraner, M.; Echavarren, A. M.; Franchino, A. Eur. J. Org. Chem. 2022, e202200518. doi:10.1002/ejoc.202200518
Return to citation in text: [1] [2] -
Coles, S. J.; Allan, D. R.; Beavers, C. M.; Teat, S. J.; Holgate, S. J. W.; Tovee, C. A. Leading Edge Chemical Crystallography Service Provision and Its Impact on Crystallographic Data Science in the Twenty-First Century. In 21st Century Challenges in Chemical Crystallography I: History and Technical Developments; Mingos, D. M. P.; Raithby, P. R., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp 69–140. doi:10.1007/430_2020_63
Return to citation in text: [1]
| 8. | Tang, Y.; Benaissa, I.; Huynh, M.; Vendier, L.; Lugan, N.; Bastin, S.; Belmont, P.; César, V.; Michelet, V. Angew. Chem., Int. Ed. 2019, 58, 7977–7981. doi:10.1002/anie.201901090 |
| 37. | Toullec, P. Y.; Genin, E.; Leseurre, L.; Genêt, J.-P.; Michelet, V. Angew. Chem., Int. Ed. 2006, 45, 7427–7430. doi:10.1002/anie.200601980 |
| 38. | Yao, T.; Zhang, X.; Larock, R. C. J. Am. Chem. Soc. 2004, 126, 11164–11165. doi:10.1021/ja0466964 |
| 39. | Martí, À.; Montesinos-Magraner, M.; Echavarren, A. M.; Franchino, A. Eur. J. Org. Chem. 2022, e202200518. doi:10.1002/ejoc.202200518 |
| 35. | Marion, N.; Ramón, R. S.; Nolan, S. P. J. Am. Chem. Soc. 2009, 131, 448–449. doi:10.1021/ja809403e |
| 8. | Tang, Y.; Benaissa, I.; Huynh, M.; Vendier, L.; Lugan, N.; Bastin, S.; Belmont, P.; César, V.; Michelet, V. Angew. Chem., Int. Ed. 2019, 58, 7977–7981. doi:10.1002/anie.201901090 |
| 37. | Toullec, P. Y.; Genin, E.; Leseurre, L.; Genêt, J.-P.; Michelet, V. Angew. Chem., Int. Ed. 2006, 45, 7427–7430. doi:10.1002/anie.200601980 |
| 1. | Hopkinson, M. N.; Richter, C.; Schedler, M.; Glorius, F. Nature 2014, 510, 485–496. doi:10.1038/nature13384 |
| 6. | Fürstner, A.; Davies, P. W. Angew. Chem., Int. Ed. 2007, 46, 3410–3449. doi:10.1002/anie.200604335 |
| 21. | Gillie, A. D.; Jannapu Reddy, R.; Davies, P. W. Adv. Synth. Catal. 2016, 358, 226–239. doi:10.1002/adsc.201500905 |
| 5. | Burstein, C.; Lehmann, C. W.; Glorius, F. Tetrahedron 2005, 61, 6207–6217. doi:10.1016/j.tet.2005.03.115 |
| 5. | Burstein, C.; Lehmann, C. W.; Glorius, F. Tetrahedron 2005, 61, 6207–6217. doi:10.1016/j.tet.2005.03.115 |
| 18. | Kriechbaum, M.; List, M.; Berger, R. J. F.; Patzschke, M.; Monkowius, U. Chem. – Eur. J. 2012, 18, 5506–5509. doi:10.1002/chem.201200465 |
| 19. | Lohre, C.; Fröhlich, R.; Glorius, F. Synthesis 2008, 2221–2228. doi:10.1055/s-2008-1067147 |
| 23. | Hintermann, L. Beilstein J. Org. Chem. 2007, 3, No. 22. doi:10.1186/1860-5397-3-22 |
| 24. | Calder, I. C.; Spotswood, T. M.; Sasse, W. H. P. Tetrahedron Lett. 1963, 4, 95–100. doi:10.1016/s0040-4039(01)90585-4 |
| 25. | Chien, C.-H.; Fujita, S.; Yamoto, S.; Hara, T.; Yamagata, T.; Watanabe, M.; Mashima, K. Dalton Trans. 2008, 916–923. doi:10.1039/b712901g |
| 26. | Samanta, T.; Kumar Rana, B.; Roymahapatra, G.; Giri, S.; Mitra, P.; Pallepogu, R.; Kumar Chattaraj, P.; Dinda, J. Inorg. Chim. Acta 2011, 375, 271–279. doi:10.1016/j.ica.2011.05.017 |
| 27. | Zhang, J.-L.; Chen, L.-A.; Xu, R.-B.; Wang, C.-F.; Ruan, Y.-P.; Wang, A.-E.; Huang, P.-Q. Tetrahedron: Asymmetry 2013, 24, 492–498. doi:10.1016/j.tetasy.2013.03.004 |
| 4. | Alcarazo, M.; Roseblade, S. J.; Cowley, A. R.; Fernández, R.; Brown, J. M.; Lassaletta, J. M. J. Am. Chem. Soc. 2005, 127, 3290–3291. doi:10.1021/ja0423769 |
| 20. | Davies, P. W.; Cremonesi, A.; Dumitrescu, L. Angew. Chem., Int. Ed. 2011, 50, 8931–8935. doi:10.1002/anie.201103563 |
| 21. | Gillie, A. D.; Jannapu Reddy, R.; Davies, P. W. Adv. Synth. Catal. 2016, 358, 226–239. doi:10.1002/adsc.201500905 |
| 2. | Reshi, N. U. D.; Bera, J. K. Coord. Chem. Rev. 2020, 422, 213334. doi:10.1016/j.ccr.2020.213334 |
| 3. | Iglesias-Sigüenza, J.; Izquierdo, C.; Díez, E.; Fernández, R.; Lassaletta, J. M. Dalton Trans. 2016, 45, 10113–10117. doi:10.1039/c6dt01700b |
| 22. | Glorius, F.; Altenhoff, G.; Goddard, R.; Lehmann, C. Chem. Commun. 2002, 2704–2705. doi:10.1039/b208045a |
| 14. | Zhang, J.-Q.; Liu, Y.; Wang, X.-W.; Zhang, L. Organometallics 2019, 38, 3931–3938. doi:10.1021/acs.organomet.9b00400 |
| 16. | Gao, P.; Xu, J.; Zhou, T.; Liu, Y.; Bisz, E.; Dziuk, B.; Lalancette, R.; Szostak, R.; Zhang, D.; Szostak, M. Angew. Chem., Int. Ed. 2023, 62, e202218427. doi:10.1002/anie.202218427 |
| 17. | Scott, S. C.; Cadge, J. A.; Boden, G. K.; Bower, J. F.; Russell, C. A. Angew. Chem., Int. Ed. 2023, 62, e202301526. doi:10.1002/anie.202301526 |
| 40. | Coles, S. J.; Allan, D. R.; Beavers, C. M.; Teat, S. J.; Holgate, S. J. W.; Tovee, C. A. Leading Edge Chemical Crystallography Service Provision and Its Impact on Crystallographic Data Science in the Twenty-First Century. In 21st Century Challenges in Chemical Crystallography I: History and Technical Developments; Mingos, D. M. P.; Raithby, P. R., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp 69–140. doi:10.1007/430_2020_63 |
| 11. | Varela, I.; Faustino, H.; Díez, E.; Iglesias-Sigüenza, J.; Grande-Carmona, F.; Fernández, R.; Lassaletta, J. M.; Mascareñas, J. L.; López, F. ACS Catal. 2017, 7, 2397–2402. doi:10.1021/acscatal.6b03651 |
| 12. | Pallova, L.; Abella, L.; Jean, M.; Vanthuyne, N.; Barthes, C.; Vendier, L.; Autschbach, J.; Crassous, J.; Bastin, S.; César, V. Chem. – Eur. J. 2022, 28, e202200166. doi:10.1002/chem.202200166 |
| 13. | Francos, J.; Grande-Carmona, F.; Faustino, H.; Iglesias-Sigüenza, J.; Díez, E.; Alonso, I.; Fernández, R.; Lassaletta, J. M.; López, F.; Mascareñas, J. L. J. Am. Chem. Soc. 2012, 134, 14322–14325. doi:10.1021/ja3065446 |
| 2. | Reshi, N. U. D.; Bera, J. K. Coord. Chem. Rev. 2020, 422, 213334. doi:10.1016/j.ccr.2020.213334 |
| 18. | Kriechbaum, M.; List, M.; Berger, R. J. F.; Patzschke, M.; Monkowius, U. Chem. – Eur. J. 2012, 18, 5506–5509. doi:10.1002/chem.201200465 |
| 19. | Lohre, C.; Fröhlich, R.; Glorius, F. Synthesis 2008, 2221–2228. doi:10.1055/s-2008-1067147 |
| 8. | Tang, Y.; Benaissa, I.; Huynh, M.; Vendier, L.; Lugan, N.; Bastin, S.; Belmont, P.; César, V.; Michelet, V. Angew. Chem., Int. Ed. 2019, 58, 7977–7981. doi:10.1002/anie.201901090 |
| 9. | Pedrazzani, R.; Pintus, A.; De Ventura, R.; Marchini, M.; Ceroni, P.; Silva López, C.; Monari, M.; Bandini, M. ACS Org. Inorg. Au 2022, 2, 229–235. doi:10.1021/acsorginorgau.1c00052 |
| 10. | Kim, Y.; Kim, Y.; Hur, M. Y.; Lee, E. J. Organomet. Chem. 2016, 820, 1–7. doi:10.1016/j.jorganchem.2016.07.023 |
| 39. | Martí, À.; Montesinos-Magraner, M.; Echavarren, A. M.; Franchino, A. Eur. J. Org. Chem. 2022, e202200518. doi:10.1002/ejoc.202200518 |
| 7. | Teixeira, P.; Bastin, S.; César, V. Isr. J. Chem. 2023, 63, e202200051. doi:10.1002/ijch.202200051 |
| 7. | Teixeira, P.; Bastin, S.; César, V. Isr. J. Chem. 2023, 63, e202200051. doi:10.1002/ijch.202200051 |
| 15. | Rawat, V. K.; Higashida, K.; Sawamura, M. ACS Catal. 2022, 12, 8325–8330. doi:10.1021/acscatal.2c01701 |
| 36. | Nieto-Oberhuber, C.; López, S.; Echavarren, A. M. J. Am. Chem. Soc. 2005, 127, 6178–6179. doi:10.1021/ja042257t |
| 7. | Teixeira, P.; Bastin, S.; César, V. Isr. J. Chem. 2023, 63, e202200051. doi:10.1002/ijch.202200051 |
| 4. | Alcarazo, M.; Roseblade, S. J.; Cowley, A. R.; Fernández, R.; Brown, J. M.; Lassaletta, J. M. J. Am. Chem. Soc. 2005, 127, 3290–3291. doi:10.1021/ja0423769 |
| 21. | Gillie, A. D.; Jannapu Reddy, R.; Davies, P. W. Adv. Synth. Catal. 2016, 358, 226–239. doi:10.1002/adsc.201500905 |
| 35. | Marion, N.; Ramón, R. S.; Nolan, S. P. J. Am. Chem. Soc. 2009, 131, 448–449. doi:10.1021/ja809403e |
| 36. | Nieto-Oberhuber, C.; López, S.; Echavarren, A. M. J. Am. Chem. Soc. 2005, 127, 6178–6179. doi:10.1021/ja042257t |
| 33. | Chianese, A. R.; Li, X.; Janzen, M. C.; Faller, J. W.; Crabtree, R. H. Organometallics 2003, 22, 1663–1667. doi:10.1021/om021029+ |
| 34. | Nelson, D. J.; Nolan, S. P. Chem. Soc. Rev. 2013, 42, 6723–6753. doi:10.1039/c3cs60146c |
| 30. | Gómez-Suárez, A.; Nelson, D. J.; Nolan, S. P. Chem. Commun. 2017, 53, 2650–2660. doi:10.1039/c7cc00255f |
| 31. | Hanasaka, F.; Tanabe, Y.; Fujita, K.-i.; Yamaguchi, R. Organometallics 2006, 25, 826–831. doi:10.1021/om050723x |
| 32. | Corberán, R.; Sanaú, M.; Peris, E. Organometallics 2006, 25, 4002–4008. doi:10.1021/om060343r |
| 28. | CCDC 2310256–2310257 contain the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via https://www.ccdc.cam.ac.uk/data_request/cif. |
| 29. | Falivene, L.; Credendino, R.; Poater, A.; Petta, A.; Serra, L.; Oliva, R.; Scarano, V.; Cavallo, L. Organometallics 2016, 35, 2286–2293. doi:10.1021/acs.organomet.6b00371 |
© 2024 Gillie et al.; licensee Beilstein-Institut.
This is an open access article licensed under the terms of the Beilstein-Institut Open Access License Agreement (https://www.beilstein-journals.org/bjoc/terms), which is identical to the Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0). The reuse of material under this license requires that the author(s), source and license are credited. Third-party material in this article could be subject to other licenses (typically indicated in the credit line), and in this case, users are required to obtain permission from the license holder to reuse the material.