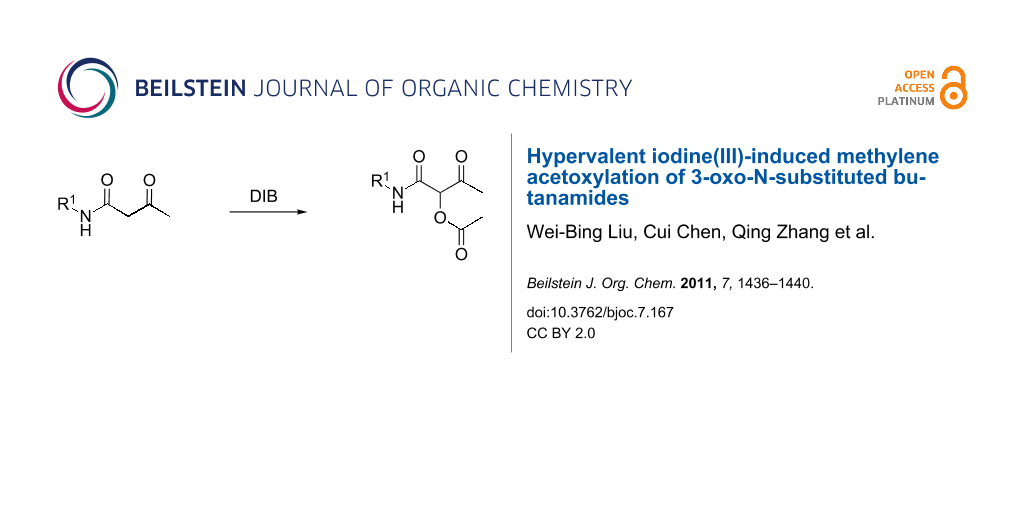
Abstract
1-Carbamoyl-2-oxopropyl acetate derivatives were synthesized through an acetoxylation process to methylene with the aid of (diacetoxyiodo)benzene (DIB) as the oxidant. Not only mild reaction conditions, but also excellent yields and good substrate scope make the present protocol potentially useful in organic synthesis.
Graphical Abstract
Introduction
Carbon–carbon, carbon–heteroatom bond formation leading to useful molecular structures is one of the most interesting and challenging research topics in organic chemistry [1-14]. Indeed, direct oxidative C–H bond functionalization provides an atom-economical and efficient pathway to achieve these goals. Representative examples have been elegantly utilized not only in academic research, but also in the production of a variety of fine chemicals, such as pharmaceuticals, agrochemicals, and intermediates [15-18]. The field of chemistry concerning organic polyvalent iodine compounds has witnessed a great expansion during the last few decades, an expansion which continues at an increasing pace [19-30]. The availability of iodine(III) and iodine(V) compounds and the development of new reagents, along with their low toxicity, ready availability, easy handling, clean transformation and reactivity, their selectivity under a variety of conditions, and their tolerance to different functional groups make these compounds valuable tools in organic synthesis [31-36]. Our interest in the chemistry of polyvalent iodine(III) reagents [37-39] prompted us to exploit the reactivity of (diacetoxyiodo)benzene (DIB). We report herein the use of DIB, as a nucleophile and oxidant, to perform an acetoxylation reaction with 3-oxo-N-substituted butanamides (Scheme 1).
Scheme 1: Synthesis of 1-carbamoyl-2-oxopropyl acetates.
Scheme 1: Synthesis of 1-carbamoyl-2-oxopropyl acetates.
Results and Discussion
Initially, we employed 3-oxo-N-phenylbutanamide (1a) as the model substrate and tried to establish an effective reaction system for the synthesis. The results are shown in Table 1. It was found that the reaction afforded the desired product 1-(phenylcarbamoyl)-2-oxopropyl acetate (2a) by using DIB as the additive, and the optimum reaction time was 2 hours (Table 1, entries 1–3), whereas almost no desired product was obtained when Lewis acids were added (Table 1, entries 4–6). Among the various solvents examined, dioxane, DCE and DMF were practical solvents (Table 1, entries 2, 7–9). It is noteworthy that the reaction led to an obvious decrease of the yield of 2a when either 0.5 or 2 equiv of DIB were used (Table 1, entries 11 and 13) compared to 1.3 equiv (Table 1, entry 12), which was found to be the optimum amount of DIB (Table 1, entries 11–13).
Table 1: Optimization of reaction conditions.a
|
|
||||
| entry | solvent | additive (1.0 equiv) | time (h) | yield (%)b |
|---|---|---|---|---|
| 1 | dioxane | — | 1 | 66 |
| 2 | dioxane | — | 2 | 80 |
| 3 | dioxane | — | 3 | 81 |
| 4 | dioxane | FeCl3 | 2 | trace |
| 5 | dioxane | ZnCl2 | 2 | trace |
| 6 | dioxane | CuCl2 | 2 | trace |
| 7 | cyclohexane | — | 2 | 36 |
| 8 | DCE | — | 2 | 82 |
| 9 | DMF | — | 2 | 71 |
| 10 | DMSO | — | 2 | 47 |
| 11c | DCE | — | 2 | 35 |
| 12d | DCE | — | 2 | 89 |
| 13e | DCE | — | 2 | 75 |
a1a (0.25 mmol), solvent (2 mL), DIB (1.0 equiv); bGC yield; cDIB (0.5 equiv); dDIB (1.3 equiv); eDIB (2.0 equiv).
To explore the substrate scope and limitations of this reaction, a range of 3-oxo-N-phenylbutanamides were then examined under the optimized reaction conditions. The results are shown in Scheme 2.
Scheme 2: The synthesis of 1-carbamoyl-2-oxopropyl acetates. Conditions: 1 (1.0 mmol), DCE (2 mL), DIB (1.3 equiv); %: Isolated yield.
Scheme 2: The synthesis of 1-carbamoyl-2-oxopropyl acetates. Conditions: 1 (1.0 mmol), DCE (2 mL), DIB (1.3 e...
We found that the reaction led to the corresponding products 2a–2l in excellent isolated yields with all substrates. The reaction appears to be quite tolerant to differences in the position, number and electronic contribution of the substituent on the benzene ring. For example, the reactions of 3-oxo-N-phenylbutanamide, N-(4-methoxyphenyl)-3-oxobutanamide, N-(2-methoxyphenyl)-3-oxobutanamide, N-(2,5-dichlorophenyl)-3-oxobutanamide, N-(2,4-dimethoxyphenyl)-3-oxobutanamide as well as N-(4-chloro-2,5-dimethoxyphenyl)-3-oxobutanamide all lead to the corresponding products (2a, 2e, 2f, 2g, 2j, and 2k, respectively) in excellent isolated yield. Similarly, the reactions of other N-(alkylsubstituted)-3-oxobutanamides were investigated, such as that of N-methyl-3-oxobutanamide (1l), which led to 1-(methylcarbamoyl)-2-oxopropyl acetate in 89% yield. Furthermore, we applied this method to non-carbamoyl 1,3-dicarbonyl compounds. These substrates, namely 1-phenylbutane-1,3-dione, 1,3-diphenylpropane-1,3-dione and ethyl 3-oxo-3-phenylpropanoate, all produced products in moderate isolated yields (2m, 2n, 2o).
A plausible mechanism for the described transformation can be rationalized as shown in Scheme 3. The reaction initiates with the attack of the lone-pair electrons of the carbamoyl nitrogen [39-41] or carbonyl oxygen [42-45] on the iodine(III) of DIB, forming intermediates 3 and 5, respectively. Alternatively, DIB attacks the C–C double bond of the enol derived from 1a and forms intermediate 6 [46,47]. The subsequent N–I, O–I and C–I bond cleavage along with the nucleophilic attack of the acetate ion on the C–N or C–C double bond of the intermediate 4, 5 or 6 affords the final product 2a.
Conclusion
In conclusion, we have shown an efficient and operationally simple method to synthesize 1-carbamoyl-2-oxopropyl acetate derivatives. The readily accessible starting materials, cheap oxidant DIB, as well as the mild reaction conditions and excellent yields make the present protocol potentially useful in organic synthesis. Further studies on the application to more valuable compounds and detailed investigations of the reaction mechanism are in progress.
Supporting Information
| Supporting Information File 1: Experimental details and copies of NMR spectra. | ||
| Format: PDF | Size: 3.8 MB | Download |
References
-
Ritleng, V.; Sirlin, C.; Pfeffer, M. Chem. Rev. 2002, 102, 1731–1770. doi:10.1021/cr0104330
Return to citation in text: [1] -
Kakiuchi, F.; Chatani, N. Adv. Synth. Catal. 2003, 345, 1077–1101. doi:10.1002/adsc.200303094
Return to citation in text: [1] -
Dilman, A. D.; Ioffe, S. L. Chem. Rev. 2003, 103, 733–772. doi:10.1021/cr020003p
Return to citation in text: [1] -
Fagnou, K.; Lautens, M. Chem. Rev. 2003, 103, 169–196. doi:10.1021/cr020007u
Return to citation in text: [1] -
Li, C.-J. Chem. Rev. 2005, 105, 3095–3166. doi:10.1021/cr030009u
Return to citation in text: [1] -
Fagnoni, M.; Dondi, D.; Ravelli, D.; Albini, A. Chem. Rev. 2007, 107, 2725–2756. doi:10.1021/cr068352x
Return to citation in text: [1] -
Beccalli, E. M.; Broggini, G.; Martinelli, M.; Sottocornola, S. Chem. Rev. 2007, 107, 5318–5365. doi:10.1021/cr068006f
Return to citation in text: [1] -
Tong, X.; Beller, M.; Tse, M. K. J. Am. Chem. Soc. 2007, 129, 4906–4907. doi:10.1021/ja070919j
Return to citation in text: [1] -
Niu, J.; Zhou, H.; Li, Z.; Xu, J.; Hu, S. J. Org. Chem. 2008, 73, 7814–7817. doi:10.1021/jo801002c
Return to citation in text: [1] -
Lee, J. M.; Park, E. J.; Cho, S. H.; Chang, S. J. Am. Chem. Soc. 2008, 130, 7824–7825. doi:10.1021/ja8031218
Return to citation in text: [1] -
Ueda, S.; Nagasawa, H. J. Org. Chem. 2009, 74, 4272–4277. doi:10.1021/jo900513z
Return to citation in text: [1] -
Liang, Z.; Hou, W.; Du, Y.; Zhang, Y.; Pan, Y.; Mao, D.; Zhao, K. Org. Lett. 2009, 11, 4978–4981. doi:10.1021/ol902157c
Return to citation in text: [1] -
Peng, Y.; Cui, L.; Zhang, G.; Zhang, L. J. Am. Chem. Soc. 2009, 131, 5062–5063. doi:10.1021/ja901048w
Return to citation in text: [1] -
Mizuhara, T.; Oishi, S.; Fujii, N.; Ohno, H. J. Org. Chem. 2010, 75, 265–268. doi:10.1021/jo902327n
Return to citation in text: [1] -
Markó, I. E.; Giles, P. R.; Tsukazaki, M.; Brown, S. M.; Urch, C. J. Science 1996, 274, 2044–2046. doi:10.1126/science.274.5295.2044
Return to citation in text: [1] -
ten Brink, G.-J.; Arends, I. W. C. E.; Sheldon, R. A. Science 2000, 287, 1636–1639. doi:10.1126/science.287.5458.1636
Return to citation in text: [1] -
Enache, D. I.; Edwards, J. K.; Landon, P.; Solsona-Espriu, B.; Carley, A. F.; Herzing, A. A.; Watanabe, M.; Kiely, C. J.; Knight, D. W.; Hutchings, G. J. Science 2006, 311, 362–365. doi:10.1126/science.1120560
Return to citation in text: [1] -
Piera, J.; Bäckvall, J.-E. Angew. Chem., Int. Ed. 2008, 47, 3506–3523. doi:10.1002/anie.200700604
Return to citation in text: [1] -
Varvoglis, A. The Organic Chemistry of Polycoordinated Iodine; VCH: New York, 1992.
Return to citation in text: [1] -
Varvoglis, A. Hypervalent Iodine in Organic Synthesis; Academic Press: London, 1997.
Return to citation in text: [1] -
Zhdankin, V. V.; Stang, P. J. Chem. Rev. 2002, 102, 2523–2584. doi:10.1021/cr010003+
Return to citation in text: [1] -
Wirth, T., Ed. Hypervalent Iodine Chemistry; Springer-Verlag: Berlin, 2003.
Return to citation in text: [1] -
Richardson, R. D.; Wirth, T. Angew. Chem., Int. Ed. 2006, 45, 4402–4404. doi:10.1002/anie.200601817
Return to citation in text: [1] -
Ciufolini, M. A.; Braun, N. A.; Canesi, S.; Ousmer, M.; Chang, J.; Chai, D. Synthesis 2007, 3759–3772. doi:10.1055/s-2007-990906
Return to citation in text: [1] -
Quideau, S.; Pouységu, L.; Deffieux, D. Synlett 2008, 467–495. doi:10.1055/s-2008-1032094
Return to citation in text: [1] -
Zhdankin, V. V.; Stang, P. J. Chem. Rev. 2008, 108, 5299–5358. doi:10.1021/cr800332c
Return to citation in text: [1] -
Koller, R.; Stanek, K.; Stolz, D.; Aardoom, R.; Niedermann, K.; Togni, A. Angew. Chem., Int. Ed. 2009, 48, 4332–4336. doi:10.1002/anie.200900974
Return to citation in text: [1] -
Uyanik, M.; Yasui, T.; Ishihara, K. Angew. Chem., Int. Ed. 2010, 49, 2175–2177. doi:10.1002/anie.200907352
Return to citation in text: [1] -
Niedermann, K.; Früh, N.; Vinogradova, E.; Wiehn, M. S.; Moreno, A.; Togni, A. Angew. Chem., Int. Ed. 2011, 50, 1059–1063. doi:10.1002/anie.201006021
Return to citation in text: [1] -
Brand, J. P.; González, D. F.; Nicolai, S.; Waser, J. Chem. Commun. 2011, 47, 102–115. doi:10.1039/c0cc02265a
Return to citation in text: [1] -
Ochiai, M.; Miyamoto, K. Eur. J. Org. Chem. 2008, 4229–4239. doi:10.1002/ejoc.200800416
Return to citation in text: [1] -
Dohi, T.; Kita, Y. Chem. Commun. 2009, 2073–2085. doi:10.1039/b821747e
Return to citation in text: [1] -
Jen, T.; Mendelsohn, B. A.; Ciufolini, M. A. J. Org. Chem. 2011, 76, 728–731. doi:10.1021/jo102241s
Return to citation in text: [1] -
Sun, Y.; Fan, R. H. Chem. Commun. 2010, 46, 6834–6836. doi:10.1039/c0cc01911a
Return to citation in text: [1] -
Chen, L.; Shi, E.; Liu, Z.; Chen, S.; Wei, W.; Li, H.; Xu, K.; Wan, X. Chem.–Eur. J. 2011, 17, 4085–4089. doi:10.1002/chem.201100192
Return to citation in text: [1] -
Uyanik, M.; Yasui, T.; Ishihara, K. Angew. Chem., Int. Ed. 2010, 49, 2175–2177. doi:10.1002/anie.200907352
Return to citation in text: [1] -
Liu, W.; Jiang, H.; Huang, L. Org. Lett. 2010, 12, 312–315. doi:10.1021/ol9026478
Return to citation in text: [1] -
Liu, W.; Chen, C.; Zhang, Q. Org. Biomol. Chem. 2011, 9, 6484–6486. doi:10.1039/c1ob05958k
Return to citation in text: [1] -
Li, X.; Du, Y.; Liang, Z.; Li, X.; Pan, Y.; Zhao, K. Org. Lett. 2009, 11, 2643–2646. doi:10.1021/ol9006663
Return to citation in text: [1] [2] -
Malamidou-Xenikaki, E.; Spyroudis, S.; Tsanakopoulou, M.; Hadjipavlou-Litina, D. J. Org. Chem. 2009, 74, 7315–7321. doi:10.1021/jo9013063
Return to citation in text: [1] -
Serna, S.; Tellitu, I.; Domínguez, E.; Moreno, I.; SanMartín, R. Org. Lett. 2005, 7, 3073–3076. doi:10.1021/ol0510623
Return to citation in text: [1] -
Harayama, Y.; Yoshida, M.; Kamimura, D.; Kita, Y. Chem. Commun. 2005, 1764–1766. doi:10.1039/b418212j
Return to citation in text: [1] -
Singh, C. B.; Ghosh, H.; Murru, S.; Patel, B. K. J. Org. Chem. 2008, 73, 2924–2927. doi:10.1021/jo702628g
Return to citation in text: [1] -
Mizukami, F.; Ando, M.; Tanaka, T.; Imamura, J. Bull. Chem. Soc. Jpn. 1978, 51, 335–336. doi:10.1246/bcsj.51.335
Return to citation in text: [1] -
Kawano, Y.; Togo, H. Synlett 2008, 217–220. doi:10.1055/s-2007-1000871
Return to citation in text: [1] -
Yu, J.; Tian, J.; Zhang, C. Adv. Synth. Catal. 2010, 352, 531–546. doi:10.1002/adsc.200900737
Return to citation in text: [1] -
Ochiai, M.; Takeuchi, Y.; Katayama, T.; Sueda, T.; Miyamoto, K. J. Am. Chem. Soc. 2005, 127, 12244–12245. doi:10.1021/ja0542800
Return to citation in text: [1]
| 1. | Ritleng, V.; Sirlin, C.; Pfeffer, M. Chem. Rev. 2002, 102, 1731–1770. doi:10.1021/cr0104330 |
| 2. | Kakiuchi, F.; Chatani, N. Adv. Synth. Catal. 2003, 345, 1077–1101. doi:10.1002/adsc.200303094 |
| 3. | Dilman, A. D.; Ioffe, S. L. Chem. Rev. 2003, 103, 733–772. doi:10.1021/cr020003p |
| 4. | Fagnou, K.; Lautens, M. Chem. Rev. 2003, 103, 169–196. doi:10.1021/cr020007u |
| 5. | Li, C.-J. Chem. Rev. 2005, 105, 3095–3166. doi:10.1021/cr030009u |
| 6. | Fagnoni, M.; Dondi, D.; Ravelli, D.; Albini, A. Chem. Rev. 2007, 107, 2725–2756. doi:10.1021/cr068352x |
| 7. | Beccalli, E. M.; Broggini, G.; Martinelli, M.; Sottocornola, S. Chem. Rev. 2007, 107, 5318–5365. doi:10.1021/cr068006f |
| 8. | Tong, X.; Beller, M.; Tse, M. K. J. Am. Chem. Soc. 2007, 129, 4906–4907. doi:10.1021/ja070919j |
| 9. | Niu, J.; Zhou, H.; Li, Z.; Xu, J.; Hu, S. J. Org. Chem. 2008, 73, 7814–7817. doi:10.1021/jo801002c |
| 10. | Lee, J. M.; Park, E. J.; Cho, S. H.; Chang, S. J. Am. Chem. Soc. 2008, 130, 7824–7825. doi:10.1021/ja8031218 |
| 11. | Ueda, S.; Nagasawa, H. J. Org. Chem. 2009, 74, 4272–4277. doi:10.1021/jo900513z |
| 12. | Liang, Z.; Hou, W.; Du, Y.; Zhang, Y.; Pan, Y.; Mao, D.; Zhao, K. Org. Lett. 2009, 11, 4978–4981. doi:10.1021/ol902157c |
| 13. | Peng, Y.; Cui, L.; Zhang, G.; Zhang, L. J. Am. Chem. Soc. 2009, 131, 5062–5063. doi:10.1021/ja901048w |
| 14. | Mizuhara, T.; Oishi, S.; Fujii, N.; Ohno, H. J. Org. Chem. 2010, 75, 265–268. doi:10.1021/jo902327n |
| 37. | Liu, W.; Jiang, H.; Huang, L. Org. Lett. 2010, 12, 312–315. doi:10.1021/ol9026478 |
| 38. | Liu, W.; Chen, C.; Zhang, Q. Org. Biomol. Chem. 2011, 9, 6484–6486. doi:10.1039/c1ob05958k |
| 39. | Li, X.; Du, Y.; Liang, Z.; Li, X.; Pan, Y.; Zhao, K. Org. Lett. 2009, 11, 2643–2646. doi:10.1021/ol9006663 |
| 31. | Ochiai, M.; Miyamoto, K. Eur. J. Org. Chem. 2008, 4229–4239. doi:10.1002/ejoc.200800416 |
| 32. | Dohi, T.; Kita, Y. Chem. Commun. 2009, 2073–2085. doi:10.1039/b821747e |
| 33. | Jen, T.; Mendelsohn, B. A.; Ciufolini, M. A. J. Org. Chem. 2011, 76, 728–731. doi:10.1021/jo102241s |
| 34. | Sun, Y.; Fan, R. H. Chem. Commun. 2010, 46, 6834–6836. doi:10.1039/c0cc01911a |
| 35. | Chen, L.; Shi, E.; Liu, Z.; Chen, S.; Wei, W.; Li, H.; Xu, K.; Wan, X. Chem.–Eur. J. 2011, 17, 4085–4089. doi:10.1002/chem.201100192 |
| 36. | Uyanik, M.; Yasui, T.; Ishihara, K. Angew. Chem., Int. Ed. 2010, 49, 2175–2177. doi:10.1002/anie.200907352 |
| 19. | Varvoglis, A. The Organic Chemistry of Polycoordinated Iodine; VCH: New York, 1992. |
| 20. | Varvoglis, A. Hypervalent Iodine in Organic Synthesis; Academic Press: London, 1997. |
| 21. | Zhdankin, V. V.; Stang, P. J. Chem. Rev. 2002, 102, 2523–2584. doi:10.1021/cr010003+ |
| 22. | Wirth, T., Ed. Hypervalent Iodine Chemistry; Springer-Verlag: Berlin, 2003. |
| 23. | Richardson, R. D.; Wirth, T. Angew. Chem., Int. Ed. 2006, 45, 4402–4404. doi:10.1002/anie.200601817 |
| 24. | Ciufolini, M. A.; Braun, N. A.; Canesi, S.; Ousmer, M.; Chang, J.; Chai, D. Synthesis 2007, 3759–3772. doi:10.1055/s-2007-990906 |
| 25. | Quideau, S.; Pouységu, L.; Deffieux, D. Synlett 2008, 467–495. doi:10.1055/s-2008-1032094 |
| 26. | Zhdankin, V. V.; Stang, P. J. Chem. Rev. 2008, 108, 5299–5358. doi:10.1021/cr800332c |
| 27. | Koller, R.; Stanek, K.; Stolz, D.; Aardoom, R.; Niedermann, K.; Togni, A. Angew. Chem., Int. Ed. 2009, 48, 4332–4336. doi:10.1002/anie.200900974 |
| 28. | Uyanik, M.; Yasui, T.; Ishihara, K. Angew. Chem., Int. Ed. 2010, 49, 2175–2177. doi:10.1002/anie.200907352 |
| 29. | Niedermann, K.; Früh, N.; Vinogradova, E.; Wiehn, M. S.; Moreno, A.; Togni, A. Angew. Chem., Int. Ed. 2011, 50, 1059–1063. doi:10.1002/anie.201006021 |
| 30. | Brand, J. P.; González, D. F.; Nicolai, S.; Waser, J. Chem. Commun. 2011, 47, 102–115. doi:10.1039/c0cc02265a |
| 15. | Markó, I. E.; Giles, P. R.; Tsukazaki, M.; Brown, S. M.; Urch, C. J. Science 1996, 274, 2044–2046. doi:10.1126/science.274.5295.2044 |
| 16. | ten Brink, G.-J.; Arends, I. W. C. E.; Sheldon, R. A. Science 2000, 287, 1636–1639. doi:10.1126/science.287.5458.1636 |
| 17. | Enache, D. I.; Edwards, J. K.; Landon, P.; Solsona-Espriu, B.; Carley, A. F.; Herzing, A. A.; Watanabe, M.; Kiely, C. J.; Knight, D. W.; Hutchings, G. J. Science 2006, 311, 362–365. doi:10.1126/science.1120560 |
| 18. | Piera, J.; Bäckvall, J.-E. Angew. Chem., Int. Ed. 2008, 47, 3506–3523. doi:10.1002/anie.200700604 |
| 46. | Yu, J.; Tian, J.; Zhang, C. Adv. Synth. Catal. 2010, 352, 531–546. doi:10.1002/adsc.200900737 |
| 47. | Ochiai, M.; Takeuchi, Y.; Katayama, T.; Sueda, T.; Miyamoto, K. J. Am. Chem. Soc. 2005, 127, 12244–12245. doi:10.1021/ja0542800 |
| 42. | Harayama, Y.; Yoshida, M.; Kamimura, D.; Kita, Y. Chem. Commun. 2005, 1764–1766. doi:10.1039/b418212j |
| 43. | Singh, C. B.; Ghosh, H.; Murru, S.; Patel, B. K. J. Org. Chem. 2008, 73, 2924–2927. doi:10.1021/jo702628g |
| 44. | Mizukami, F.; Ando, M.; Tanaka, T.; Imamura, J. Bull. Chem. Soc. Jpn. 1978, 51, 335–336. doi:10.1246/bcsj.51.335 |
| 45. | Kawano, Y.; Togo, H. Synlett 2008, 217–220. doi:10.1055/s-2007-1000871 |
| 39. | Li, X.; Du, Y.; Liang, Z.; Li, X.; Pan, Y.; Zhao, K. Org. Lett. 2009, 11, 2643–2646. doi:10.1021/ol9006663 |
| 40. | Malamidou-Xenikaki, E.; Spyroudis, S.; Tsanakopoulou, M.; Hadjipavlou-Litina, D. J. Org. Chem. 2009, 74, 7315–7321. doi:10.1021/jo9013063 |
| 41. | Serna, S.; Tellitu, I.; Domínguez, E.; Moreno, I.; SanMartín, R. Org. Lett. 2005, 7, 3073–3076. doi:10.1021/ol0510623 |
© 2011 Liu et al; licensee Beilstein-Institut.
This is an Open Access article under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
The license is subject to the Beilstein Journal of Organic Chemistry terms and conditions: (http://www.beilstein-journals.org/bjoc)