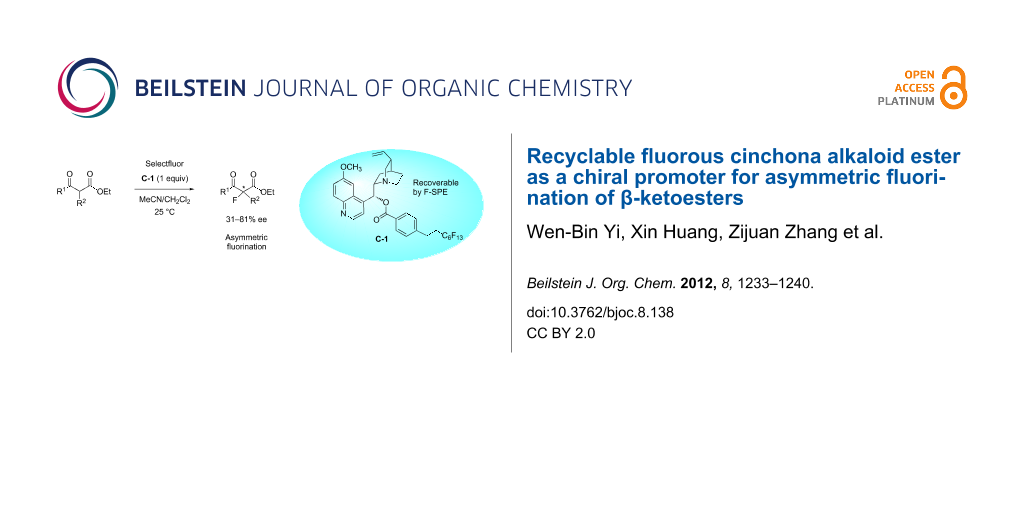
Abstract
A fluorous cinchona alkaloid ester has been developed as a chiral promoter for the asymmetric fluorination of β-ketoesters. It has comparable reactivity and selectivity to the nonfluorous versions of cinchona alkaloids and can be easily recovered from the reaction mixture by simple fluorous solid-phase extraction (F-SPE) and used for the next round of reaction without further purification.
Graphical Abstract
Introduction
Fluorinated organic compounds have unique properties because fluorine forms a strong carbon–fluorine bond with a small covalent radius and high electronegativity. Other than fluorinated polymers in materials science, organofluorine compounds have gained increasing popularity in medical chemistry and agricultural chemistry. Introducing one or a few fluorine atoms to biologically interesting molecules can significantly change the physical, chemical and biological properties [1,2]. The significant amount of publications on fluorinated small molecules, amino acids, carbohydrates, steroids and nucleosides indicates that organofluorine chemistry plays an important role in the life sciences [3,4].
A fluorine atom has been introduced to the α-position of some biologically interesting β-ketoesters, such as erythromycin and sesquiterpenic drimane (Figure 1) [5,6]. The achiral fluorination of β-ketoesters can be achieved by electrophilic reaction with Selectfluor (F-TEDA-BF4, 1-chloromethyl-4-fluoro-1,4-diazoniabicyclo[2.2.2]octane bis(tetrafluoroborate)), as developed by Bank [7-9]. The Cahard [10-12] and Shibata [13,14] groups combined cinchona alkaloids and Selectfluor for asymmetric fluorination of substrates such as imido-protected phenylglycines (up to 94% ee), indanones and tetralones (up to 91% ee), ethyl α-cyanotolyl acetates (up to 87% ee), and cyclic β-ketoesters (up to 80% ee) [15]. A catalytic approach for the cinchona alkaloids and Selectfluor combinations has also been developed [16]. The Togni group employed chiral titanium Lewis acid TiCl2(TADDOLate) for the asymmetric fluorination of β-ketoesters (up to 96% ee) [17-20]. Most Selectfluor-promoted asymmetric fluorinations require a stoichiometric amount of chiral promoters to suppress the competitively direct achiral fluorination. Different supported cinchona alkaloids have been developed as recyclable chiral promoters or organocatalysts. Among them, the Cahard group developed soluble polymer- and ionic-liquid-supported cinchona alkaloids for electrophilic fluorination [21,22]. The Fache and Soόs groups developed fluorous tag-attached cinchona alkaloids for catalytic Diels–Alder reactions [23,24]. Introduced in this paper is a new fluorous cinchona alkaloid ester for flourination of β-ketoesters. It is part of our recent effort on the development of recyclable fluorous reagents and organocatalysts for asymmetric synthesis [25-27].
Figure 1: Biologically interesting α-fluorinated β-ketoesters.
Figure 1: Biologically interesting α-fluorinated β-ketoesters.
Results and Discussion
Cinchona alkaloids and their derivatives have been well-explored in asymmetric synthesis [28]. We envisioned that the introduction of a fluorous tag could facilitate the recycling of cinchona alkaloids. The synthesis of fluorous quinine ester C-1 was accomplished by the reaction of quinine with a fluorous acid chloride (Scheme 1). This compound was easily purified by fluorous-solid phase extraction (F-SPE) with a cartridge charged with fluorous silica gels [29,30]. It is stable in air and soluble in solvents such as CH2Cl2, CH3OH, and CH3CN.
Scheme 1: Preparation of quinine ester C-1.
Scheme 1: Preparation of quinine ester C-1.
With the fluorous quinine ester C-1 in hand, we explored the fluorination reaction using ethyl 2-methyl-3-oxo-3-phenylpropanoate (1a) as a model compound. Nonfluorous quinine esters, such as C-2 and C-3, cinchona alkaloids C-4 and C-5, and fluorous pyrrolidine ester C-6, were also evaluated (Figure 2). The results of the fluorination of β-ketoester 1a with Selectfluor and different promoters are listed in Table 1. It was found that using MeCN as a solvent with 1 equiv of C-1 gave fluorinated product 2a in 49% yield and 65% ee (Table 1, entry 1). Compared to other promoters (Table 1, entries 2–5), C-1 gave fluorinated products in a slightly low yield but better enantioselectivity. This may be attributed to the stereo and the electronic effect of the fluorous tag. Fluorous pyrrolidine C-6 (Table 1, entry 6) gave the lowest product yield and ee among all six promoters. Reducing the amount of C-1 from 1 equiv to 0.5 and 0.2 equiv significantly reduced the ee of the product (Table 1, entries 7 and 8). A control reaction without C-1 gave 2a in 35% yield as a racemic product (Table 1, entry 9). The results suggest that a stoichiometric amount of C-1 is required to minimize the formation of achiral fluorination product by direct fluorination. Solvent screening indicated that using 1:1 CH3CN/CH2Cl2 gave product 2a in 51% yield and 70% ee (Table 1, entry 15), which is better than using CH3CN alone. Other single or binary solvent systems containing toluene, THF, H2O, and CF3C6H5 did not afford better results (Table 1, entries 10–14). It was also found that lowering of the reaction temperature from 25 to 10 or 0 °C did not necessarily improve the enantioselectivity of the fluorination (Table 1, entries 16 and 17).
Figure 2: Promoters for asymmetric fluorination.
Figure 2: Promoters for asymmetric fluorination.
Table 1: Asymmetric fluorination of 1a.a
|
|
|||||
| Entry | Cat. (equiv) | Solvent | t (h) | Yield (%) | ee (%) |
|---|---|---|---|---|---|
| 1 | C-1 (1.0) | MeCN | 72 | 49 | 65 |
| 2 | C-2 (1.0) | MeCN | 72 | 52 | 56 |
| 3 | C-3 (1.0) | MeCN | 72 | 54 | 51 |
| 4 | C-4 (1.0) | MeCN | 72 | 62 | 46 |
| 5 | C-5 (1.0) | MeCN | 72 | 65 | 48 |
| 6 | C-6 (1.0) | MeCN | 96 | 41 | 18 |
| 7 | C-1 (0.5) | MeCN | 60 | 51 | 26 |
| 8 | C-1 (0.2) | MeCN | 60 | 41 | <5 |
| 9 | – | MeCN | 96 | 35 | 0 |
| 10 | C-1 (1.0) | Toluene | 72 | 16 | 23 |
| 11 | C-1 (1.0) | THF | 72 | 32 | 41 |
| 12 | C-1 (1.0) | H2O | 96 | – | – |
| 13 | C-1 (1.0) | MeCN/THF | 60 | 38 | 45 |
| 14 | C-1 (1.0) | MeCN/CF3C6H5 | 60 | 43 | 59 |
| 15 | C-1 (1.0) | MeCN/CH2Cl2 | 60 | 51 | 70 |
| 16b | C-1 (1.0) | MeCN/CH2Cl2 | 72 | 46 | 69 |
| 17c | C-1 (1.0) | MeCN/CH2Cl2 | 72 | 39 | 71 |
aReaction temperature 25 °C unless otherwise indicated. bReaction temperature 10 °C. cReaction temperature 0 °C.
Recycling of promoter C-1 is an important part of this project. In our previous work we have demonstrated that fluorous organocatalysts and reagents can be readily recovered by F-SPE [19,20]. In the current work, upon completion of the fluorination reaction, a base such as aqueous NaOH or KOH was added to the reaction mixture to convert the cinchona alkaloid/Selectfluor complex to free cinchona alkaloid. The organic phase was loaded onto a fluorous silica gel cartridge for F-SPE. Promoter C-1 was recovered in high yield (94%) and excellent purity (98%). It was used for five rounds without significant change of product yield and ee (Scheme 2).
Scheme 2: Preparation of 2a by using recycled quinine ester C-1.
Scheme 2: Preparation of 2a by using recycled quinine ester C-1.
The scope of fluorous quinine ester C-1-mediated fluorination was evaluated by carrying out the reactions with a number of α-substituted ethyl benzoylacetates 1a–e and 1g–i as well as ethyl 2-cyclohexanonecarboxylate (1f). Results summarized in Figure 3 indicate that benzoylacetates bearing R2 such as Me, PhCH2, Cl, and Br gave fluorination products 2a–d in 43–71% yields and 60–70% ee. The nonsubstituted benzoylacetate 1e gave product 2e in good yield 69% but low ee (31%). Ethyl 2-cyclohexanonecarboxylate (1f) afforded product 2f in 73% yield and 63% ee. Reactions of ethyl benzoylacetates with bigger substitution groups, such as phenylsulfonyl and maleimide derivatives, were also attempted and gave products 2g–i in 74–83% yields and 78–81% ee.
Figure 3: The asymmetric fluorination of various β-ketoesters.
Figure 3: The asymmetric fluorination of various β-ketoesters.
Conclusion
A fluorous cinchona alkaloid-ester has been introduced as a promoter for Selectfluor-based asymmetric fluorination of β-ketoesters. The fluorous promoter has slightly lower reactivity but better enantioselectivity than the nonfluorous cinchona alkaloids. It can be easily recovered by simple fluorous solid-phase extraction for reuse.
Experimental
General
Chemicals and solvents were purchased from commercial suppliers and used as received. 1H and 13C NMR spectra were recorded on a 300 MHz Varian NMR spectrometer. Chemical shifts were reported in parts per million (ppm), and the residual solvent peak was used as an internal reference, i.e., proton (chloroform δ 7.26), carbon (chloroform δ 77.0). Multiplicity was indicated as follows: s (singlet), d (doublet), t (triplet), q (quartet), m (multiplet), dd (doublet of doublet), br s (broad singlet). Coupling constants were reported in hertz (Hz). LC–MS were performed on an Agilent 2100 system. A C18 column (5.0 μm, 6.0 × 50 mm) was used for the separation. The mobile phases were methanol and water, both containing 0.05% trifluoroacetic acid. A linear gradient was used to increase from 25:75 v/v methanol/water to 100% methanol over 7.0 min at a flow rate of 0.7 mL/min. UV detections were conducted at 210, 254 and 365 nm. Low-resolution mass spectra were recorded in APCI (atmospheric pressure chemical ionization). The high-resolution mass spectra were obtained on a Finnigan/MAT 95XL-T spectrometer. Sorbent silica gel XHL TLC plates (130815) were used for the thin-layer chromatography (TLC). Flash chromatography separations were performed on YAMAZEN AI-580 flash column system with Agela silica gel columns (230–400 μm mesh). The enantiomeric excesses of products were determined by chiral phase HPLC analysis on an SHIMADZU LC-20AD system.
Synthesis of fluorous quinine ester C-1
Thionyl chloride (1.19 g, 10 mmol) was added to a mixture of (1H,1H,2H,2H-perfluorooctyl)benzoic acid (0.468 g, 1 mmol) and pyridine (75 mg, 1 mmol). After stirring of the mixture for 4 h at 50 °C, the reaction container was flushed with nitrogen gas to remove unreacted thionyl chloride. Quinine (0.275 g, 0.85 mmol) and N,N-diisopropylethylamine (129 mg, 1 mmol) in CH2Cl2 (3 mL) was added, and the solution was stirred for 24 h under reflux. After the reaction had been quenched with H2O (2 mL) for 1 h, aqueous K2CO3 (2 M, 10 mL) was added, and the mixture was extracted with CH2Cl2 (3 × 10 mL). The CH2Cl2 layer was washed with aqueous HCl (ca. 2 M, 10 mL) and H2O (20 mL). The combined extracts were dried over K2CO3 and evaporated. The slightly yellow residue was purified by a fluorous silica gel cartridge (5 g). It was first eluted with 80:20 MeOH/H2O (20 mL) and then with 100% MeOH. The MeOH fraction was concentrated to give C-1 as a yellowish solid (0.625 g, 95%). Mp 175–177 °C; 1H NMR (CDCl3, 300 MHz) δ 1.51–2.05 (m, 6H), 2.30–2.42 (m, 3H), 2.65–2.70 (m, 2H), 2.97–3.18 (m, 4H), 3.50 (q, J = 6.9 Hz, 1H), 3.98 (s, 3H), 5.02 (m, 2H), 5.83 (m, 1H), 6.72 (d, J = 6.9 Hz, 1H), 7.32–7.51 (m, 5H), 8.01–8.07 (m, 3H), 8.72–8.73 (d, 1H); 13C NMR (CDCl3, 75 MHz) δ 24.2, 26.5, 27.6, 27.9, 32.4, 39.7, 42.6, 55.6, 56.7, 59.4, 74.5, 101.3, 114.6, 117.3, 118.6, 121.9, 126.9, 128.3, 128.63, 130.2, 131.9, 141.7, 143.6, 144.8, 145.0, 147.5, 156.0, 165.3; APCIMS m/z: 775.1 (M+ + 1); HRMS–ESI (m/z): [M + H]+ calcd. for C35H32F13N2O3, 775.2205; found, 775.2214.
Synthesis of quinine benzoate catalyst C-2
Benzoyl chloride (28 mg, 0.2 mmol) was added to a mixture of quinine (65 mg, 0.2 mmol) in CH2Cl2 (0.5 mL). After stirring at rt for 4 h, aqueous K2CO3 (2 M, 1 mL) was added. The reaction mixture was extracted with CH2Cl2 (2 × 3 mL). The CH2Cl2 layer was washed with aqueous HCl (2 M, 2 mL) and H2O (3 mL). The combined organic extracts were dried (K2CO3) and evaporated. The white residue was purified by flash column chromatography (18:1 CH2Cl2/MeOH) to give quinine benzoate C-2 (77 mg, 90%) as a colorless solid. 1H NMR (CDCl3, 300 MHz) δ 1.69–2.00 (m, 5H), 2.42 (m, 1H), 2.82 (m, 2H), 3.19–3.40 (m, 2H), 3.49–3.56 (q, J = 7.2 Hz, 1H), 4.00 (s, 3H), 5.04 (m, 2H), 5.82 (m, 1H), 6.97 (d, J = 7.2, 1H), 7.40–7.65 (m, 6H), 8.01–8.13 (m, 3H), 8.73 (d, 1H); 13C NMR (CDCl3, 75 MHz) δ 23.1, 27.2, 27.5, 39.0, 42.5, 56.0, 56.1, 59.0, 73.5, 101.2, 115.2, 117.2, 122.3, 126.6, 127.9, 128.7, 129.6, 129.6, 131.6, 131.8, 133.6, 140.6, 144.7, 147.2, 158.3, 165.1, 200.2; APCIMS m/z: 429.2 (M+ + 1).
Synthesis of quinine acetate C-3
Acetic anhydride (30 mg, 0.3 mmol) was added to a mixture of quinine (65 mg, 0.2 mmol) in CH2Cl2 (0.5 mL). After stirring at rt for 8 h, aqueous K2CO3 (2 M, 1 mL) was added, and the mixture was extracted with CH2Cl2 (2 × 3 mL). The CH2Cl2 layer was washed with aqueous HCl (2 M, 2 mL) and H2O (3 mL). The combined organic extracts were dried (K2CO3) and evaporated. The white residue was purified by flash column chromatography (18:1 CH2Cl2/MeOH) to give quinine acetate C-3 (67 mg, 92%) as a colorless oil. 1H NMR (CDCl3, 300 MHz) δ 1.26–1.89 (m, 5H), 2.42 (m, 1H), 2.12 (s, 3H), 2.23–2.36 (m, 2H), 2.37–2.70 (m, 2H), 3.00–3.16 (m, 2H), 3.34–3.42 (q, J = 7.2 Hz, 1H), 3.96 (s, 3H), 5.03 (m, 2H), 5.86 (m, 1H), 6.50 (d, J = 7.2 Hz, 1H), 7.35–7.44 (m, 3H), 8.02 (d, J = 9.0 Hz, 3H), 8.74 (d, 1H); 13C NMR (CDCl3, 75 MHz) δ 21.1, 24.3, 27.5, 27.7, 39.6, 42.4, 55.6, 56.5, 59.0, 73.7, 101.4, 114.5, 118.9, 121.8, 127.0, 131.8, 141.7, 143.5, 144.8, 147.4, 149.6, 157.9, 170.0, 199.5, 200.2; ACPIMS m/z: 367.2 (M+ + 1).
Synthesis of fluorous pyrrolidine ester C-6
N,N'-Dicyclohexylcarbodiimide (DCC) (0.206 g, 1 mmol) was added to a mixture of (1H,1H,2H,2H-perfluorooctyl)benzoic acid (0.468 g, 1 mmol), N-Boc-L-prolinol (0.221 g, 1.1 mmol), 4-dimethylaminopyridine (DMAP) (0.122 g, 1 mmol) in THF. After being stirred for 24 h at rt, the mixture was directly loaded onto a fluorous silica-gel cartridge (5 g; eluted by 100% methanol) to give the N-Boc-L-prolinyl (1H,1H,2H,2H-perfluorooctyl)benzoate (0.618 g, 95%). The N-Boc ester was then added to a mixture of TFA in CH2Cl2. After being stirred for 12 h at 0 °C, the reaction mixture was loaded onto a fluorous silica-gel cartridge (5 g) again to give the title compound L-prolinyl (1H,1H,2H,2H-perfluorooctyl)benzoate (0.496 g, 90%). 1H NMR (CDCl3, 300 MHz) δ 1.62–1.89 (m, 3H), 2.16–2.21 (m, 1H), 2.29–2.47 (m, 2H), 2.92–2.98 (m, 2H), 3.48–3.54 (m, 2H), 3.71–3.84 (m, 2H), 4.38–4.42 (m, 1H), 4.95–4.97 (m, 2H), 7.27–7.31 (d, 2H), 7.48–7.50 (d, 2H); 13C NMR (CDCl3, 75 MHz) δ 25.0, 26.2, 26.3, 26.3, 28.5, 32.2, 32.6, 51.1, 61.6, 67.2, 127.3, 127.6, 128.3, 128.3, 128.4, 130.1, 135.1, 141.4, 171.9; APCIMS m/z: 552.1 (M+ + 1).
General procedure for fluorination reaction
A mixture of Selectfluor (0.057 g, 0.16 mmol) and fluorous quinine ester C-1 (0.124 g, 0.16 mmol) in CH3CN and CH2Cl2 was stirred at rt for 1 h. Ethyl 2-methyl-3-oxo-3-phenylpropanoate (1a) (0.033 g, 0.16 mmol) was added. After stirring of the mixture at rt for 32 h, the reaction was quenched with H2O. After F-SPE, the mixture was extracted with EtOAc. The organic layer was washed with aqueous HCl (2 M, 5 mL) and H2O (5 mL), and then dried over Na2SO4. After evaporation of the solvent, the residue was purified by flash column chromatography (8:1 hexane/EtOAc) to give (S)-ethyl 2-methyl-2-fluoro-3-oxo-3-phenylpropanoate (2a) as a colorless oil.
(S)-Ethyl 2-methyl-2-fluoro-3-oxo-3-phenylpropanoate (2a)
51% yield, 70% ee. The enantiomeric excess was determined by HPLC on (R,R)-WHELK-O1 with hexane/iPrOH (92:8) as the eluent. Flow rate: 0.6 mL/min, λ = 254 nm; tminor = 20.132 min, tmajor = 17.924 min; 1H NMR (CDCl3, 300 MHz) δ 1.00 (t, J = 7.2 Hz, 3H), 1.93 (s, 1H), 4.11 (q, J = 7.2 Hz, 2H), 7.33–7.38 (m, 2H), 7.46 (m, 1H), 7.90–7.92 (m, 2H); APCIMS m/z: 225.2 (M+ + 1).
(S)-Ethyl 2-benzyl-2-fluoro-3-oxo-3-phenylpropanoate (2b)
59% yield, 60% ee. The enantiomeric excess was determined by HPLC on Regis Chiral 5 Micron with hexane/iPrOH (90:10) as the eluent. Flow rate: 0.8 mL/min, λ = 254 nm; tminor = 8.732 min, tmajor = 10.352 min; 1H NMR (CDCl3, 300 MHz) δ 0.92 (t, J = 7.2 Hz, 3H), 3.48 (d, J = 14.1 Hz, 1H), 3.67 (d, J = 14.1 Hz, 1H), 4.01 (q, J = 7.2 Hz, 2H), 7.14–7.23 (m, 5H), 7.26 (m, 2H), 7.36 (m, 1H), 7.91 (d, 2H); APCIMS m/z: 301.1 (M+ + 1).
(R)-Ethyl 2-chloro-2-fluoro-3-oxo-3-phenylpropanoate (2c)
71% yield, 66% ee. The enantiomeric excess was determined by HPLC on Regis Chiral 5 Micron with hexane/iPrOH (90:10) as the eluent. Flow rate: 0.8 mL/min, λ = 254 nm; tminor = 12.220 min, tmajor = 14.492 min; 1H NMR (CDCl3, 300 MHz) δ 1.18 (t, J = 7.2 Hz, 3H), 4.32 (q, J = 7.2 Hz, 2H), 7.47–7.49 (m, 2H), 7.60 (m, 1H), 8.02–8.05 (m, 2H); APCIMS m/z: 245.0 (M+ + 1).
(R)-Ethyl 2-bromo-2-fluoro-3-oxo-3-phenylpropanoate (2d)
43% yield, 66% ee. The enantiomeric excess was determined by HPLC on Regis Chiral 5 Micron with hexane/iPrOH (90:10) as the eluent. Flow rate: 1.2 mL/min, λ = 254 nm; tminor = 5.912 min, tmajor = 7.004 min; 1H NMR (CDCl3, 300 MHz) δ 1.28 (t, J = 7.2 Hz, 3H), 4.38 (q, J = 7.2 Hz, 2H), 7.48–7.53 (m, 2H), 7.64 (m, 1H), 8.06–8.10 (m, 2H); APCIMS m/z: 289.0 (M+ + 1).
(S)-Ethyl 2-fluoro-3-oxo-3-phenylpropanoate (2e)
69% yield, 31% ee. The enantiomeric excess was determined by HPLC on (R,R)-WHELK-O1with hexane/iPrOH (95:5) as the eluent. Flow rate: 1.0 mL/min, λ = 254 nm; tminor = 5.904 min, tmajor = 5.380 min; 1H NMR (CDCl3, 300 MHz) δ 1.15 (t, J = 7.2 Hz, 3H), 4.23 (q, J = 7.2 Hz, 2H), 5.70–5.87 (s, J = 48.9 Hz, 1H), 7.18–7.45 (m, 2H), 7.53 (m, 1H), 7.94–7.98 (m, 2H); APCIMS m/z: 211.1 (M+ + 1).
(R)-Ethyl 2-fluoro-2-cyclohexanonecarboxylate (2f)
73% yield, 63% ee. The enantiomeric excess was determined by HPLC on (R,R)-WHELK-O1 with hexane/iPrOH (90:10) as the eluent. Flow rate: 0.8 mL/min, λ = 210 nm; tminor = 10.848 min, tmajor = 12.440 min; 1H NMR (CDCl3, 300 MHz) δ 1.32 (t, J = 7.2 Hz, 3H), 1.61–1.89 (m, 2H), 2.06–2.10 (m, 1H), 2.51–2.73 (m, 3H), 4.30 (q, J = 7.2 Hz, 2H).
(S)-Ethyl 2-(4’-methylbenzenesulfonyl)-2-fluoro-3-oxo-3-phenylpropanoate (2g)
74% yield, 78% ee. The enantiomeric excess was determined by HPLC on Venusil Chiral OD-H with hexane/iPrOH (92:8) as the eluent. Flow rate: 0.3 mL/min, λ = 254 nm; tminor = 27.176 min, tmajor = 24.288 min; 1H NMR (CDCl3, 300 MHz) δ 1.41 (t, J = 7.2 Hz, 3H), 2.36 (s, 3H), 4.38 (q, J = 7.2 Hz, 2H), 7.09–7.18 (m, 4H), 7.20–7.35 (m, 3H), 7.54 (s, 2H); APCIMS m/z: 365.1 (M+ + 1).
(S)-Ethyl 2-(N-ethylmaleimide)-2-fluoro-3-oxo-3-phenylpropanoate (2h)
78% yield, 82% ee. The enantiomeric excess was determined by HPLC on Venusil Chiral OD-H with hexane/iPrOH (94:6) as the eluent. Flow rate: 0.5 mL/min, λ = 254 nm; tminor = 13.832 min, tmajor = 12.432 min; 1H NMR (CDCl3, 300 MHz) δ 1.16 (t, J = 7.2 Hz, 3H), 1.28 (t, J = 7.2 Hz, 3H), 2.58 (dd, J = 18.3 Hz, 1H), 3.05 (dd, J = 18.3 Hz, 1H), 3.61 (q, J = 7.2 Hz, 2H), 4.14 (m, 1H ), 4.42 (m, J = 7.2 Hz, 2H), 7.46–7.51 (m, 2H), 7.62 (m, 1H), 8.12 (m, 2H); 13C NMR (CDCl3, 75 MHz) δ 12.9, 13.8, 30.8, 34.1, 44.9, 63.5, 128.5, 128.8, 130.1, 130.2, 134.8, 168.5, 174.8; APCIMS m/z: 336.1 (M+ + 1).
(S)-Ethyl 2-(N-benzylmaleimide)-2-fluoro-3-oxo-3-phenylpropanoate (2i)
83% yield, 81% ee. The enantiomeric excess was determined by HPLC on Venusil Chiral OD-H with hexane/iPrOH (92:8) as the eluent. Flow rate: 0.3 mL/min, λ = 254 nm: tminor = 27.176 min, tmajor = 24.288 min; 1H NMR (CDCl3, 300 MHz) δ 1.27 (t, J = 7.2 Hz, 3H), 2.58 (dd, J = 18.3 Hz, 1H), 3.05 (dd, J = 18.3 Hz, 1H), 4.14 (m, 1H ), 4.42 (m, J = 7.2 Hz, 2H), 7.67 (q, J = 15 Hz, 2H), 7.25–7.37 (m, 5H), 7.46–7.51 (m, 2H), 7.62 (m, 1H), 8.12 (m, 2H); 13C NMR (CDCl3, 75 MHz) δ 13.8, 30.8, 42.7, 45.0, 45.3, 63.5, 128.0, 128.6, 128.7, 128.8, 130.1, 130.2, 134.9, 174.6; APCIMS m/z: 398.1 (M+ + 1).
Synthesis of racemic samples
The mixture of Selectfluor (0.057 g, 0.16 mmol) and ethyl benzoylacetate (0.031 g, 0.16 mmol) in CH3CN (1 mL) was stirred at 90 °C under microwave irradiation for 40 min. The reaction was quenched by water. The mixture was extracted with ethyl acetate (3 mL). The organic layer was washed with aqueous HCl (2 M, 5 mL) and water (5 mL), and dried over Na2SO4. After evaporation of the solvent, the residue was purified by flash column chromatography (8:1 hexane/EtOAc) to give ethyl 2-methyl-2-fluoro-3-oxo-3-phenylpropanoate (0.032 g, 94%) as a colorless oil.
General procedure for recycling of C-1
The reaction mixture was loaded onto a fluorous silica-gel cartridge (5 g) and eluted by 80:20 MeOH/H2O to collect nonfluorous components, including the fluorinated product. The cartridge was eluted with MeOH to collect C-1. After concentration of the MeOH fraction and drying at 60 °C for 8 h, the recovered promoter was ready for the next round of reactions.
Supporting Information
| Supporting Information File 1: Chiral HPLC chromatograms for fluorination products 2a–i. LC–MS, NMR spectra for fluorination products 2a–i and cinchona alkaloid derivatives C-1, C-2, C-3 and C-6. LC–MS spectra for 2h and HRMS spectra for C-1. | ||
| Format: PDF | Size: 3.0 MB | Download |
Acknowledgements
W. Z. thanks the University of Massachusetts Boston for a grant support. W. Y. thanks for support from “NUST Excellence Initiative”, NUST Research Funding (2011ZDJH07), Jiangsu Provincial Natural Science Foundation of China for Key Projects (BK2010070) and National Natural Science Foundation of China (20902047).
References
-
Banks, R. E.; Tatlow, J. C., Eds. Organofluorine Chemistry: Principles and Commercial Applications; Topics in Applied Chemistry; Plenum Press: New York, 1994.
Return to citation in text: [1] -
Ojima, I.; McCarthy, J. R.; Welch, J. T., Eds. Biomedical Frontiers of Fluorine chemistry; ACS Symposium Series, Vol. 639; American Chemical Society: Washington, 1996. doi:10.1021/bk-1996-0639
Return to citation in text: [1] -
Ojima, I. Fluorine in Medicinal Chemistry and Chemical Biology; Wiley-Blackwell: Hoboken, 2009.
Return to citation in text: [1] -
Qiu, X.-L.; Xu, X.-H.; Qing, F.-L. Tetrahedron 2010, 66, 789–843. doi:10.1016/j.tet.2009.11.001
Return to citation in text: [1] -
Phan, L. T.; Clark, R. F.; Rupp, M.; Or, Y. S.; Chu, D. T. W.; Ma, Z. Org. Lett. 2000, 2, 2951–2954. doi:10.1021/ol006226o
Return to citation in text: [1] -
Abad, A.; Agulló, C.; Cuñat, A. C.; González-Coloma, A.; Pardo, D. Eur. J. Org. Chem. 2010, 2182–2198. doi:10.1002/ejoc.200901499
Return to citation in text: [1] -
Banks, R. E. J. Fluorine Chem. 1998, 87, 1–17. doi:10.1016/S0022-1139(97)00127-9
Return to citation in text: [1] -
Nyffeler, P. T.; Gonzalez Durón, S.; Burkart, M. D.; Vincent, S. P.; Wong, C.-H. Angew. Chem., Int. Ed. 2005, 44, 192–212. doi:10.1002/anie.200400648
Return to citation in text: [1] -
Singh, R. P.; Shreeve, J. M. Acc. Chem. Res. 2004, 37, 31–44. doi:10.1021/ar030043v
Return to citation in text: [1] -
Cahard, D.; Audouard, C.; Plaquevent, J.-C.; Roques, N. Org. Lett. 2000, 2, 3699–3701. doi:10.1021/ol006610l
Return to citation in text: [1] -
Mohar, B.; Baudoux, J.; Plaquevent, J.-C.; Cahard, D. Angew. Chem., Int. Ed. 2001, 40, 4214–4216. doi:10.1002/1521-3773(20011119)40:22<4214::AID-ANIE4214>3.0.CO;2-B
Return to citation in text: [1] -
Cahard, D.; Audouard, C.; Plaquevent, J.-C.; Toupet, L.; Roques, N. Tetrahedron Lett. 2001, 42, 1867–1869. doi:10.1016/S0040-4039(01)00017-X
Return to citation in text: [1] -
Shibata, N.; Suzuki, E.; Takeuchi, Y. J. Am. Chem. Soc. 2000, 122, 10728–10729. doi:10.1021/ja002732x
Return to citation in text: [1] -
Shibata, N.; Suzuki, E.; Asahi, T.; Shiro, M. J. Am. Chem. Soc. 2001, 123, 7001–7009. doi:10.1021/ja010789t
Return to citation in text: [1] -
Ma, J.-A.; Cahard, D. Chem. Rev. 2008, 108, PR1–PR43. doi:10.1021/cr800221v
Return to citation in text: [1] -
Fukuzumi, T.; Shibata, N.; Sugiura, M.; Nakamura, S.; Toru, T. J. Fluorine Chem. 2006, 127, 548–551. doi:10.1016/j.jfluchem.2006.01.004
Return to citation in text: [1] -
Hintermann, L.; Togni, A. Angew. Chem., Int. Ed. 2000, 39, 4359–4362. doi:10.1002/1521-3773(20001201)39:23<4359::AID-ANIE4359>3.0.CO;2-P
Return to citation in text: [1] -
Hintermann, L.; Togni, A. Catalytic halogenation of activated methylene and methane compound. Eur. Pat. Appl. EP1151980 A1, Nov 7, 2001.
Return to citation in text: [1] -
Muñiz, K. Angew. Chem., Int. Ed. 2001, 40, 1653–1656. doi:10.1002/1521-3773(20010504)40:9<1653::AID-ANIE16530>3.0.CO;2-W
Return to citation in text: [1] [2] -
Hintermann, L.; Perseghini, M.; Togni, A. Beilstein J. Org. Chem. 2011, 7, 1421–1435. doi:10.3762/bjoc.7.166
Return to citation in text: [1] [2] -
Thierry, B.; Audouard, C.; Plaquevent, J.-C.; Cahard, D. Synlett 2004, 856–860. doi:10.1055/s-2004-817781
Return to citation in text: [1] -
Baudequin, C.; Plaquevent, J.-C.; Audouard, C.; Cahard, D. Green Chem. 2002, 4, 584–586. doi:10.1039/b208817g
Return to citation in text: [1] -
Fache, F.; Piva, O. Tetrahedron Lett. 2001, 42, 5655–5657. doi:10.1016/S0040-4039(01)01036-X
Return to citation in text: [1] -
Kaleta, Z.; Egyed, O.; Soós, T. Org. Biomol. Chem. 2005, 3, 2228–2230. doi:10.1039/b504973c
Return to citation in text: [1] -
Wang, L.; Cai, C.; Curran, D. P.; Zhang, W. Synlett 2010, 433–436. doi:10.1055/s-0029-1219198
Return to citation in text: [1] -
Chu, Q.; Zhang, W.; Curran, D. P. Tetrahedron Lett. 2006, 47, 9287–9290. doi:10.1016/j.tetlet.2006.10.101
Return to citation in text: [1] -
Zhang, W. Top. Curr. Chem. 2012, 308, 175–190. doi:10.1007/128_2011_257
Return to citation in text: [1] -
Song, C. E., Ed. Cinchona Alkaloids in Synthesis and Catalysis: Ligands, Immobilization and Organocatalysis; Wiley-VCH: Weinheim, Germany, 2009.
Return to citation in text: [1] -
Zhang, W.; Curran, D. P. Tetrahedron 2006, 62, 11837–11865. doi:10.1016/j.tet.2006.08.051
Return to citation in text: [1] -
Fluorous SPE cartridges are available from Fluorous Technologies, Inc. http://www.fluorous.com and http://www.silicycle.com
Return to citation in text: [1]
| 1. | Banks, R. E.; Tatlow, J. C., Eds. Organofluorine Chemistry: Principles and Commercial Applications; Topics in Applied Chemistry; Plenum Press: New York, 1994. |
| 2. | Ojima, I.; McCarthy, J. R.; Welch, J. T., Eds. Biomedical Frontiers of Fluorine chemistry; ACS Symposium Series, Vol. 639; American Chemical Society: Washington, 1996. doi:10.1021/bk-1996-0639 |
| 10. | Cahard, D.; Audouard, C.; Plaquevent, J.-C.; Roques, N. Org. Lett. 2000, 2, 3699–3701. doi:10.1021/ol006610l |
| 11. | Mohar, B.; Baudoux, J.; Plaquevent, J.-C.; Cahard, D. Angew. Chem., Int. Ed. 2001, 40, 4214–4216. doi:10.1002/1521-3773(20011119)40:22<4214::AID-ANIE4214>3.0.CO;2-B |
| 12. | Cahard, D.; Audouard, C.; Plaquevent, J.-C.; Toupet, L.; Roques, N. Tetrahedron Lett. 2001, 42, 1867–1869. doi:10.1016/S0040-4039(01)00017-X |
| 19. | Muñiz, K. Angew. Chem., Int. Ed. 2001, 40, 1653–1656. doi:10.1002/1521-3773(20010504)40:9<1653::AID-ANIE16530>3.0.CO;2-W |
| 20. | Hintermann, L.; Perseghini, M.; Togni, A. Beilstein J. Org. Chem. 2011, 7, 1421–1435. doi:10.3762/bjoc.7.166 |
| 7. | Banks, R. E. J. Fluorine Chem. 1998, 87, 1–17. doi:10.1016/S0022-1139(97)00127-9 |
| 8. | Nyffeler, P. T.; Gonzalez Durón, S.; Burkart, M. D.; Vincent, S. P.; Wong, C.-H. Angew. Chem., Int. Ed. 2005, 44, 192–212. doi:10.1002/anie.200400648 |
| 9. | Singh, R. P.; Shreeve, J. M. Acc. Chem. Res. 2004, 37, 31–44. doi:10.1021/ar030043v |
| 5. | Phan, L. T.; Clark, R. F.; Rupp, M.; Or, Y. S.; Chu, D. T. W.; Ma, Z. Org. Lett. 2000, 2, 2951–2954. doi:10.1021/ol006226o |
| 6. | Abad, A.; Agulló, C.; Cuñat, A. C.; González-Coloma, A.; Pardo, D. Eur. J. Org. Chem. 2010, 2182–2198. doi:10.1002/ejoc.200901499 |
| 28. | Song, C. E., Ed. Cinchona Alkaloids in Synthesis and Catalysis: Ligands, Immobilization and Organocatalysis; Wiley-VCH: Weinheim, Germany, 2009. |
| 3. | Ojima, I. Fluorine in Medicinal Chemistry and Chemical Biology; Wiley-Blackwell: Hoboken, 2009. |
| 4. | Qiu, X.-L.; Xu, X.-H.; Qing, F.-L. Tetrahedron 2010, 66, 789–843. doi:10.1016/j.tet.2009.11.001 |
| 29. | Zhang, W.; Curran, D. P. Tetrahedron 2006, 62, 11837–11865. doi:10.1016/j.tet.2006.08.051 |
| 30. | Fluorous SPE cartridges are available from Fluorous Technologies, Inc. http://www.fluorous.com and http://www.silicycle.com |
| 17. | Hintermann, L.; Togni, A. Angew. Chem., Int. Ed. 2000, 39, 4359–4362. doi:10.1002/1521-3773(20001201)39:23<4359::AID-ANIE4359>3.0.CO;2-P |
| 18. | Hintermann, L.; Togni, A. Catalytic halogenation of activated methylene and methane compound. Eur. Pat. Appl. EP1151980 A1, Nov 7, 2001. |
| 19. | Muñiz, K. Angew. Chem., Int. Ed. 2001, 40, 1653–1656. doi:10.1002/1521-3773(20010504)40:9<1653::AID-ANIE16530>3.0.CO;2-W |
| 20. | Hintermann, L.; Perseghini, M.; Togni, A. Beilstein J. Org. Chem. 2011, 7, 1421–1435. doi:10.3762/bjoc.7.166 |
| 23. | Fache, F.; Piva, O. Tetrahedron Lett. 2001, 42, 5655–5657. doi:10.1016/S0040-4039(01)01036-X |
| 24. | Kaleta, Z.; Egyed, O.; Soós, T. Org. Biomol. Chem. 2005, 3, 2228–2230. doi:10.1039/b504973c |
| 16. | Fukuzumi, T.; Shibata, N.; Sugiura, M.; Nakamura, S.; Toru, T. J. Fluorine Chem. 2006, 127, 548–551. doi:10.1016/j.jfluchem.2006.01.004 |
| 25. | Wang, L.; Cai, C.; Curran, D. P.; Zhang, W. Synlett 2010, 433–436. doi:10.1055/s-0029-1219198 |
| 26. | Chu, Q.; Zhang, W.; Curran, D. P. Tetrahedron Lett. 2006, 47, 9287–9290. doi:10.1016/j.tetlet.2006.10.101 |
| 27. | Zhang, W. Top. Curr. Chem. 2012, 308, 175–190. doi:10.1007/128_2011_257 |
| 13. | Shibata, N.; Suzuki, E.; Takeuchi, Y. J. Am. Chem. Soc. 2000, 122, 10728–10729. doi:10.1021/ja002732x |
| 14. | Shibata, N.; Suzuki, E.; Asahi, T.; Shiro, M. J. Am. Chem. Soc. 2001, 123, 7001–7009. doi:10.1021/ja010789t |
| 21. | Thierry, B.; Audouard, C.; Plaquevent, J.-C.; Cahard, D. Synlett 2004, 856–860. doi:10.1055/s-2004-817781 |
| 22. | Baudequin, C.; Plaquevent, J.-C.; Audouard, C.; Cahard, D. Green Chem. 2002, 4, 584–586. doi:10.1039/b208817g |
© 2012 Yi et al; licensee Beilstein-Institut.
This is an Open Access article under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
The license is subject to the Beilstein Journal of Organic Chemistry terms and conditions: (http://www.beilstein-journals.org/bjoc)