Abstract
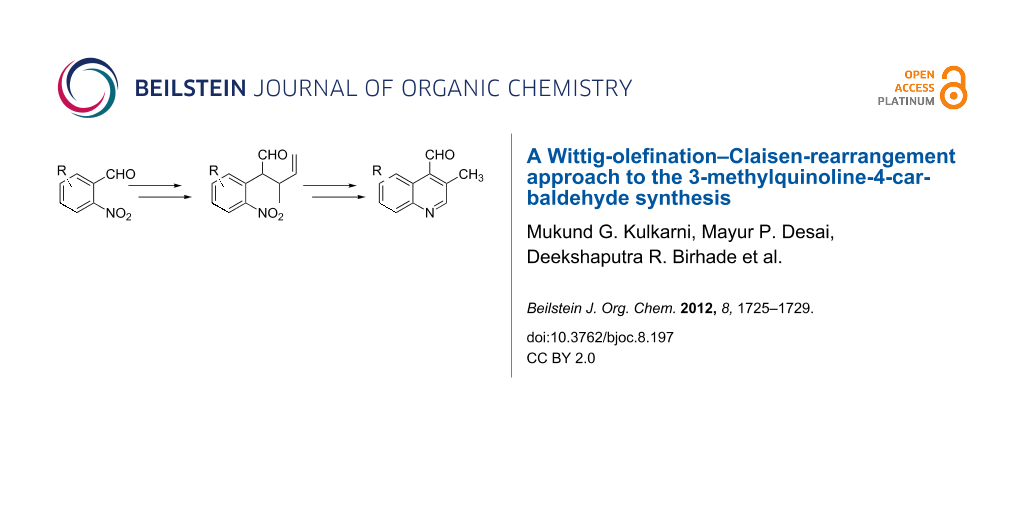
Efficient syntheses are described for the synthetically important 3-methylquinoline-4-carbaldehydes 6a–h from o-nitrobenzaldehydes 1a–h employing a Wittig-olefination–Claisen-rearrangement protocol. The Wittig reaction of o-nitrobenzaldehydes with crotyloxymethylene triphenylphosphorane afforded crotyl vinyl ethers 2a–h, which on heating under reflux in xylene underwent Claisen rearrangement to give 4-pentenals 3a–h. Protection of the aldehyde group of the 4-pentenals as acetals 4a–h and subsequent oxidative cleavage of the terminal olefin furnished nitroaldehydes 5a–h. Reductive cyclization of these nitroaldehydes yielded the required 3-methylquinoline-4-carbaldehydes 6a–h in excellent yields. Therefore, an efficient method was developed for the preparation of 3-methylquinoline-4-carbaldehydes from o-nitrobenzaldehydes in a simple five-step procedure.
Graphical Abstract
Introduction
Quinoline aldehydes are important synthetic intermediates in the synthesis of heterocyclic compounds that are used in the manufacturing of dyes [1] and pharmaceuticals [2,3]. 3-Substituted and 2,3-di-substituted quinoline-4-carbaldehyde derivatives are used in the synthesis of immunosuppressant KF20444 [4] and 5-HT3 receptor antagonists [5]. Quinoline mevalonolactones, prepared from 3-methylquinoline-4-carbaldehyde, act as inhibitors of HMG-CoA reductase [6]. 3-Substituted quinoline-4-carbaldehyde derivatives are used in the development of molecular probes for the identification of extra interaction sites in the midgorge and peripheral sites of butyrylcholinesterase (BuChE) [7]. These derivatives are also exploited in the synthesis of DNA binders [8], macrolides [9], antitumor agents [10] and for the treatment of viral and parasitic infections [11].
Though there are a number of quite efficient methods for the preparation of quinoline-4-carbaldehyde [12-24], only a few methods [6,9] are available for the preparation of 3-methylquinoline-4-carbaldehyde derivatives. In connection with the synthesis of camptothecin, we needed a general, high-yielding method for the synthesis of 3-methylquinoline-4-carbaldehyde. It was considered that a properly substituted 2-(2-nitrophenyl)pent-4-enal [25-27] could be a fitting intermediate for this purpose. Such an intermediate is easily accessible through the Wittig-olefination–Claisen-rearrangement protocol developed in our group [28,29].
Results and Discussion
Reaction of the o-nitrobenzaldehydes 1a–h with crotyloxymethylene triphenylphosphorane under optimized reaction conditions (Scheme 1) gave crotyl vinyl ethers in good yields (Table 1). The geometrical isomers of the crotyl vinyl ethers 2a–d were well separated on TLC, and it was possible to separate them by column chromatography. In the case of other crotyl vinyl ethers 2e–h, all attempts to separate these (E)- or (Z)-isomers were unsuccessful.
Scheme 1: Reagents and conditions: (i) Ph3P+CH2OCH2CH=CHCH3Cl−, t-BuOK, dry THF, 0 °C; (ii) xylene, reflux, 5–7 h; (iii) 3 equiv ethylene glycol, cat. p-TSA, toluene, reflux, 3–4 h; (iv) cat. potassium osmate, 2 equiv NMO, 2 equiv NaIO4, aq THF, 3–4 h; (v) 5 equiv Zn, AcOH, reflux, 0.5 h.
Scheme 1: Reagents and conditions: (i) Ph3P+CH2OCH2CH=CHCH3Cl−, t-BuOK, dry THF, 0 °C; (ii) xylene, reflux, 5...
Table 1: Synthesis of 3-methylquinoline-4-carbaldehydes.
| Aldehydes 1 | % Yields of | ||||
|---|---|---|---|---|---|
| 2a | 3b | 4b | 5b | 6 | |
|
1a |
90
(Z/E = 1:1.48) |
85
(dr = 1:1.21) |
92
(dr = 1:1.5) |
91
(dr = 1:2.46) |
85 |
|
1b |
91
(Z/E = 1:1.42) |
83
(dr = 1:1.36) |
91
(dr = 1:1.12) |
90
(dr = 1:1.03) |
84 |
|
1c |
92
(Z/E = 1:1.87) |
84
(dr = 1:1.74) |
90
(dr = 1:1.24) |
91
(dr = 1:1.52) |
85 |
|
1d |
93
(Z/E = 1:1.93) |
86
(dr = 1:1.63) |
92
(dr = 1:1.66) |
91
(dr = 1:1.41) |
83 |
|
1e |
91c |
85
(dr = 1:1.16) |
91
(dr = 1:2.63) |
90
(dr = 1:6) |
80d |
|
1f |
90
(only (E)-isomer) |
83
(dr = 1:9.01) |
92
(dr = 1:1) |
91
(dr = 1:6.58) |
79 |
|
1g |
92
(Z/E = 1:2.06) |
83
(dr = 1:2.42) |
91
(dr = 1:1.11) |
90
(dr = 1:1.5) |
80 |
|
1h |
91
(Z/E = 1:1.94) |
85
(dr = 1:1.87) |
90
(dr = 1:1.86) |
91
(dr = 1:1.44) |
82 |
a2a–d are separable geometrical isomers, whereas 2e, 2g and 2h are mixtures of inseparable geometrical isomers. The Z/E ratio of the geometrical isomers of 2g–h was calculated from their NMR signals.
b3a–h, 4a–h and 5a–h are mixtures of diastereomers, and the diastereomeric ratio (dr) was calculated from their NMR signals.
cThe Z/E ratio of the geometrical isomers of 2e cannot be calculated from their NMR signals.
dKnown compound [6,9].
Claisen rearrangement on either (E)- or (Z)-isomers 2a–d also led to a diastereomeric mixture of 4-pentenals. However, these diastereomers remained inseparable. The crotyl vinyl ethers 2e–h, on heating under reflux in anhydrous xylene, underwent the Claisen rearrangement smoothly to give the diastereomeric mixture of the corresponding 4-pentenals 3e–h in good yields (Table 1).
Treatment of the 4-pentenals 3a–h with ethylene glycol furnished the corresponding acetals 4a–h in good yields (Table 1). From the NMR spectra of these acetals, it was clear that they were also a mixture of diastereomers, although they appeared to be homogeneous on TLC. All attempts to separate the diastereomers at this stage were also unsuccessful. Subjecting these acetals to oxidative cleavage in aq THF furnished the aldehydes 5a–h in good yields (Table 1). The NMR of these aldehydes revealed them again to be a mixture of diastereomers, although they appeared to be homogeneous on TLC. Reductive cyclization of these nitroaldehydes furnished the required 3-methylquinoline-4-carbaldehydes 6a–h.
Conclusion
A new and efficient methodology for the construction of a 3-methylquinoline-4-carbaldehyde framework, with 50–55% overall yield, through a Wittig-olefination–Claisen-rearrangement protocol has been developed.
Experimental
General
Silica gel (100–200 mesh) was used for column chromatography. IR spectra were recorded on a Perkin Elmer model 1600 series FTIR instrument. 1H and 13C NMR (ppm, TMS, internal standard) in CDCl3 were recorded on a JEOL FX 90Q, Varian Mercury 300 MHz and 75 MHz, respectively. CHN analysis was performed on a Thermo FLASH EA model 1112 series. TLC was checked either under UV light and/or charring after dipping into anisaldehyde solution.
General procedure for the Wittig olefination
To a suspension of the o-nitrobenzaldehyde (20 mmol) and crotyloxymethylenetriphenylphosphonium chloride (24 mmol, 1.2 equiv) in dry THF (40 mL) at 0 °C was added t-BuOK (24 mmol, 1.2 equiv) in small portions. After 40–45 min (TLC, ethyl acetate/petroleum ether 1:9), THF was removed under vacuum. Water (25 mL) was added to the reaction mixture, and then the aqueous layer was extracted with ethyl acetate (3 × 15 mL), the combined organic layer was dried over sodium sulfate, and ethyl acetate was evaporated under vacuum. The crude product, i.e., crotyl vinyl ether, was purified by using silica-gel column chromatography (mobile phase 1–3% ethyl acetate in petroleum ether). Crotyl vinyl ethers (a–h were obtained in 84–89% yield.
General procedure for the Claisen rearrangement
The crotyl vinyl ethers 2a–h (17 mmol) obtained from the Wittig reaction were dissolved in anhydrous xylene (35 mL) and the solution was heated under reflux for 5–7 h (TLC, ethyl acetate/petroleum ether 1:9). Then, the solvent was removed under reduced pressure. The crude aldehyde was purified by using silica-gel column chromatography (mobile phase 2–5% ethyl acetate in pet. ether). 4-Pentenals 3a–h were obtained in 83–89% yield.
General procedure for the protection of aldehyde
Aldehydes 3a–h obtained from Claisen rearrangement (15 mmol) were dissolved in anhydrous toluene (25 mL). To this solution, a catalytic amount of p-TSA (1.5 mmol, 0.1 equiv) and ethylene glycol (45 mmol, 3 equiv) were added. The reaction mixture was heated under reflux for 3–4 h by using a Dean–Stark condenser (TLC, ethyl acetate/petroleum ether 1:9). After removal of the solvent under reduced pressure, water (20 mL) was added to the reaction mixture, and then the aqueous layer was extracted with ethyl acetate (3 × 15 mL), the combined organic layer was dried over sodium sulfate, and ethyl acetate was evaporated under vacuum. Finally, the product was purified by silica-gel column chromatography (mobile phase 1–3% ethyl acetate in petroleum ether). The products 4a–h were obtained in 89–93% yield.
General procedure for the oxidative cleavage of alkene
Alkenes 4a–h (13.5 mmol), obtained as described above, were dissolved in aq. THF (30 mL, THF/H2O 1:1). N-Methylmorpholine-N-oxide (NMO) (27 mmol, 2 equiv) and potassium osmate (0.027 mmol, 2 mol %) were added to this solution. The mixture was stirred at room temperature for 2–3 h until the starting compound disappeared (TLC, ethyl acetate/petroleum ether 1:9). Then, sodium metaperiodate was added (27 mmol, 2 equiv) and stirring was continued for 1 h (TLC, ethyl acetate/petroleum ether 1:9). THF was removed under reduced pressure. Water (20 mL) was added to the reaction mixture, and then the aqueous layer was extracted with ethyl acetate (3 × 10 mL), the combined organic layer was dried over sodium sulfate, and ethyl acetate was evaporated under vacuum. The crude product was obtained after removal of the solvent under reduced pressure. The product was purified by using silica-gel column chromatography (mobile phase 4–7% ethyl acetate in petroleum ether). The products 5a–h were obtained in 89–95% yield.
General procedure for the reductive cyclization
Aldehydes 5a–h (11 mmol) were dissolved in glacial acetic acid (20 mL) and heated under reflux with zinc dust (5 equiv) for 0.5 h (TLC, ethyl acetate/petroleum ether 1:9). Acetic acid was evaporated under vacuum, and chloroform was added to the residue. The solution was filtered through a celite bed. CHCl3 was removed under reduced pressure. Water (25 mL) was added to the reaction mixture, and then the aqueous layer was extracted with ethyl acetate (3 × 15 mL), the combined organic layer was dried over sodium sulfate, and ethyl acetate was evaporated under vacuum. The crude product was obtained and purified by silica-gel column chromatography (mobile phase 2–3% ethyl acetate in petroleum ether). The products 6a–h were obtained in 84–87% yield.
Supporting Information
| Supporting Information File 1:
IR, 1H NMR, 13C NMR and CHN analysis and spectral data of synthesized compounds.
The geometric isomeric ratios for 2g and 2h and diastereomeric ratios for 3a–h, 4a–h and 5a–h were calculated from their NMR signals. |
||
| Format: PDF | Size: 2.8 MB | Download |
References
-
Gross, W.; Oberkobusch, D.; Höffkes, H.; Moch, M. Hair Colourants. W.O. Patent 2007/073923, July 5, 2007.
Return to citation in text: [1] -
Allen, D. R.; Buckley, G. M.; Bürli, R.; Davenport, R. J.; Kinsella, N.; Lock, C. J.; Lowe, C.; Mack, S. R.; Pitt, W. R.; Ratcliffe, A. J.; Richard, M. D.; Savin, V. M.; Sharpe, A.; Tait, L. J.; Warrelow, G. J.; Williams, S. C. Quinoxaline and Quinoline Derivatives as Kinase Inhibitors. W.O. Patent 2009/081105, July 2, 2009.
Return to citation in text: [1] -
Schmitt, M.; Klotz, E.; Macher, J.-P.; Bourguignon, J.-J. Compositions Derived from Quinoline and Quinoxaline, Preparation and Use Thereof. W.O. Patent 2003/010146, Feb 6, 2003.
Return to citation in text: [1] -
Chujo, I.; Masuda, Y.; Fujino, K.; Kato, S.; Ogasa, T.; Mohri, S.-i.; Kasai, M. Bioorg. Med. Chem. 2001, 9, 3273–3286. doi:10.1016/S0968-0896(01)00238-3
Return to citation in text: [1] -
Cappelli, A.; Anzini, M.; Vomero, S.; Mennuni, L.; Markovec, F.; Doucet, E.; Hamon, M.; Menziani, M. C.; De Benedetti, P. G.; Giorgi, G.; Ghelardini, C.; Collina, S. Bioorg. Med. Chem. 2002, 10, 779–801. doi:10.1016/S0968-0896(01)00332-7
Return to citation in text: [1] -
Sliskovic, D. R.; Picard, J. A.; Roark, W. H.; Roth, B. D.; Ferguson, E.; Krause, B. R.; Newton, R. S.; Sekerke, C.; Shaw, M. K. J. Med. Chem. 1991, 34, 367–373. doi:10.1021/jm00105a057
Return to citation in text: [1] [2] [3] -
Campiani, G.; Fattorusso, C.; Butini, S.; Gaeta, A.; Agnusdei, M.; Gemma, S.; Persico, M.; Catalanotti, B.; Savini, L.; Nacci, V.; Novellino, E.; Holloway, H. W.; Greig, N. H.; Belinskaya, T.; Fedorko, J. M.; Saxena, A. J. Med. Chem. 2005, 48, 1919–1929. doi:10.1021/jm049510k
Return to citation in text: [1] -
Lyakhova, E. A.; Lyakhov, S. A.; Litvinova, L. A.; Andronati, S. A.; Lebedyuk, M. N.; Fedchuk, V. P.; Khorokhorina, G. A. Pharm. Chem. J. 2005, 39, 183–187. doi:10.1007/s11094-005-0113-0
Return to citation in text: [1] -
Chupak, L. S.; Flanagan, M. E.; Kaneko, T.; Magee, T. V.; Noe, M. C.; Reilly, U. Macrolides. U.S. Patent 2006/0135447, June 22, 2006.
Return to citation in text: [1] [2] [3] -
Da Rocha Pitta, I.; Alves De Lima, M. d. C.; Lins Galdino, S.; Barbe, J. Molecules with Antitumor Activity and Chemical Synthesis. W.O. Patent 2004/024058, March 25, 2004.
Return to citation in text: [1] -
Giannini, G.; Pisano, C.; Vesci, L.; Tinti, M. O.; Merlini, L.; Penco, S.; Zunino, F. 7- Polyaminoalkyl(oxy) Iminomethylcamptothecins Bearing Protective Groups. U.S. Patent 2007/0043067, Feb 22, 2007.
Return to citation in text: [1] -
Pérez-Melero, C.; Maya, A. B. S.; del Rey, B.; Peláez, R.; Caballero, E.; Medarde, M. Bioorg. Med. Chem. Lett. 2004, 14, 3771–3774. doi:10.1016/j.bmcl.2004.04.098
Return to citation in text: [1] -
Ramadas, S. R.; Krishna, M. V. Curr. Sci. 1981, 50, 120–123.
Return to citation in text: [1] -
Kwartler, C. E.; Lindwall, H. G. J. Am. Chem. Soc. 1937, 59, 524–526. doi:10.1021/ja01282a027
Return to citation in text: [1] -
MacDonald, S. F. J. Am. Chem. Soc. 1947, 69, 1219–1220. doi:10.1021/ja01197a511
Return to citation in text: [1] -
Plobeck, N.; Delorme, D.; Wei, Z.-Y.; Yang, H.; Zhou, F.; Schwarz, P.; Gawell, L.; Gagnon, H.; Pelcman, B.; Schmidt, R.; Yue, S. Y.; Walpole, C.; Brown, W.; Zhou, E.; Labarre, M.; Payza, K.; St-Onge, S.; Kamassah, A.; Morin, P.-E.; Projean, D.; Ducharme, J.; Roberts, E. J. Med. Chem. 2000, 43, 3878–3894. doi:10.1021/jm000228x
Return to citation in text: [1] -
Achremowicz, L. Synth. Commun. 1996, 26, 1681–1684. doi:10.1080/00397919608002606
Return to citation in text: [1] -
Nishikawa, T.; Ino, A.; Isobe, M.; Goto, T. Chem. Lett. 1991, 7, 1271–1274. doi:10.1246/cl.1991.1271
Return to citation in text: [1] -
Rodriguez, J. G.; Benito, Y. J. Heterocycl. Chem. 1988, 25, 819–821. doi:10.1002/jhet.5570250323
Return to citation in text: [1] -
Mathes, W.; Sauermilch, W. Chem. Ber. 1954, 87, 1179–1183. doi:10.1002/cber.19540870820
Return to citation in text: [1] -
Ihara, M.; Noguchi, K.; Fukumoto, K.; Kametani, T. Tetrahedron 1985, 41, 2109–2114. doi:10.1016/S0040-4020(01)96581-0
Return to citation in text: [1] -
Clemo, G. R.; Hoggarth, E. J. Chem. Soc. 1939, 1241–1244. doi:10.1039/JR9390001241
Return to citation in text: [1] -
Giordano, C.; Minisci, F.; Vismara, E.; Levi, S. J. Org. Chem. 1986, 51, 536–537. doi:10.1021/jo00354a026
Return to citation in text: [1] -
Vismara, E.; Francesca, F.; Francesco, M. Gazz. Chim. Ital. 1987, 117, 135–136.
Return to citation in text: [1] -
Kulkarni, M. G.; Davawala, S. I.; Dhondge, A. P.; Gaikwad, D. D.; Borhade, A. S.; Chavhan, S. W. Tetrahedron Lett. 2006, 47, 1003–1005. doi:10.1016/j.tetlet.2005.11.134
Return to citation in text: [1] -
Kulkarni, M. G.; Chavhan, S. W.; Desai, M. P.; Shaikh, Y. B.; Gaikwad, D. D.; Dhondge, A. P.; Borhade, A. S.; Ningdale, V. B.; Birhade, D. R.; Dhatrak, N. R. Tetrahedron Lett. 2010, 51, 4494–4496. doi:10.1016/j.tetlet.2010.06.068
Return to citation in text: [1] -
Kulkarni, M. G.; Dhondge, A. P.; Chavhan, S. W.; Borhade, A. S.; Shaikh, Y. B.; Birhade, D. R.; Desai, M. P.; Dhatrak, M. P. Beilstein J. Org. Chem. 2010, 6, 876–879. doi:10.3762/bjoc.6.103
Return to citation in text: [1] -
Kulkarni, M. G.; Pendharkar, D. S.; Rasne, R. M. Tetrahedron Lett. 1997, 38, 1459–1462. doi:10.1016/S0040-4039(97)00057-9
Return to citation in text: [1] -
Kulkarni, M. G.; Davawala, S. I.; Doke, A. K.; Pendharkar, D. S. Synthesis 2004, 17, 2919–2926. doi:10.1055/s-2004-831208
Return to citation in text: [1]
| 1. | Gross, W.; Oberkobusch, D.; Höffkes, H.; Moch, M. Hair Colourants. W.O. Patent 2007/073923, July 5, 2007. |
| 6. | Sliskovic, D. R.; Picard, J. A.; Roark, W. H.; Roth, B. D.; Ferguson, E.; Krause, B. R.; Newton, R. S.; Sekerke, C.; Shaw, M. K. J. Med. Chem. 1991, 34, 367–373. doi:10.1021/jm00105a057 |
| 6. | Sliskovic, D. R.; Picard, J. A.; Roark, W. H.; Roth, B. D.; Ferguson, E.; Krause, B. R.; Newton, R. S.; Sekerke, C.; Shaw, M. K. J. Med. Chem. 1991, 34, 367–373. doi:10.1021/jm00105a057 |
| 9. | Chupak, L. S.; Flanagan, M. E.; Kaneko, T.; Magee, T. V.; Noe, M. C.; Reilly, U. Macrolides. U.S. Patent 2006/0135447, June 22, 2006. |
| 5. | Cappelli, A.; Anzini, M.; Vomero, S.; Mennuni, L.; Markovec, F.; Doucet, E.; Hamon, M.; Menziani, M. C.; De Benedetti, P. G.; Giorgi, G.; Ghelardini, C.; Collina, S. Bioorg. Med. Chem. 2002, 10, 779–801. doi:10.1016/S0968-0896(01)00332-7 |
| 4. | Chujo, I.; Masuda, Y.; Fujino, K.; Kato, S.; Ogasa, T.; Mohri, S.-i.; Kasai, M. Bioorg. Med. Chem. 2001, 9, 3273–3286. doi:10.1016/S0968-0896(01)00238-3 |
| 25. | Kulkarni, M. G.; Davawala, S. I.; Dhondge, A. P.; Gaikwad, D. D.; Borhade, A. S.; Chavhan, S. W. Tetrahedron Lett. 2006, 47, 1003–1005. doi:10.1016/j.tetlet.2005.11.134 |
| 26. | Kulkarni, M. G.; Chavhan, S. W.; Desai, M. P.; Shaikh, Y. B.; Gaikwad, D. D.; Dhondge, A. P.; Borhade, A. S.; Ningdale, V. B.; Birhade, D. R.; Dhatrak, N. R. Tetrahedron Lett. 2010, 51, 4494–4496. doi:10.1016/j.tetlet.2010.06.068 |
| 27. | Kulkarni, M. G.; Dhondge, A. P.; Chavhan, S. W.; Borhade, A. S.; Shaikh, Y. B.; Birhade, D. R.; Desai, M. P.; Dhatrak, M. P. Beilstein J. Org. Chem. 2010, 6, 876–879. doi:10.3762/bjoc.6.103 |
| 2. | Allen, D. R.; Buckley, G. M.; Bürli, R.; Davenport, R. J.; Kinsella, N.; Lock, C. J.; Lowe, C.; Mack, S. R.; Pitt, W. R.; Ratcliffe, A. J.; Richard, M. D.; Savin, V. M.; Sharpe, A.; Tait, L. J.; Warrelow, G. J.; Williams, S. C. Quinoxaline and Quinoline Derivatives as Kinase Inhibitors. W.O. Patent 2009/081105, July 2, 2009. |
| 3. | Schmitt, M.; Klotz, E.; Macher, J.-P.; Bourguignon, J.-J. Compositions Derived from Quinoline and Quinoxaline, Preparation and Use Thereof. W.O. Patent 2003/010146, Feb 6, 2003. |
| 28. | Kulkarni, M. G.; Pendharkar, D. S.; Rasne, R. M. Tetrahedron Lett. 1997, 38, 1459–1462. doi:10.1016/S0040-4039(97)00057-9 |
| 29. | Kulkarni, M. G.; Davawala, S. I.; Doke, A. K.; Pendharkar, D. S. Synthesis 2004, 17, 2919–2926. doi:10.1055/s-2004-831208 |
| 10. | Da Rocha Pitta, I.; Alves De Lima, M. d. C.; Lins Galdino, S.; Barbe, J. Molecules with Antitumor Activity and Chemical Synthesis. W.O. Patent 2004/024058, March 25, 2004. |
| 12. | Pérez-Melero, C.; Maya, A. B. S.; del Rey, B.; Peláez, R.; Caballero, E.; Medarde, M. Bioorg. Med. Chem. Lett. 2004, 14, 3771–3774. doi:10.1016/j.bmcl.2004.04.098 |
| 13. | Ramadas, S. R.; Krishna, M. V. Curr. Sci. 1981, 50, 120–123. |
| 14. | Kwartler, C. E.; Lindwall, H. G. J. Am. Chem. Soc. 1937, 59, 524–526. doi:10.1021/ja01282a027 |
| 15. | MacDonald, S. F. J. Am. Chem. Soc. 1947, 69, 1219–1220. doi:10.1021/ja01197a511 |
| 16. | Plobeck, N.; Delorme, D.; Wei, Z.-Y.; Yang, H.; Zhou, F.; Schwarz, P.; Gawell, L.; Gagnon, H.; Pelcman, B.; Schmidt, R.; Yue, S. Y.; Walpole, C.; Brown, W.; Zhou, E.; Labarre, M.; Payza, K.; St-Onge, S.; Kamassah, A.; Morin, P.-E.; Projean, D.; Ducharme, J.; Roberts, E. J. Med. Chem. 2000, 43, 3878–3894. doi:10.1021/jm000228x |
| 17. | Achremowicz, L. Synth. Commun. 1996, 26, 1681–1684. doi:10.1080/00397919608002606 |
| 18. | Nishikawa, T.; Ino, A.; Isobe, M.; Goto, T. Chem. Lett. 1991, 7, 1271–1274. doi:10.1246/cl.1991.1271 |
| 19. | Rodriguez, J. G.; Benito, Y. J. Heterocycl. Chem. 1988, 25, 819–821. doi:10.1002/jhet.5570250323 |
| 20. | Mathes, W.; Sauermilch, W. Chem. Ber. 1954, 87, 1179–1183. doi:10.1002/cber.19540870820 |
| 21. | Ihara, M.; Noguchi, K.; Fukumoto, K.; Kametani, T. Tetrahedron 1985, 41, 2109–2114. doi:10.1016/S0040-4020(01)96581-0 |
| 22. | Clemo, G. R.; Hoggarth, E. J. Chem. Soc. 1939, 1241–1244. doi:10.1039/JR9390001241 |
| 23. | Giordano, C.; Minisci, F.; Vismara, E.; Levi, S. J. Org. Chem. 1986, 51, 536–537. doi:10.1021/jo00354a026 |
| 24. | Vismara, E.; Francesca, F.; Francesco, M. Gazz. Chim. Ital. 1987, 117, 135–136. |
| 9. | Chupak, L. S.; Flanagan, M. E.; Kaneko, T.; Magee, T. V.; Noe, M. C.; Reilly, U. Macrolides. U.S. Patent 2006/0135447, June 22, 2006. |
| 6. | Sliskovic, D. R.; Picard, J. A.; Roark, W. H.; Roth, B. D.; Ferguson, E.; Krause, B. R.; Newton, R. S.; Sekerke, C.; Shaw, M. K. J. Med. Chem. 1991, 34, 367–373. doi:10.1021/jm00105a057 |
| 9. | Chupak, L. S.; Flanagan, M. E.; Kaneko, T.; Magee, T. V.; Noe, M. C.; Reilly, U. Macrolides. U.S. Patent 2006/0135447, June 22, 2006. |
| 8. | Lyakhova, E. A.; Lyakhov, S. A.; Litvinova, L. A.; Andronati, S. A.; Lebedyuk, M. N.; Fedchuk, V. P.; Khorokhorina, G. A. Pharm. Chem. J. 2005, 39, 183–187. doi:10.1007/s11094-005-0113-0 |
| 7. | Campiani, G.; Fattorusso, C.; Butini, S.; Gaeta, A.; Agnusdei, M.; Gemma, S.; Persico, M.; Catalanotti, B.; Savini, L.; Nacci, V.; Novellino, E.; Holloway, H. W.; Greig, N. H.; Belinskaya, T.; Fedorko, J. M.; Saxena, A. J. Med. Chem. 2005, 48, 1919–1929. doi:10.1021/jm049510k |
| 11. | Giannini, G.; Pisano, C.; Vesci, L.; Tinti, M. O.; Merlini, L.; Penco, S.; Zunino, F. 7- Polyaminoalkyl(oxy) Iminomethylcamptothecins Bearing Protective Groups. U.S. Patent 2007/0043067, Feb 22, 2007. |
© 2012 Kulkarni et al; licensee Beilstein-Institut.
This is an Open Access article under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
The license is subject to the Beilstein Journal of Organic Chemistry terms and conditions: (http://www.beilstein-journals.org/bjoc)