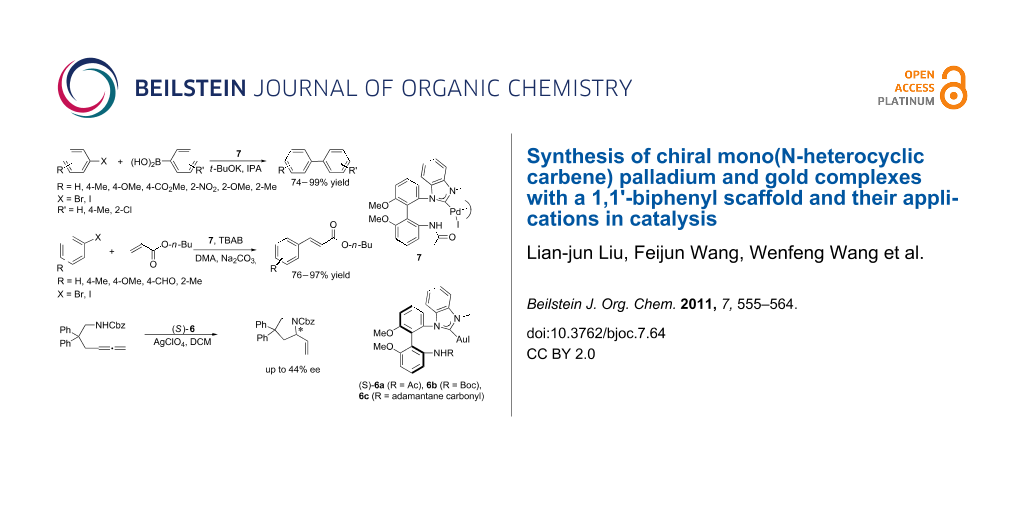
Abstract
Axially chiral mono(NHC)–Pd(II) and mono(NHC)–Au(I) complexes with one side shaped 1,1'-biphenyl backbone have been prepared from chiral 6,6'-dimethoxybiphenyl-2,2'-diamine. The complexes were characterized by X-ray crystal structure diffraction. The Pd(II) complex showed good catalytic activities in the Suzuki–Miyaura and Heck–Mizoroki coupling reactions, and the (S)-Au(I) complexes also showed good catalytic activities in the asymmetric intramolecular hydroamination reaction to give the corresponding product in moderate ee.
Graphical Abstract
Introduction
N-heterocyclic carbene (NHC) ligands, which have intrinsic characteristics such as strong σ-donor but poor π-acceptor abilities, easy preparation, air and thermal stability of their metal complexes, and convenient introduction of chiral elements, have been widely used as promising ligands in metal-catalyzed transformations [1-13]. Numbers of novel chiral NHCs and NHC–metal-catalyzed asymmetric transformations have been developed in a dramatic expansion of this area of chemistry during the past decade; however, up to 2010 only a very few efficient chiral NHCs or NHCs metal catalysts have been described [14-18]. From the typical configuration of NHC metal complex 1 (Figure 1), NHCs are generally more or less cone-shaped with flat heterocyclic structures, and that R1, R2 and M can rotate flexibly around the R1–N, R2–N and C–M bonds, respectively. Such internal rotations cause the active chiral space at the metal center to be relatively ill-defined, which is a key factor for their low enantioselectivity in asymmetric catalysis. As a result, many monodentate NHCs (Figure 1) with sterically hindered R1, R2 groups have been designed, and these have been shown to be good to excellent catalysts in chiral induction reactions [19-25].
Figure 1: Monodentate NHCs with sterically hindered N-substituents.
Figure 1: Monodentate NHCs with sterically hindered N-substituents.
The axially chiral biaryl framework, widely used in the design of chiral ligands such as BINAP [26,27], BINOL [28,29], and boxax [30], has proved to be very rigid, and was introduced in the development of NHC ligands by the Hoveyda group [31-36] and ours [37-44] (Figure 2). Several highly efficient asymmetric catalytic processes with these novel chiral NHCs-bonded metal catalysts have so far been reported. Encouraged by these results, we attempted to develop a new type of mono(NHC) metal complex 2 with a biaryl framework, in which one of biaryl groups bearing a substituent might provide steric hindrance to limit the rotation of the N–Ar bond. Herein we wish to report the synthesis of novel chiral [(NHC)Pd(allyl)I] and mono(NHC)–Au complexes bearing an axially chiral biphenyl framework, and their application in catalysis.
Figure 2: NHCs with axially chiral biaryl frameworks.
Figure 2: NHCs with axially chiral biaryl frameworks.
Results and Discussion
Synthesis of the NHC–Pd(II) and NHC–Au(I) complexes
The synthesis of the chiral benzimidazolium salt (S)-5a is shown in Scheme 1. Thus (S)-6,6'-dimethoxybiphenyl-2,2'-diamine was reacted with acetic anhydride in the presence of acetic acid at room temperature (25 °C) in DCM to afford the corresponding amide (S)-1a in 56% yield. The coupling reaction of (S)-1a with 2-bromonitrobenzene was achieved by the use of bis(2-diphenylphosphinophenyl)ether (DPEphos) as a ligand and Pd2(dba)3 as the catalyst in the presence of Cs2CO3 to give the desired compound (S)-2a in 98% yield. Reduction of (S)-2a by means of Pd-C/H2 for 8 h gave (S)-3a in 98% yield. Subsequent cyclization with triethyl orthoformate catalyzed by p-toluenesulfonic acid at 100 °C for 5 h afforded (S)-4a in 83% yield. Quaternization of the benzimidazole ring of (S)-4a by heating with methyl iodide in acetonitrile provided the corresponding benzimidazolium salt (S)-5a in quantitative yield.
Scheme 1: Synthesis of N-heterocyclic carbene precursor.
Scheme 1: Synthesis of N-heterocyclic carbene precursor.
With the NHC precursor (S)-5a in hand, its coordination with Pd or Au metal salts was examined. Benzimidazolium salt (S)-5a was treated with (η3-C3H5PdCl)2 in tetrahydrofuran (THF) in the presence of t-BuOK at 50 °C to give [(NHC)Pd(allyl)I] complex (S)-7 in 70% yield as a yellow solid after purification by silica gel column chromatography (Scheme 2) [45,46]. Due to the stereochemical orientation of π-allyl group relative to the unsymmetrical carbene ligand, this NHC–Pd complex exists as two stereoisomers in solution, (S)-7a and (S)-7b, which could be easily distinguished in its 1H NMR spectrum recorded at 23 °C [47-50]. The ratio of (S)-7a and (S)-7b was found to be 1.2:1 on the basis of 1H NMR spectroscopic data. It appears that (S)-7a is slightly more stable than (S)-7b presumably due to the steric repulsion between the π-allyl group and the acetylated amino group in another phenyl group. However, we were unable to isolate either stereoisomer in a pure form by silica gel column chromatography. After recrystallization from DCM and pentane (1:3), one of the two stereoisomers, [(NHC)Pd(allyl)I] (S)-7a, was obtained as a crystalline compound and its structure was confirmed by the X-ray single crystal diffraction (Figure 3) [51].
Scheme 2: Synthesis of mono(NHC)–Pd(II) complex.
Scheme 2: Synthesis of mono(NHC)–Pd(II) complex.
![[1860-5397-7-64-3]](/bjoc/content/figures/1860-5397-7-64-3.png?scale=2.0&max-width=1024&background=FFFFFF)
Figure 3: ORTEP drawing of NHC–Pd(II) complex (S)-7a with thermal ellipsoids at the 30% probability level. Selected bond distances (Å) and angles (deg): Pd–C1 = 2.050(5), Pd–C25 = 2.154(7), Pd–C26 = 2.128(9), Pd–C27 = 2.168(8), Pd–I1 = 2.6404(7), N1–C1–Pd = 125.6(4), N2–C1–Pd = 128.8(4), C1–Pd–I1 = 99.52(13), C1–Pd–C25 = 98.3(3), C1–Pd–C26 = 132.3(5), C1–Pd–C27 = 163.7(4), I1–Pd–C25 = 161.9(3), I1–Pd–C26 = 126.5(5), I1–Pd–C27 = 95.9(3), C25–Pd–C27 = 66.0(4), C25–Pd–C26 = 37.5(4), C26–Pd–C27 = 34.8(4).
Figure 3: ORTEP drawing of NHC–Pd(II) complex (S)-7a with thermal ellipsoids at the 30% probability level. Se...
Benzimidazolium salt (S)-5a also complexed Au(I). According to the previously reported procedure [52-55], (S)-5a reacted with AuCl·S(Me) 2 on heating in THF for 8 h in the presence of KI and t-BuOK to give the expected chiral NHC–Au(I) complex (S)-6a in 65% yield (Scheme 3). Its structure was also confirmed by X-ray diffraction (Figure 4) [56]. It was found that the Au–carbene distance is 2.036 Å which is consistent with other reported NHC–Au complexes [57-59].
Scheme 3: Synthesis of mono(NHC)–Au(I) complex.
Scheme 3: Synthesis of mono(NHC)–Au(I) complex.
![[1860-5397-7-64-4]](/bjoc/content/figures/1860-5397-7-64-4.png?scale=2.0&max-width=1024&background=FFFFFF)
Figure 4: ORTEP drawing of NHC–Au(I) complex (S)-6a with thermal ellipsoids at the 30% probability level. Selected bond distances (Å) and angles (deg): Au1–C1 = 2.036(14), Au1–I1 = 2.5093(13), C1–Au1–I1 = 176.5(3), N1–C1–Au1 = 125.7(9), N2–C1–Au = 126.1(9).
Figure 4: ORTEP drawing of NHC–Au(I) complex (S)-6a with thermal ellipsoids at the 30% probability level. Sel...
The NHC–metal complex [(NHC)Pd(allyl)I] complex 7 and NHC–Au(I) complex (S)-6a, are air and moisture stable both in the solid state and in solution, and can be used as catalysts. Therefore, the catalytic activities of these complexes were investigated in Pd-catalyzed coupling reactions and a Au-catalyzed asymmetric reaction, respectively.
Suzuki–Miyaura and Heck–Mizoroki coupling reactions catalyzed by NHC–Pd(II) complex
The Pd-catalyzed coupling reaction is one of the most powerful methods for the formation of carbon–carbon bonds in organic synthesis [60-66]. NHC–Allylpalladium complexes have been employed and showed good catalytic activities in carbon–carbon bond coupling reactions [67-70]. Stereoisomeric complex 7 was firstly applied as the catalyst in the catalyze Suzuki–Miyaura coupling reaction. On the basis of screening of the solvent and base in the reaction of phenylboronic acid with bromobenzene, it was found that using t-BuOK as the base in iPrOH at 50 °C for 10 h, the coupling product 9a was obtained in the highest yield (81% yield) (Supporting Information File 1). Under the optimized conditions, the reactions of various aryl halides with arylboronic acids were carried out, and it was found that the electronic properties of the R groups and halide atoms significantly affected the reaction yield of the Suzuki–Miyaura reactions. The results have been summarized in Table 1.
Table 1: Suzuki–Miyaura reaction catalyzed by NHC–Pd(II) complex 7a.
|
|
|||||
| Entry | R | R' | X | Product | Yield (%)b |
|---|---|---|---|---|---|
| 1 | 4-Me | H | Br | 9a | 81 |
| 2 | 4-COMe | H | Br | 9b | 98 |
| 3 | 2-NO2 | H | Br | 9c | 96 |
| 4 | 2-MeO | H | Br | 9d | 89 |
| 5 | 2-Me | H | Br | 9e | 80 |
| 6 | 4-COMe | 4-Me | Br | 9f | 97 |
| 7 | 4-MeO | 4-Me | Br | 9g | 81 |
| 8 | 4-Me | 2-Cl | Br | 9h | 74 |
| 9 | H | 4-Me | I | 9a | >99 |
| 10 | H | 4-Me | Cl | 9a | <5 |
aReaction conditions: 1 mmol aryl halide, 1.3 mmol arylboronic acid, 1.3 mmol t-BuOK, 0.01 mmol NHC–Pd(II) complex, 2.0 mL IPA.
bIsolated yields.
The [(NHC)Pd(allyl)I] complex 7 was also examined in the Heck–Mizoroki coupling reaction. Under optimized conditions (Supporting Information File 1), the reactions of various aryl halides with n-butyl acrylate were carried out in the presence of Na2CO3 in N,N-dimethylacetamide (DMA) at 140 °C. [(NHC)Pd(allyl)I] complex 7 showed good catalytic activities in the reaction of arylbromides or iodobenzene with n-butyl acrylate to afford the coupling products 10 in up to 97% yield. The results have been summarized in Table 2.
Table 2: Heck–Mizoroki reaction catalyzed by NHC–Pd(II) complex 7a.
|
|
|||||
| Entry | R | X | Time (h) | Product | Yield (%)b |
|---|---|---|---|---|---|
| 1 | H | Br | 18 | 10a | 82 |
| 2 | 4-Me | Br | 18 | 10b | 79 |
| 3 | 4-MeO | Br | 18 | 10c | 80 |
| 4 | 4-CHO | Br | 18 | 10d | 85 |
| 5 | 2-Me | Br | 18 | 10e | 76 |
| 6 | H | I | 18 | 10a | 97 |
| 7 | H | Cl | 18 | 10a | <5 |
aReaction conditions: 7 (1 mol % Pd), Na2CO3 (2.0 mmol), TBAB (0.1 mmol), aryl halide (1.0 mmol) and n-butyl acrylate (1.5 mmol) in DMA (3.0 mL) at 140 °C for 18 h.
bIsolated yield after silica gel column chromatography.
Intramolecular hydroamination reaction catalyzed by NHC–Au(I) complex (S)-6a
Since the first example of NHC–Au complex was reported in 1989 [71], a variety of neutral or cationic NHC–Au complexes has been synthesized and applied in many catalytic reactions [72,73]. For example, recently, NHC–Au showed good catalytic activity in the intramolecular [4 + 2] cycloadditions of 1,3-enynes or arylalkynes [74], rearrangement of allylic acetates [75,76], carbene-transfer reactions from ethyl diazoacetate [77], formation of conjugated enones and enals [78], regio- and stereoselective synthesis of fluoroalkenes [79], and so on [80-85]. However, reports on NHC–Au catalyzed asymmetric reactions are rare [86]. The NHC–Au(I) complex (S)-6a was consequently investigated as the catalyst in the asymmetric intramolecular hydroamination of allenes. This reaction has been achieved with high enantioselectivity by a chiral phosphine–Au(I) complex [87-93]. Treatment of allene 11 with (S)-6a and AgSbF6 (5 mol %) in DCM at room temperature for 36 h afforded pyrrolidine derivative 12 in 53% yield with an ee of only 10%. When THF or toluene was used as solvent, only traces of compound 12 were formed. Further screening of AgX revealed that the combination of (S)-6a and AgClO4 gave the best catalytic activity in this reaction (Table 3).
Table 3: NHC–Au complex (S)-6a catalyzed asymmetric intramolecular hydroamination.
|
|
|||||
| Entry | Solvent | AgX | Time (h) | Yield (%)a | ee (%)b |
|---|---|---|---|---|---|
| 1 | THF | AgSbF6 | 36 | trace | —c |
| 2 | Toluene | AgSbF6 | 36 | trace | — |
| 3 | DCM | AgSbF6 | 36 | 53 | 10 |
| 4 | DCE | AgSbF6 | 36 | 43 | 7 |
| 5 | DCM | AgClO4 | 36 | 63 | 10 |
| 6 | DCM | AgOTf | 36 | 42 | 10 |
| 7 | DCM | AgOTs | 36 | —d | — |
| 8 | DCM | AgBF4 | 36 | 46 | 0 |
aIsolated yield.
bDetermined by chiral HPLC.
cnot determined.
dno reaction.
We assumed that the ill-defined chiral space at the Au center may be the cause of the low ee, and that a more sterically bulky group than an acetyl group in another phenyl framework may be required to improve the enantioselectivity. Accordingly, the original acetyl group was replaced by a more sterically bulky group such as tert-butoxycarbonyl group and adamantanecarbonyl group.
Synthesis of the NHC–Au(I) complexes (S)-6b and 6c
The synthesis of (S)-6b is shown in Scheme 4: Thus (S)-4a was heated under refux with 4 M HCl in EtOH to afford the corresponding amide (S)-8 in 98% yield. Amine (S)-8 was then treated with (Boc)2O in the presence of Et3N at room temperature for 24 h to give the corresponding BOC derivative (S)-4b in 87% yield. Quaternization of the benzimidazole ring of (S)-4b with methyl iodide in acetonitrile gave the corresponding benzimidazolium salt (S)-5b in quantitative yield. Benzimidazolium salt (S)-5b was then complexed with Au (I) as described above for (S)-6a (AuCl·S(Me)2 in the presence of KI and t-BuOK in THF for 8 h) to produce the expected chiral NHC–Au(I) complex (S)-6b in 32% yield.
Scheme 4: Synthesis of mono(NHC)–Au(I) complex (S)-6b.
Scheme 4: Synthesis of mono(NHC)–Au(I) complex (S)-6b.
For the preparation of (S)-6c, (S)-6,6'-dimethoxybiphenyl-2,2'-diamine was treated with adamantane-2-carbonyl chloride in the presence of Et3N at room temperature (25 °C) in DCM to afford the corresponding amide (S)-1c in 71% yield. According to the synthetic method for the synthesis of compound (S)-6a, NHC–Au complex (S)-6c was successfully prepared in 45% yield (Scheme 5).
Scheme 5: Synthesis of mono(NHC)–Au(I) complex (S)-6c.
Scheme 5: Synthesis of mono(NHC)–Au(I) complex (S)-6c.
Under the optimized conditions, (S)-6b and (S)-6c were used as catalysts to examine their chiral induction abilities in the intramolecular hydroamination reaction (Scheme 6). As expected, the corresponding pyrrolidine derivative 12 was obtained in higher ee value but in lower isolated yield: With (S)-6c as catalyst, 12 was obtained in 47% yield with an ee of 44% whereas with (S)-6b as catalyst, 12 was obtained in 55% yield but the ee was only 16%.
Scheme 6: The application of catalysts (S)-6b and 6c in the intramolecular hydroamination reaction.
Scheme 6: The application of catalysts (S)-6b and 6c in the intramolecular hydroamination reaction.
Conclusion
Axially chiral mono(NHC)–Pd(II) and mono(NHC)–Au(I) complexes with one side shaped 1,1'-biphenyl backbone have been prepared from chiral 6,6'-dimethoxybiphenyl-2,2'-diamine. The Pd(II) complex showed good catalytic activity in the Suzuki–Miyaura and Heck–Mizoroki coupling reactions. The (S)-Au(I) complex also showed moderate catalytic activities along with moderate chiral inductions in the asymmetric intramolecular hydroamination reaction. Using chiral Au complex (S)-6c, having a sterically hindered 2-adamantanecarbonyl group, as the catalyst gave the corresponding intramolecular hydroamination product in higher enantioselectivity (44% ee). Efforts are underway to extend the scope and limitations of these chiral (NHC) Pd(II) and Au(I) complexes in other asymmetric catalytic reactions.
Experimental
Synthesis of NHC–Pd(II) complex 7
Compound 5a (105.8 mg, 0.2 mmol) and [PdCl(η3-allyl)]2 (109.1 mg, 0.3 mmol), t-BuOK (56 mg, 0.5 mmol) were heated under reflux in THF (10 mL) for 8 h. The volatiles were then removed under reduced pressure and the residue purified by a silica gel flash column chromatography (eluent: petroleum ether/ethyl acetate, 2:1–0:1) to give 7 as a mixture of two isomers (117.0 mg, 70%). A single crystal grown from racemic complex 7 in a saturated solution of DCM:pentane (1/3) was suitable for X-ray crystal structure analysis. (S)-7, light yellow solid; mp: 124.6–125.3 °C; [α]D20 +13.0 (c 0.25, CHCl3); IR (DCM) ν 3303, 3037, 2933, 2838, 1688, 1688, 1594, 1520, 1464, 1378, 1256, 1125, 1090, 1062, 1004, 976, 939, 733, 560, 530 cm−1; 1H NMR (400 MHz, CDCl3, TMS): δ [2.07 (s, CH3), 2.14 (s, CH3), 1:1.2, 3H], [2.53 (d, J = 12.0 Hz, CH2), 2.71 (d, J = 13.2 Hz, CH2), 1:1.2, 1H], [2.84 (s, OCH3), 2.88 (s, OCH3), 1:1.2, 3H], [3.07 (d, J = 13.2. Hz, CH2), 3.65 (d, J = 6.8 Hz, CH2), 1.2:1, 1H], [3.76 (s, OCH3), 3.79 (s, OCH3), 1:1.2, 3H], [3.81 (s, CH3), 3.92 (s, CH3), 1:1.2, 3H], 4.23 (brs, 1H, CH2), [4.86–4.95 (m, CH), 5.19–5.29 (m, CH), 1:1.2, 1H], [6.35 (d, J = 8.4 Hz, CH2), 6.39 (d, J = 8.0. Hz, CH2), 1:1.2, 1H], 7.09–7.23 (m, 3H, ArH), 7.29–7.36 (m, 4H, ArH), 7.43–7.69 (m, 3H, ArH), [8.10 (s, NH), 8.18 (s, NH), 1:1.2, 1H]; MS (ESI) m/z (%): 675 (M+, 60.07), 402 (M+−273, 100), 274 (M+−401, 28.80); Anal. Calcd. for C27H28IN3O3Pd requires: C, 47.98; H, 4.18; N, 6.22%. Found: C27H28IN3O3Pd, C 47.78, H 4.68, N 5.78%.
Synthesis of NHC–Au(I) complex (S)-6a
Compound (S)-5a (105.8 mg, 0.2 mmol) and AuCl·S(Me)2 (58.8 mg, 0.2 mmol), KI (49.8 mg, 0.3 mmol) t-BuOK (56 mg, 0.5 mmol) were heated under reflux in THF (10 mL) for 8 h. The volatiles were then removed under reduced pressure and the residue purified by a silica gel flash column chromatography (eluent: petroleum ether/ethyl acetate, 2:1–0:1) to give 8 as a white solid (94 mg, 65%). A single crystal grown from racemic complex 6a in a saturated solution of DCM/pentane (1:3) was suitable for X-ray crystal structure analysis. (S)-6a: white solid; mp: 184.3–129.6 °C; [α]D20 +5.0 (c 0.25, CHCl3); IR (DCM) ν 3407, 2929, 2835, 1697, 1591, 1468, 1438, 1286, 1083, 1002, 852, 779, 747, 657 cm−1; 1H NMR (400 MHz, CDCl3, TMS): δ 2.19 (s, CH3, 3H), 3.21 (s, OCH3, 3H), 3.81 (s, OCH3, 3H), 3.97 (s, CH3, 3H), 6.36 (d, J = 8.4 Hz, Ar, 1H), 7.11–7.25 (m, Ar and NH, 5H), 7.32–7.37 (m, Ar, 4H), 7.47 (d, J = 8.0 Hz, Ar, 1H), 7.62 (t, J = 8.0 Hz, Ar, 1H); MS (ESI) m/z (%): 551 (M+, 10.05), 598 (M+−127, 100), 612 (M+−113, 22.10); Anal. Calcd. for C24H23IN3O3Au requires: C, 39.74; H, 3.20; N, 5.79%. Found: C24H23IN3O3Au C 40.64, H 3.08, N 5.72%.
General procedure for the intramolecular hydroamination reaction catalyzed by NHC–Au(I) complex (S)-6a
A mixture of NHC–Au(I) (S)-6a (7.2 mg, 5 mol %) and AgX (5 mol %) in DCM (0.4 mL) was stirred at rt for 5 min. A solution of compound 11 (76.6 mg, 0.20 mmol) in DCM (0.6 mL) was added to the resulting solution and the mixture stirred at rt for 36 h. Column chromatography of the reaction mixture gave the desired product. The enantiomeric purity of the product was determined by chiral HPLC analysis.
Supporting Information
| Supporting Information File 1: Experimental procedures and characterization data of compounds given in this article. | ||
| Format: PDF | Size: 1.3 MB | Download |
| Supporting Information File 2: Crystal structure data for NHC–Pd(II) complex 7a. | ||
| Format: PDF | Size: 2.0 MB | Download |
| Supporting Information File 3: Crystal structure data for NHC–Au(I) complex 6a. | ||
| Format: PDF | Size: 261.9 KB | Download |
| Supporting Information File 4: Crystal structure information file of compound 6a. | ||
| Format: CIF | Size: 21.3 KB | Download |
| Supporting Information File 5: Crystal structure information file of compound 7a. | ||
| Format: CIF | Size: 30.5 KB | Download |
Acknowledgements
Financial support from the Shanghai Municipal Committee of Science and Technology (08dj1400100-2), the Fundamental Research Funds for the Central Universities, National Basic Research Program of China (973)-2010CB833302, the Fundamental Research Funds for the Central Universities and the National Natural Science Foundation of China (21072206, 20902019, 20472096, 20872162, 20672127, 20732008, 20821002, and 20702013) are gratefully acknowledged.
References
-
Rogers, M. M.; Stahl, S. S. N-Heterocyclic Carbenes as Ligands for High-Oxidation-State Metal Complexes and Oxidation Catalysis. In N-Heterocyclic Carbenes in Transition Metal Catalysis; Glorius, F., Ed.; Topics in Organometallic Chemistry, Vol. 21; Springer: Berlin Heidelberg, 2007. doi:10.1007/3418_025
Return to citation in text: [1] -
Glorius, F., Ed. N-Heterocyclic Carbenes as Ligands for High-Oxidation-State Metal Complexes and Oxidation Catalysis; Topics in Organometallic Chemistry, Vol. 21; Springer: Berlin Heidelberg, 2007. doi:10.1007/11603795
Return to citation in text: [1] -
Arduengo, A. J.; Bertrand, G. Chem. Rev. 2009, 109, 3209–3210. doi:10.1021/cr900241h
Return to citation in text: [1] -
Schuster, O.; Yang, L.; Raubenheimer, H. G.; Albrecht, M. Chem. Rev. 2009, 109, 3445–3478. doi:10.1021/cr8005087
Return to citation in text: [1] -
Lin, J. C. Y.; Huang, R. T. W.; Lee, C. S.; Bhattacharyya, A.; Hwang, W. S.; Lin, I. J. B. Chem. Rev. 2009, 109, 3561–3598. doi:10.1021/cr8005153
Return to citation in text: [1] -
Arnold, P. L.; Casely, I. J. Chem. Rev. 2009, 109, 3599–3611. doi:10.1021/cr8005203
Return to citation in text: [1] -
Poyatos, M.; Mata, J. A.; Peris, E. Chem. Rev. 2009, 109, 3677–3707. doi:10.1021/cr800501s
Return to citation in text: [1] -
Corberán, R.; Mas-Marzá, E.; Peris, E. Eur. J. Inorg. Chem. 2009, 1700–1716. doi:10.1002/ejic.200801095
Return to citation in text: [1] -
Hahn, F. E.; Jahnke, M. C. Angew. Chem. 2008, 120, 3166–3216. doi:10.1002/ange.200703883
Angew. Chem., Int. Ed. 2008, 47, 3122–3172. doi:10.1002/anie.200703883
Return to citation in text: [1] -
Bourissou, D.; Guerret, O.; Gabba, F. P.; Bertrand, G. Chem. Rev. 2000, 100, 39–92. doi:10.1021/cr940472u
Return to citation in text: [1] -
Pugh, D.; Danopoulos, A. A. Coord. Chem. Rev. 2007, 251, 610–641. doi:10.1016/j.ccr.2006.08.001
Return to citation in text: [1] -
Braband, H.; Kückmann, T. I.; Abram, U. J. Organomet. Chem. 2005, 690, 5421–5429. doi:10.1016/j.jorganchem.2005.07.014
Return to citation in text: [1] -
Herrmann, W. A. Angew. Chem. 2002, 114, 1342–1363. doi:10.1002/1521-3757(20020415)114:8<1342::AID-ANGE1342>3.0.CO;2-A
Angew. Chem., Int. Ed. 2002, 41, 1290–1309. doi:10.1002/1521-3773(20020415)41:8<1290::AID-ANIE1290>3.0.CO;2-Y
Return to citation in text: [1] -
Powell, M. T.; Hou, D.-R.; Perry, M. C.; Cui, X.; Burgess, K. J. Am. Chem. Soc. 2001, 123, 8878–8879. doi:10.1021/ja016011p
Return to citation in text: [1] -
Seiders, T. J.; Ward, D. W.; Grubbs, R. H. Org. Lett. 2001, 3, 3225–3228. doi:10.1021/ol0165692
Return to citation in text: [1] -
He, M.; Bode, J. W. J. Am. Chem. Soc. 2008, 130, 418–419. doi:10.1021/ja0778592
Return to citation in text: [1] -
Lee, Y.; Hoveyda, A. H. J. Am. Chem. Soc. 2009, 131, 3160–3161. doi:10.1021/ja809382c
Return to citation in text: [1] -
Hauwert, P.; Boerleider, R.; Warsink, S.; Weigand, J. J.; Elsevier, C. J. J. Am. Chem. Soc. 2010, 132, 16900–16910. doi:10.1021/ja1062407
Return to citation in text: [1] -
Song, C.; Ma, C.; Ma, Y.; Feng, W.; Ma, S.; Chai, Q.; Andrus, M. B. Tetrahedron Lett. 2005, 46, 3241–3244. doi:10.1016/j.tetlet.2005.03.026
Return to citation in text: [1] -
Kündig, E. P.; Seidel, T. M.; Jia, Y.-X.; Bernardinelli, G. Angew. Chem. 2007, 119, 8636–8639. doi:10.1002/ange.200703408
Angew. Chem., Int. Ed. 2007, 46, 8484–8487.doi:10.1002/anie.200703408
Return to citation in text: [1] -
Jia, Y.-X.; Hillgren, J. M.; Watson, E. L.; Marsden, S. P.; Kündig, E. P. Chem. Commun. 2008, 4040–4042. doi:10.1039/b810858g
Return to citation in text: [1] -
Marsden, S. P.; Watson, E. L.; Raw, S. A. Org. Lett. 2008, 10, 2905–2908. doi:10.1021/ol801028e
Return to citation in text: [1] -
Hillgren, J. M.; Marsden, S. P. J. Org. Chem. 2008, 73, 6459–6461. doi:10.1021/jo8010842
Return to citation in text: [1] -
Lee, S.; Hartwig, J. F. J. Org. Chem. 2001, 66, 3402–3415. doi:10.1021/jo005761z
Return to citation in text: [1] -
Würtz, S.; Lohre, C.; Fröhlich, R.; Bergander, K.; Glorius, F. J. Am. Chem. Soc. 2009, 131, 8344–8345. doi:10.1021/ja901018g
Return to citation in text: [1] -
Miyashita, A.; Yasuda, A.; Takaya, H.; Toriumi, K.; Ito, T.; Souchi, T.; Noyori, R. J. Am. Chem. Soc. 1980, 102, 7932–7934. doi:10.1021/ja00547a020
Return to citation in text: [1] -
Noyori, R. Angew. Chem., Int. Ed. 2002, 41, 2008–2022. doi:10.1002/1521-3773(20020617)41:12<2008::AID-ANIE2008>3.0.CO;2-4
Return to citation in text: [1] -
Chen, Y.; Yekta, S.; Yudin, A. K. Chem. Rev. 2003, 103, 3155–3212. doi:10.1021/cr020025b
Return to citation in text: [1] -
Brunel, J. M. Chem. Rev. 2005, 105, 857–898. doi:10.1021/cr040079g
Return to citation in text: [1] -
Uozumi, Y.; Kyota, H.; Kishi, E.; Kitayama, K.; Hayashi, T. Tetrahedron: Asymmetry 1996, 7, 1603–1606. doi:10.1016/0957-4166(96)00193-0
Return to citation in text: [1] -
Hoveyda, A. H. Catalytic Asymmetric Olefin Metathesis. In Handbook of Metathesis; Grubbs, R. H., Ed.; Wiley-VCH: Weinheim, Germany, 2003.
Return to citation in text: [1] -
Schrock, R. R.; Hoveyda, A. H. Angew. Chem. 2003, 115, 4740–4782. doi:10.1002/ange.200300576
Angew. Chem., Int. Ed. 2003, 42, 4592–4633. doi:10.1002/anie.200300576
Return to citation in text: [1] -
Hoveyda, A. H.; Schrock, R. R. Chem.–Eur. J. 2001, 7, 945–950. doi:10.1002/1521-3765(20010302)7:5<945::AID-CHEM945>3.0.CO;2-3
Return to citation in text: [1] -
Luchaco-Cullis, C. A.; Mizutani, H.; Murphy, K. E.; Hoveyda, A. H. Angew. Chem. 2001, 113, 1504–1508. doi:10.1002/1521-3757(20010417)113:8<1504::AID-ANGE1504>3.0.CO;2-V
Angew. Chem., Int. Ed. 2001, 40, 1456–1460.doi:10.1002/1521-3773(20010417)40:8<1456::AID-ANIE1456>3.0.CO;2-T
Return to citation in text: [1] -
Murphy, K. E.; Hoveyda, A. H. J. Am. Chem. Soc. 2003, 125, 4690–4691. doi:10.1021/ja0300618
Return to citation in text: [1] -
Kacprzynski, M. A.; Hoveyda, A. H. J. Am. Chem. Soc. 2004, 126, 10676–10681. doi:10.1021/ja0478779
Return to citation in text: [1] -
Duan, W.-L.; Shi, M.; Rong, G.-B. Chem. Commun. 2003, 2916–2917. doi:10.1039/b309185f
Return to citation in text: [1] -
Chen, T.; Jiang, J.-J.; Xu, Q.; Shi, M. Org. Lett. 2007, 9, 865–868. doi:10.1021/ol063061w
Return to citation in text: [1] -
Xu, Q.; Gu, X.; Liu, S.; Dou, Q.; Shi, M. J. Org. Chem. 2007, 72, 2240–2242. doi:10.1021/jo062453d
Return to citation in text: [1] -
Zhang, T.; Shi, M. Chem.–Eur. J. 2008, 14, 3759–3764. doi:10.1002/chem.200701982
Return to citation in text: [1] -
Ma, G.-N.; Zhang, T.; Shi, M. Org. Lett. 2009, 11, 875–878. doi:10.1021/ol802861s
Return to citation in text: [1] -
Liu, L.-J.; Wang, F.; Shi, M. Eur. J. Inorg. Chem. 2009, 1723–1728. doi:10.1002/ejic.200801207
Return to citation in text: [1] -
Liu, L.-J.; Wang, F.; Shi, M. Organometallics 2009, 28, 4416–4420. doi:10.1021/om900320c
Return to citation in text: [1] -
Liu, Z.; Shi, M. Organometallics 2010, 29, 2831–2834. doi:10.1021/om100331z
Return to citation in text: [1] -
Viciu, M. S.; Navarro, O.; Germaneau, R. F.; Kelly, R. A., III; Sommer, W.; Marion, N.; Stevens, E. D.; Cavallo, L.; Nolan, S. P. Organometallics 2004, 23, 1629–1635. doi:10.1021/om034319e
Return to citation in text: [1] -
Viciu, M. S.; Germaneau, R. F.; Navarro-Fernandez, O.; Stevens, E. D.; Nolan, S. P. Organometallics 2002, 21, 5470–5472. doi:10.1021/om020804i
Return to citation in text: [1] -
Roland, S.; Cotet, W.; Mangeney, P. Eur. J. Inorg. Chem. 2009, 1796–1805. doi:10.1002/ejic.200801157
Return to citation in text: [1] -
Wang, C.-Y.; Liu, Y.-H.; Peng, S.-M.; Chen, J.-T.; Liu, S.-T. J. Organomet. Chem. 2007, 692, 3976–3983. doi:10.1016/j.jorganchem.2007.06.007
Return to citation in text: [1] -
Peng, H. M.; Song, G. Y.; Li, Y. X.; Li, X. W. Inorg. Chem. 2008, 47, 8031–8043. doi:10.1021/ic800361v
Return to citation in text: [1] -
Ketz, B. E.; Cole, A. P.; Waymouth, R. M. Organometallics 2004, 23, 2835–2837. doi:10.1021/om049838b
Return to citation in text: [1] -
The crystal data of 7 have been deposited at the CCDC with number 754776. Empirical Formula: C27H28IN3O3Pd; Formula Weight: 675.82; Crystal Color, Habit: colorless, prismatic; Crystal Dimensions: 0.309 x 0.250 x 0.191 mm; Crystal System: Monoclinic; Lattice Type: Primitive; Lattice Parameters: a = 9.8872(8)Å, b = 16.8643(14)Å, c = 16.8744(15)Å, α = 90°, β = 100.713(2)°, γ = 90°, V = 2764.6(4)Å3; Space group: P2(1)/c; Z = 4; Dcalc= 1.624 g/cm3; F000 = 1336; Diffractometer: Bruker Smart CCD.
Return to citation in text: [1] -
Baker, M. V.; Barnard, P. J.; Berners-Price, S. J.; Brayshaw, S. K.; Hickey, J. L.; Skelton, B. W.; White, A. H. J. Organomet. Chem. 2005, 690, 5625–5635. doi:10.1016/j.jorganchem.2005.07.013
Return to citation in text: [1] -
Lemke, J.; Pinto, A.; Niehoff, P.; Vasylyeva, V.; Metzler-Nolte, N. Dalton Trans. 2009, 7063–7070. doi:10.1039/b906140a
Return to citation in text: [1] -
de Frémont, P.; Scott, N. M.; Stevens, E. D.; Nolan, S. P. Organometallics 2005, 24, 2411–2418. doi:10.1021/om050111c
Return to citation in text: [1] -
Ray, L.; Shaikh, M. M.; Ghosh, P. Organometallics 2007, 26, 958–964. doi:10.1021/om060834b
Return to citation in text: [1] -
The crystal data of 8 have been deposited at the CCDC with number 782844. Empirical formula: C24H23AuIN3O3; formula weight: 725.32; crystal size: 0.277 x 0.268 x 0.224 mm; crystal color, habit: colorless, prismatic; crystal system: monoclinic; lattice type: primitive; lattice parameters: a = 9.2786(7) Å, b = 25.5371 (19) Å, c = 10.1633 (14) Å, α = 90o, β = 104.9480(10)°, γ = 90o, V = 2326.7(3) A3; Space group: P-1; Z = 4; Dcalc= 2.071 g/cm3; F000 = 1376; R1 = 0.0478, wR2 = 0.1273. Diffractometer: Bruker Smart CCD.
Return to citation in text: [1] -
Ray, L.; Katiyar, V.; Barman, S.; Raihan, M. J.; Nanavati, H.; Shaikh, M. M.; Ghosh, P. J. Organomet. Chem. 2007, 692, 4259–4269. doi:10.1016/j.jorganchem.2007.06.033
Return to citation in text: [1] -
Jothibasu, R.; Huynh, H. V.; Koh, L. L. J. Organomet. Chem. 2008, 693, 374–380. doi:10.1016/j.jorganchem.2007.11.003
Return to citation in text: [1] -
Zhang, X.; Gu, S.; Xia, Q.; Chen, W. J. Organomet. Chem. 2009, 694, 2359–2367. doi:10.1016/j.jorganchem.2009.03.031
Return to citation in text: [1] -
Eisnor, C. R.; Gossage, R. A.; Yadav, P. N. Tetrahedron 2006, 62, 3395–3401. doi:10.1016/j.tet.2006.01.046
Return to citation in text: [1] -
Taylor, S. R.; Ung, A. T.; Pyne, S. G. Tetrahedron 2007, 63, 10889–10895. doi:10.1016/j.tet.2007.08.080
Return to citation in text: [1] -
Yip, K.-T.; Yang, M.; Law, K.-L.; Zhu, N.-Y.; Yang, D. J. Am. Chem. Soc. 2006, 128, 3130–3131. doi:10.1021/ja060291x
Return to citation in text: [1] -
Cropper, E. L.; Yuen, A.-P.; Ford, A.; White, A. J. P.; Hii, K. K. Tetrahedron 2009, 65, 525–530. doi:10.1016/j.tet.2008.10.098
Return to citation in text: [1] -
Pratap, R.; Parrish, D.; Gunda, P.; Venkataraman, D.; Lakshman, M. K. J. Am. Chem. Soc. 2009, 131, 12240–12249. doi:10.1021/ja902679b
Return to citation in text: [1] -
Marion, N.; Ecarnot, E. C.; Navarro, O.; Amoroso, D.; Bell, A.; Nolan, S. P. J. Org. Chem. 2006, 71, 3816–3821. doi:10.1021/jo060190h
Return to citation in text: [1] -
Chai, D. I.; Lautens, M. J. Org. Chem. 2009, 74, 3054–3061. doi:10.1021/jo900053b
Return to citation in text: [1] -
Marion, N.; Navarro, O.; Mei, J.; Stevens, E. D.; Scott, N. M.; Nolan, S. P. J. Am. Chem. Soc. 2006, 128, 4101–4111. doi:10.1021/ja057704z
Return to citation in text: [1] -
Viciu, M. S.; Germaneau, R. F.; Nolan, S. P. Org. Lett. 2002, 4, 4053–4056. doi:10.1021/ol026745m
Return to citation in text: [1] -
Winkelmann, O. H.; Riekstins, A.; Nolan, S. P.; Navarro, O. Organometallics 2009, 28, 5809–5813. doi:10.1021/om900677v
Return to citation in text: [1] -
Navarro, O.; Kaur, H.; Mahjoor, P.; Nolan, S. P. J. Org. Chem. 2004, 69, 3173–3180. doi:10.1021/jo035834p
Return to citation in text: [1] -
Bonati, F.; Burini, A.; Pietroni, B. R.; Bovio, B. J. J. Organomet. Chem. 1989, 375, 147–160. doi:10.1016/0022-328X(89)85094-6
Return to citation in text: [1] -
Marion, N.; Nolan, S. P. Chem. Soc. Rev. 2008, 37, 1776–1782. doi:10.1039/b711132k
Return to citation in text: [1] -
Gorin, D. J.; Sherry, B. D.; Toste, F. D. Chem. Rev. 2008, 108, 3351–3378. doi:10.1021/cr068430g
Return to citation in text: [1] -
Nieto-Oberhuber, C.; López, S.; Echavarren, A. M. J. Am. Chem. Soc. 2005, 127, 6178–6179. doi:10.1021/ja042257t
Return to citation in text: [1] -
Gourlaouen, C.; Marion, N.; Nolan, S. P.; Maseras, F. Org. Lett. 2009, 11, 81–84. doi:10.1021/ol802430m
Return to citation in text: [1] -
Marion, N.; Gealageas, R.; Nolan, S. P. Org. Lett. 2007, 9, 2653–2656. doi:10.1021/ol070843w
Return to citation in text: [1] -
Fructos, M. R.; Belderrain, T. R.; de Frémont, P.; Scott, N. M.; Nolan, S. P.; Díaz-Requejo, M. M.; Pérez, P. J. Angew. Chem. 2005, 117, 5418–5422. doi:10.1002/ange.200501056
Angew. Chem., Int. Ed. 2005, 44, 5284–5288.doi:10.1002/anie.200501056
Return to citation in text: [1] -
Marion, N.; Carlqvist, P.; Gealageas, R.; de Frémont, P.; Maseras, F.; Nolan, S. P. Chem.–Eur. J. 2007, 13, 6437–6451. doi:10.1002/chem.200700134
Return to citation in text: [1] -
Gorske, B. C.; Mbofana, C. T.; Miller, S. J. Org. Lett. 2009, 11, 4318–4321. doi:10.1021/ol9016782
Return to citation in text: [1] -
Lavallo, V.; Frey, G. D.; Donnadieu, B.; Soleilhavoup, M.; Bertrand, G. Angew. Chem. 2008, 120, 5302–5306. doi:10.1002/ange.200801136
Angew. Chem., Int. Ed. 2008, 47, 5224–5228.doi:10.1002/anie.200801136
Return to citation in text: [1] -
Zeng, X.; Frey, G. D.; Kinjo, R.; Donnadieu, B.; Bertrand, G. J. Am. Chem. Soc. 2009, 131, 8690–8696. doi:10.1021/ja902051m
Return to citation in text: [1] -
van der Vlugt, J. I. Chem. Soc. Rev. 2010, 39, 2302–2322. doi:10.1039/b925794m
Return to citation in text: [1] -
Zeng, X.; Kinjo, R.; Kinjo, R.; Donnadieu, B.; Bertrand, G. Angew. Chem. 2010, 122, 954–957. doi:10.1002/ange.200905341
Angew. Chem., Int. Ed. 2010, 49, 942–945.doi:10.1002/anie.200905341
Return to citation in text: [1] -
Klinkenberg, J. L.; Hartwig, J. F. Angew. Chem. 2011, 123, 88–98. doi:10.1002/ange.201002354
Angew. Chem., Int. Ed. 2011, 50, 86–95. doi:10.1002/anie.201002354
Return to citation in text: [1] -
Lavallo, V.; Frey, G. D.; Kousar, S.; Donnadieu, B.; Bertrand, G. Proc. Natl. Acad. Sci. U. S. A. 2007, 104, 13569–13573. doi:10.1073/pnas.0705809104
Return to citation in text: [1] -
Matsumoto, Y.; Selim, K. B.; Nakanishi, H.; Yamada, K.; Yamamoto, Y.; Tomioka, K. Tetrahedron Lett. 2010, 51, 404–406. doi:10.1016/j.tetlet.2009.11.039
Return to citation in text: [1] -
LaLonde, R. L.; Sherry, B. D.; Kang, E. J.; Toste, F. D. J. Am. Chem. Soc. 2007, 129, 2452–2453. doi:10.1021/ja068819l
Return to citation in text: [1] -
Johansson, M. J.; Gorin, D. J.; Staben, S. T.; Toste, F. D. J. Am. Chem. Soc. 2005, 127, 18002–18003. doi:10.1021/ja0552500
Return to citation in text: [1] -
Zhang, Z.; Liu, C.; Kinder, R. E.; Han, X.; Qian, H.; Widenhoefer, R. A. J. Am. Chem. Soc. 2006, 128, 9066–9073. doi:10.1021/ja062045r
Return to citation in text: [1] -
Zhang, Z.; Bender, C. F.; Widenhoefer, R. A. Org. Lett. 2007, 9, 2887–2889. doi:10.1021/ol071108n
Return to citation in text: [1] -
Zhang, Z.; Bender, C. F.; Widenhoefer, R. A. Org. Lett. 2007, 9, 2887–2889. doi:10.1021/ol071108n
Return to citation in text: [1] -
Li, H.; Widenhoefer, R. A. Org. Lett. 2009, 11, 2671–2674. doi:10.1021/ol900730w
Return to citation in text: [1] -
Zhang, Z.; Bender, C. F.; Widenhoefer, R. A. J. Am. Chem. Soc. 2007, 129, 14148–14149. doi:10.1021/ja0760731
Return to citation in text: [1]
| 1. | Rogers, M. M.; Stahl, S. S. N-Heterocyclic Carbenes as Ligands for High-Oxidation-State Metal Complexes and Oxidation Catalysis. In N-Heterocyclic Carbenes in Transition Metal Catalysis; Glorius, F., Ed.; Topics in Organometallic Chemistry, Vol. 21; Springer: Berlin Heidelberg, 2007. doi:10.1007/3418_025 |
| 2. | Glorius, F., Ed. N-Heterocyclic Carbenes as Ligands for High-Oxidation-State Metal Complexes and Oxidation Catalysis; Topics in Organometallic Chemistry, Vol. 21; Springer: Berlin Heidelberg, 2007. doi:10.1007/11603795 |
| 3. | Arduengo, A. J.; Bertrand, G. Chem. Rev. 2009, 109, 3209–3210. doi:10.1021/cr900241h |
| 4. | Schuster, O.; Yang, L.; Raubenheimer, H. G.; Albrecht, M. Chem. Rev. 2009, 109, 3445–3478. doi:10.1021/cr8005087 |
| 5. | Lin, J. C. Y.; Huang, R. T. W.; Lee, C. S.; Bhattacharyya, A.; Hwang, W. S.; Lin, I. J. B. Chem. Rev. 2009, 109, 3561–3598. doi:10.1021/cr8005153 |
| 6. | Arnold, P. L.; Casely, I. J. Chem. Rev. 2009, 109, 3599–3611. doi:10.1021/cr8005203 |
| 7. | Poyatos, M.; Mata, J. A.; Peris, E. Chem. Rev. 2009, 109, 3677–3707. doi:10.1021/cr800501s |
| 8. | Corberán, R.; Mas-Marzá, E.; Peris, E. Eur. J. Inorg. Chem. 2009, 1700–1716. doi:10.1002/ejic.200801095 |
| 9. |
Hahn, F. E.; Jahnke, M. C. Angew. Chem. 2008, 120, 3166–3216. doi:10.1002/ange.200703883
Angew. Chem., Int. Ed. 2008, 47, 3122–3172. doi:10.1002/anie.200703883 |
| 10. | Bourissou, D.; Guerret, O.; Gabba, F. P.; Bertrand, G. Chem. Rev. 2000, 100, 39–92. doi:10.1021/cr940472u |
| 11. | Pugh, D.; Danopoulos, A. A. Coord. Chem. Rev. 2007, 251, 610–641. doi:10.1016/j.ccr.2006.08.001 |
| 12. | Braband, H.; Kückmann, T. I.; Abram, U. J. Organomet. Chem. 2005, 690, 5421–5429. doi:10.1016/j.jorganchem.2005.07.014 |
| 13. |
Herrmann, W. A. Angew. Chem. 2002, 114, 1342–1363. doi:10.1002/1521-3757(20020415)114:8<1342::AID-ANGE1342>3.0.CO;2-A
Angew. Chem., Int. Ed. 2002, 41, 1290–1309. doi:10.1002/1521-3773(20020415)41:8<1290::AID-ANIE1290>3.0.CO;2-Y |
| 28. | Chen, Y.; Yekta, S.; Yudin, A. K. Chem. Rev. 2003, 103, 3155–3212. doi:10.1021/cr020025b |
| 29. | Brunel, J. M. Chem. Rev. 2005, 105, 857–898. doi:10.1021/cr040079g |
| 60. | Eisnor, C. R.; Gossage, R. A.; Yadav, P. N. Tetrahedron 2006, 62, 3395–3401. doi:10.1016/j.tet.2006.01.046 |
| 61. | Taylor, S. R.; Ung, A. T.; Pyne, S. G. Tetrahedron 2007, 63, 10889–10895. doi:10.1016/j.tet.2007.08.080 |
| 62. | Yip, K.-T.; Yang, M.; Law, K.-L.; Zhu, N.-Y.; Yang, D. J. Am. Chem. Soc. 2006, 128, 3130–3131. doi:10.1021/ja060291x |
| 63. | Cropper, E. L.; Yuen, A.-P.; Ford, A.; White, A. J. P.; Hii, K. K. Tetrahedron 2009, 65, 525–530. doi:10.1016/j.tet.2008.10.098 |
| 64. | Pratap, R.; Parrish, D.; Gunda, P.; Venkataraman, D.; Lakshman, M. K. J. Am. Chem. Soc. 2009, 131, 12240–12249. doi:10.1021/ja902679b |
| 65. | Marion, N.; Ecarnot, E. C.; Navarro, O.; Amoroso, D.; Bell, A.; Nolan, S. P. J. Org. Chem. 2006, 71, 3816–3821. doi:10.1021/jo060190h |
| 66. | Chai, D. I.; Lautens, M. J. Org. Chem. 2009, 74, 3054–3061. doi:10.1021/jo900053b |
| 26. | Miyashita, A.; Yasuda, A.; Takaya, H.; Toriumi, K.; Ito, T.; Souchi, T.; Noyori, R. J. Am. Chem. Soc. 1980, 102, 7932–7934. doi:10.1021/ja00547a020 |
| 27. | Noyori, R. Angew. Chem., Int. Ed. 2002, 41, 2008–2022. doi:10.1002/1521-3773(20020617)41:12<2008::AID-ANIE2008>3.0.CO;2-4 |
| 67. | Marion, N.; Navarro, O.; Mei, J.; Stevens, E. D.; Scott, N. M.; Nolan, S. P. J. Am. Chem. Soc. 2006, 128, 4101–4111. doi:10.1021/ja057704z |
| 68. | Viciu, M. S.; Germaneau, R. F.; Nolan, S. P. Org. Lett. 2002, 4, 4053–4056. doi:10.1021/ol026745m |
| 69. | Winkelmann, O. H.; Riekstins, A.; Nolan, S. P.; Navarro, O. Organometallics 2009, 28, 5809–5813. doi:10.1021/om900677v |
| 70. | Navarro, O.; Kaur, H.; Mahjoor, P.; Nolan, S. P. J. Org. Chem. 2004, 69, 3173–3180. doi:10.1021/jo035834p |
| 19. | Song, C.; Ma, C.; Ma, Y.; Feng, W.; Ma, S.; Chai, Q.; Andrus, M. B. Tetrahedron Lett. 2005, 46, 3241–3244. doi:10.1016/j.tetlet.2005.03.026 |
| 20. |
Kündig, E. P.; Seidel, T. M.; Jia, Y.-X.; Bernardinelli, G. Angew. Chem. 2007, 119, 8636–8639. doi:10.1002/ange.200703408
Angew. Chem., Int. Ed. 2007, 46, 8484–8487.doi:10.1002/anie.200703408 |
| 21. | Jia, Y.-X.; Hillgren, J. M.; Watson, E. L.; Marsden, S. P.; Kündig, E. P. Chem. Commun. 2008, 4040–4042. doi:10.1039/b810858g |
| 22. | Marsden, S. P.; Watson, E. L.; Raw, S. A. Org. Lett. 2008, 10, 2905–2908. doi:10.1021/ol801028e |
| 23. | Hillgren, J. M.; Marsden, S. P. J. Org. Chem. 2008, 73, 6459–6461. doi:10.1021/jo8010842 |
| 24. | Lee, S.; Hartwig, J. F. J. Org. Chem. 2001, 66, 3402–3415. doi:10.1021/jo005761z |
| 25. | Würtz, S.; Lohre, C.; Fröhlich, R.; Bergander, K.; Glorius, F. J. Am. Chem. Soc. 2009, 131, 8344–8345. doi:10.1021/ja901018g |
| 56. | The crystal data of 8 have been deposited at the CCDC with number 782844. Empirical formula: C24H23AuIN3O3; formula weight: 725.32; crystal size: 0.277 x 0.268 x 0.224 mm; crystal color, habit: colorless, prismatic; crystal system: monoclinic; lattice type: primitive; lattice parameters: a = 9.2786(7) Å, b = 25.5371 (19) Å, c = 10.1633 (14) Å, α = 90o, β = 104.9480(10)°, γ = 90o, V = 2326.7(3) A3; Space group: P-1; Z = 4; Dcalc= 2.071 g/cm3; F000 = 1376; R1 = 0.0478, wR2 = 0.1273. Diffractometer: Bruker Smart CCD. |
| 14. | Powell, M. T.; Hou, D.-R.; Perry, M. C.; Cui, X.; Burgess, K. J. Am. Chem. Soc. 2001, 123, 8878–8879. doi:10.1021/ja016011p |
| 15. | Seiders, T. J.; Ward, D. W.; Grubbs, R. H. Org. Lett. 2001, 3, 3225–3228. doi:10.1021/ol0165692 |
| 16. | He, M.; Bode, J. W. J. Am. Chem. Soc. 2008, 130, 418–419. doi:10.1021/ja0778592 |
| 17. | Lee, Y.; Hoveyda, A. H. J. Am. Chem. Soc. 2009, 131, 3160–3161. doi:10.1021/ja809382c |
| 18. | Hauwert, P.; Boerleider, R.; Warsink, S.; Weigand, J. J.; Elsevier, C. J. J. Am. Chem. Soc. 2010, 132, 16900–16910. doi:10.1021/ja1062407 |
| 57. | Ray, L.; Katiyar, V.; Barman, S.; Raihan, M. J.; Nanavati, H.; Shaikh, M. M.; Ghosh, P. J. Organomet. Chem. 2007, 692, 4259–4269. doi:10.1016/j.jorganchem.2007.06.033 |
| 58. | Jothibasu, R.; Huynh, H. V.; Koh, L. L. J. Organomet. Chem. 2008, 693, 374–380. doi:10.1016/j.jorganchem.2007.11.003 |
| 59. | Zhang, X.; Gu, S.; Xia, Q.; Chen, W. J. Organomet. Chem. 2009, 694, 2359–2367. doi:10.1016/j.jorganchem.2009.03.031 |
| 45. | Viciu, M. S.; Navarro, O.; Germaneau, R. F.; Kelly, R. A., III; Sommer, W.; Marion, N.; Stevens, E. D.; Cavallo, L.; Nolan, S. P. Organometallics 2004, 23, 1629–1635. doi:10.1021/om034319e |
| 46. | Viciu, M. S.; Germaneau, R. F.; Navarro-Fernandez, O.; Stevens, E. D.; Nolan, S. P. Organometallics 2002, 21, 5470–5472. doi:10.1021/om020804i |
| 51. | The crystal data of 7 have been deposited at the CCDC with number 754776. Empirical Formula: C27H28IN3O3Pd; Formula Weight: 675.82; Crystal Color, Habit: colorless, prismatic; Crystal Dimensions: 0.309 x 0.250 x 0.191 mm; Crystal System: Monoclinic; Lattice Type: Primitive; Lattice Parameters: a = 9.8872(8)Å, b = 16.8643(14)Å, c = 16.8744(15)Å, α = 90°, β = 100.713(2)°, γ = 90°, V = 2764.6(4)Å3; Space group: P2(1)/c; Z = 4; Dcalc= 1.624 g/cm3; F000 = 1336; Diffractometer: Bruker Smart CCD. |
| 37. | Duan, W.-L.; Shi, M.; Rong, G.-B. Chem. Commun. 2003, 2916–2917. doi:10.1039/b309185f |
| 38. | Chen, T.; Jiang, J.-J.; Xu, Q.; Shi, M. Org. Lett. 2007, 9, 865–868. doi:10.1021/ol063061w |
| 39. | Xu, Q.; Gu, X.; Liu, S.; Dou, Q.; Shi, M. J. Org. Chem. 2007, 72, 2240–2242. doi:10.1021/jo062453d |
| 40. | Zhang, T.; Shi, M. Chem.–Eur. J. 2008, 14, 3759–3764. doi:10.1002/chem.200701982 |
| 41. | Ma, G.-N.; Zhang, T.; Shi, M. Org. Lett. 2009, 11, 875–878. doi:10.1021/ol802861s |
| 42. | Liu, L.-J.; Wang, F.; Shi, M. Eur. J. Inorg. Chem. 2009, 1723–1728. doi:10.1002/ejic.200801207 |
| 43. | Liu, L.-J.; Wang, F.; Shi, M. Organometallics 2009, 28, 4416–4420. doi:10.1021/om900320c |
| 44. | Liu, Z.; Shi, M. Organometallics 2010, 29, 2831–2834. doi:10.1021/om100331z |
| 52. | Baker, M. V.; Barnard, P. J.; Berners-Price, S. J.; Brayshaw, S. K.; Hickey, J. L.; Skelton, B. W.; White, A. H. J. Organomet. Chem. 2005, 690, 5625–5635. doi:10.1016/j.jorganchem.2005.07.013 |
| 53. | Lemke, J.; Pinto, A.; Niehoff, P.; Vasylyeva, V.; Metzler-Nolte, N. Dalton Trans. 2009, 7063–7070. doi:10.1039/b906140a |
| 54. | de Frémont, P.; Scott, N. M.; Stevens, E. D.; Nolan, S. P. Organometallics 2005, 24, 2411–2418. doi:10.1021/om050111c |
| 55. | Ray, L.; Shaikh, M. M.; Ghosh, P. Organometallics 2007, 26, 958–964. doi:10.1021/om060834b |
| 31. | Hoveyda, A. H. Catalytic Asymmetric Olefin Metathesis. In Handbook of Metathesis; Grubbs, R. H., Ed.; Wiley-VCH: Weinheim, Germany, 2003. |
| 32. |
Schrock, R. R.; Hoveyda, A. H. Angew. Chem. 2003, 115, 4740–4782. doi:10.1002/ange.200300576
Angew. Chem., Int. Ed. 2003, 42, 4592–4633. doi:10.1002/anie.200300576 |
| 33. | Hoveyda, A. H.; Schrock, R. R. Chem.–Eur. J. 2001, 7, 945–950. doi:10.1002/1521-3765(20010302)7:5<945::AID-CHEM945>3.0.CO;2-3 |
| 34. |
Luchaco-Cullis, C. A.; Mizutani, H.; Murphy, K. E.; Hoveyda, A. H. Angew. Chem. 2001, 113, 1504–1508. doi:10.1002/1521-3757(20010417)113:8<1504::AID-ANGE1504>3.0.CO;2-V
Angew. Chem., Int. Ed. 2001, 40, 1456–1460.doi:10.1002/1521-3773(20010417)40:8<1456::AID-ANIE1456>3.0.CO;2-T |
| 35. | Murphy, K. E.; Hoveyda, A. H. J. Am. Chem. Soc. 2003, 125, 4690–4691. doi:10.1021/ja0300618 |
| 36. | Kacprzynski, M. A.; Hoveyda, A. H. J. Am. Chem. Soc. 2004, 126, 10676–10681. doi:10.1021/ja0478779 |
| 30. | Uozumi, Y.; Kyota, H.; Kishi, E.; Kitayama, K.; Hayashi, T. Tetrahedron: Asymmetry 1996, 7, 1603–1606. doi:10.1016/0957-4166(96)00193-0 |
| 47. | Roland, S.; Cotet, W.; Mangeney, P. Eur. J. Inorg. Chem. 2009, 1796–1805. doi:10.1002/ejic.200801157 |
| 48. | Wang, C.-Y.; Liu, Y.-H.; Peng, S.-M.; Chen, J.-T.; Liu, S.-T. J. Organomet. Chem. 2007, 692, 3976–3983. doi:10.1016/j.jorganchem.2007.06.007 |
| 49. | Peng, H. M.; Song, G. Y.; Li, Y. X.; Li, X. W. Inorg. Chem. 2008, 47, 8031–8043. doi:10.1021/ic800361v |
| 50. | Ketz, B. E.; Cole, A. P.; Waymouth, R. M. Organometallics 2004, 23, 2835–2837. doi:10.1021/om049838b |
| 74. | Nieto-Oberhuber, C.; López, S.; Echavarren, A. M. J. Am. Chem. Soc. 2005, 127, 6178–6179. doi:10.1021/ja042257t |
| 71. | Bonati, F.; Burini, A.; Pietroni, B. R.; Bovio, B. J. J. Organomet. Chem. 1989, 375, 147–160. doi:10.1016/0022-328X(89)85094-6 |
| 72. | Marion, N.; Nolan, S. P. Chem. Soc. Rev. 2008, 37, 1776–1782. doi:10.1039/b711132k |
| 73. | Gorin, D. J.; Sherry, B. D.; Toste, F. D. Chem. Rev. 2008, 108, 3351–3378. doi:10.1021/cr068430g |
| 87. | LaLonde, R. L.; Sherry, B. D.; Kang, E. J.; Toste, F. D. J. Am. Chem. Soc. 2007, 129, 2452–2453. doi:10.1021/ja068819l |
| 88. | Johansson, M. J.; Gorin, D. J.; Staben, S. T.; Toste, F. D. J. Am. Chem. Soc. 2005, 127, 18002–18003. doi:10.1021/ja0552500 |
| 89. | Zhang, Z.; Liu, C.; Kinder, R. E.; Han, X.; Qian, H.; Widenhoefer, R. A. J. Am. Chem. Soc. 2006, 128, 9066–9073. doi:10.1021/ja062045r |
| 90. | Zhang, Z.; Bender, C. F.; Widenhoefer, R. A. Org. Lett. 2007, 9, 2887–2889. doi:10.1021/ol071108n |
| 91. | Zhang, Z.; Bender, C. F.; Widenhoefer, R. A. Org. Lett. 2007, 9, 2887–2889. doi:10.1021/ol071108n |
| 92. | Li, H.; Widenhoefer, R. A. Org. Lett. 2009, 11, 2671–2674. doi:10.1021/ol900730w |
| 93. | Zhang, Z.; Bender, C. F.; Widenhoefer, R. A. J. Am. Chem. Soc. 2007, 129, 14148–14149. doi:10.1021/ja0760731 |
| 80. |
Lavallo, V.; Frey, G. D.; Donnadieu, B.; Soleilhavoup, M.; Bertrand, G. Angew. Chem. 2008, 120, 5302–5306. doi:10.1002/ange.200801136
Angew. Chem., Int. Ed. 2008, 47, 5224–5228.doi:10.1002/anie.200801136 |
| 81. | Zeng, X.; Frey, G. D.; Kinjo, R.; Donnadieu, B.; Bertrand, G. J. Am. Chem. Soc. 2009, 131, 8690–8696. doi:10.1021/ja902051m |
| 82. | van der Vlugt, J. I. Chem. Soc. Rev. 2010, 39, 2302–2322. doi:10.1039/b925794m |
| 83. |
Zeng, X.; Kinjo, R.; Kinjo, R.; Donnadieu, B.; Bertrand, G. Angew. Chem. 2010, 122, 954–957. doi:10.1002/ange.200905341
Angew. Chem., Int. Ed. 2010, 49, 942–945.doi:10.1002/anie.200905341 |
| 84. |
Klinkenberg, J. L.; Hartwig, J. F. Angew. Chem. 2011, 123, 88–98. doi:10.1002/ange.201002354
Angew. Chem., Int. Ed. 2011, 50, 86–95. doi:10.1002/anie.201002354 |
| 85. | Lavallo, V.; Frey, G. D.; Kousar, S.; Donnadieu, B.; Bertrand, G. Proc. Natl. Acad. Sci. U. S. A. 2007, 104, 13569–13573. doi:10.1073/pnas.0705809104 |
| 86. | Matsumoto, Y.; Selim, K. B.; Nakanishi, H.; Yamada, K.; Yamamoto, Y.; Tomioka, K. Tetrahedron Lett. 2010, 51, 404–406. doi:10.1016/j.tetlet.2009.11.039 |
| 78. | Marion, N.; Carlqvist, P.; Gealageas, R.; de Frémont, P.; Maseras, F.; Nolan, S. P. Chem.–Eur. J. 2007, 13, 6437–6451. doi:10.1002/chem.200700134 |
| 79. | Gorske, B. C.; Mbofana, C. T.; Miller, S. J. Org. Lett. 2009, 11, 4318–4321. doi:10.1021/ol9016782 |
| 75. | Gourlaouen, C.; Marion, N.; Nolan, S. P.; Maseras, F. Org. Lett. 2009, 11, 81–84. doi:10.1021/ol802430m |
| 76. | Marion, N.; Gealageas, R.; Nolan, S. P. Org. Lett. 2007, 9, 2653–2656. doi:10.1021/ol070843w |
| 77. |
Fructos, M. R.; Belderrain, T. R.; de Frémont, P.; Scott, N. M.; Nolan, S. P.; Díaz-Requejo, M. M.; Pérez, P. J. Angew. Chem. 2005, 117, 5418–5422. doi:10.1002/ange.200501056
Angew. Chem., Int. Ed. 2005, 44, 5284–5288.doi:10.1002/anie.200501056 |
© 2011 Liu et al; licensee Beilstein-Institut.
This is an Open Access article under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
The license is subject to the Beilstein Journal of Organic Chemistry terms and conditions: (http://www.beilstein-journals.org/bjoc)