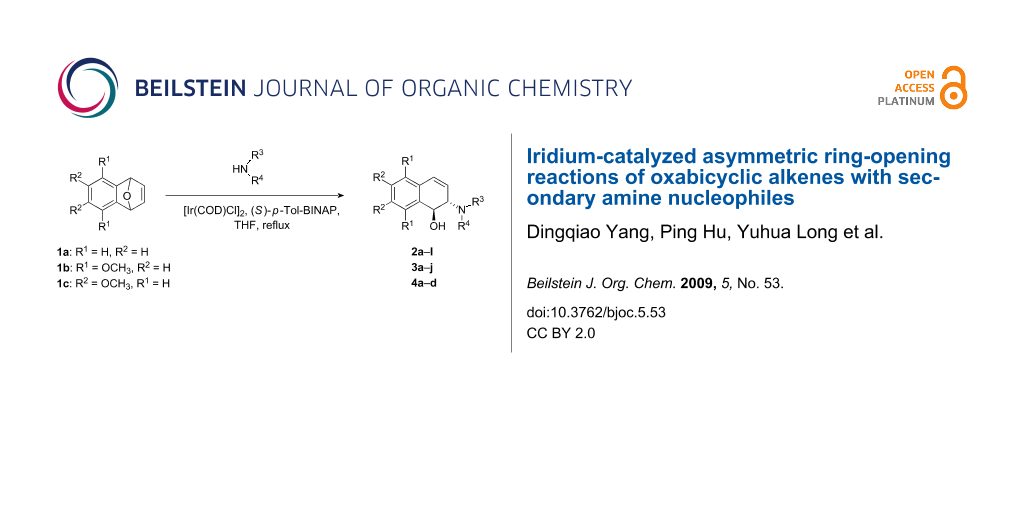
Abstract
Iridium-catalyzed asymmetric ring-opening reactions of oxabicyclic alkenes with various aliphatic and aromatic secondary amines are reported for the first time. The reaction gave the corresponding trans-1,2-dihydronaphthalenol derivatives in good yields with moderate enantioselectivities in the presence of 2.5 mol % [Ir(COD)Cl]2 and 5 mol % bisphosphine ligand (S)-p-Tol-BINAP. The trans-configuration of 3f was confirmed by X-ray crystallography.
Graphical Abstract
Introduction
Substituted dihydronaphthalenes are important molecules with different biological activities [1-5]. Therefore, the synthesis of these molecules has been an attractive research topic in recent years. Among the many reported methods for the preparation of the dihydronaphthalene skeleton, transition metal-catalyzed asymmetric ring-opening (ARO) of oxabicyclic alkenes is one of the most attractive because this reaction could potentially create two chiral centers in a single step.
Pioneering work in this field was first described by Caple et al. [6] and the group of Lautens [7-9]. In the past decades, the group of Lautens and others reported rhodium-catalyzed asymmetric ring-opening of oxabenzonorbornadiene with a wide range of nucleophiles including thiols [10], phenols [11], organoboronic acids [12,13], dialkylzincs [14,15], carboxylates [16], sulfur nucleophiles [17], and various amines [18,19].
In addition to rhodium catalysts, other transition metal catalysts may be used for asymmetric ring-opening reactions of oxabicyclic alkenes. These include complexes of copper [20-25], palladium [14,15,26-31], iron [32], and nickel [33-36]. Recently, we reported for the first time iridium-catalyzed asymmetric ring-opening of N-Boc-azabenzonorbornadiene with a wide range of secondary amines [37].
In this article, we will report ARO reactions of oxabicyclic alkenes with aliphatic and aromatic secondary amine nucleophiles using iridium-complex catalysts, which provide a fast and efficient access to chiral molecules with the dihydronaphthalene skeleton.
Results and Discussion
The ARO reaction involves many components of the chemical agents; we first attempted to optimize the ligand to iridium catalyst system (Scheme 1). In our initial experiments, we chose an achiral 1,1′-bis(diphenylphosphino)ferrocene (DPPF) ligand to validate the catalytic activity of the iridium complex. The product 2a was obtained in high yield (80%) in the presence of 2.5 mol % [Ir(COD)Cl]2 and 5 mol % DPPF in THF after 5 h.
Scheme 1: Identification of optimal chiral ligand for iridium-catalyzed asymmetric ring-opening of oxabenzonorbornadiene 1a with N-methylaniline.
Scheme 1: Identification of optimal chiral ligand for iridium-catalyzed asymmetric ring-opening of oxabenzono...
Encouraged by this result, we then ran the asymmetric version of the same reaction using different kinds of chiral bisphosphine ligands as shown in Scheme 1. We first examined ferrocenyl bisphosphine ligand, (R,S)-PPF-PtBu2, which was identified as the ligand giving the best reactivity and excellent enantioselectivity in Rh-catalyzed system [11-15]. Unfortunately, the desired ring-opened product 2a was obtained only in low yield (47%) with reasonable enantioselectivity (51% ee) in the iridium-catalyzed system. This suggested that (R,S)-PPF-PtBu2 was not an ideal ligand in iridium-catalyzed reactions, which prompted us to screen other ligands. Among the several chiral ligands we had tested, (S)-BINAP and (S)-p-Tol-BINAP were found to give better yields and reasonable enantioselectivities. Moreover, in the case of (S)-p-Tol-BINAP, the enantioselectivity is slightly higher than for (S)-BINAP (52% ee vs 35% ee); therefore, we decided to use (S)-p-Tol-BINAP as the ligand for this ring-opening reaction.
Using (S)-p-Tol-BINAP as our standard ligand, we next investigated the effect of different solvents on reactivity and enantioselectivity (Table 1). Among the solvents examined for asymmetric ring-opening of oxabenzonorbornadiene 1a, THF was found to be the best in terms of yield and enantioselectivity (Table 1, entry 6). Reactions in toluene and dioxane afforded the desired product 2a in 70% and 76% yields, respectively. However, the enantioselectivities were slightly lower (Table 1, entries 3 and 4). On the other hand, temperature had a remarkable impact on reactivity and enantioselectivity. At room temperature, the reaction failed to give the corresponding ring-opened product 2a (Table 1, entry 7). A sluggish reaction was observed at 65 °C (Table 1, entry 8), whereas at a higher temperature, such as 100 °C, the reaction gave a better yield and moderate enantioselectivity (Table 1, entry 9).
Table 1: Screening conditions for iridium-catalyzed asymmetric ring-opening of oxabenzonorbornadiene 1a with N-methylaniline.a
| Entry | Solvent | Temperature (°C) | Time (h) | Yield (%)b | ee (%)c |
|---|---|---|---|---|---|
| 1 | DME | 100 | 12 | 48 | 16 |
| 2 | CH3CN | 90 | 12 | 36 | 21 |
| 3 | Toluene | 110 | 8 | 70 | 34 |
| 4 | Dioxane | 110 | 8 | 76 | 46 |
| 5 | THF | 100 | 12 | 65 | 50 |
| 6 | THF | 80 | 8 | 87 | 52 |
| 7 | THF | 25 | 48 | n.r. | – |
| 8 | THF | 65 | 12 | 58 | 53 |
| 9 | THF | 100 | 5 | 89 | 46 |
aThe reaction was carried out with 1a (0.34 mmol) and 3.0 equiv of N-methylaniline (1.0 mmol) in a solvent (2.0 mL) in the presence of [Ir(COD)Cl]2 (2.5 mol %) and (S)-p-Tol-BINAP (5.0 mol %). bIsolated yields after silica gel column chromatography. cDetermined by HPLC with a Chiralcel AD column.
Based on the above findings, we decided to use the following reaction condition as a standard to run the ARO reactions, which consisted of 2.5 mol % [Ir(COD)Cl]2, 5 mol % (S)-p-Tol-BINAP, and 3.0 equiv of substituted N-alkylaniline in THF at 80 °C. The results of the ARO of oxabenzonorbornadiene 1a are shown in Table 2.
Table 2: Scope of ring-opening of oxabenzonorbornadiene 1a with substituted N-alkylaniline.a
|
|
||||||
| Entry | R3 | R4 | Product | Time (h) | Yield (%)b | ee (%)c |
|---|---|---|---|---|---|---|
| 1 | CH3 | H | 2a | 8 | 87 | 52 |
| 2 | CH2CH3 | H | 2b | 12 | 75 | 56 |
| 3 | CH2CH=CH2 | H | 2c | 24 | 48 | 74 |
| 4 | Cyclohexyl | H | – | 60 | n.r. | – |
| 5 | CH3 | 2-Cl | – | 24 | n.r. | – |
| 6 | CH3 | 3-Cl | 2d | 24 | 56 | 72 |
| 7 | CH3 | 4-Cl | 2e | 12 | 70 | 55 |
| 8 | CH3 | 4-F | 2f | 12 | 65 | 51 |
| 9 | CH3 | 4-Br | 2g | 12 | 71 | 65 |
| 10 | CH3 | 4-NO2 | – | 60 | n.r. | – |
| 11 | CH3 | 4-OCH3 | 2h | 6 | 85 | 50 |
aThe reaction was carried out with 1a (0.34 mmol) and 3.0 equiv of substituted N-alkylaniline (1.0 mmol) in THF (2.0 mL) at 80 °C in the presence of [Ir(COD)Cl]2 (2.5 mol %) and (S)-p-Tol-BINAP (5.0 mol %). bIsolated yields after silica gel column chromatography. cDetermined by HPLC with a Chiralcel AD column.
The results demonstrated that the size of the alkyl group on nitrogen in the nucleophile significantly influenced the reactivity and enantioselectivity. For instance, in the reaction of 1a with N-methylaniline, which has a small group on N-atom, the ring-opening product 2a was obtained in 87% yield and 52% ee (Table 2, entry 1), whereas in the reaction of 1a with N-ethylaniline, which has a larger group on N-atom, the yield decreased to 75%, but the enantioselectivity was slightly increased (Table 2, entry 2). When N-allylaniline was used as a nucleophile, we obtained the product 2c in 48% yield with 74% ee (Table 2, entry 3). The reaction using N-cyclohexylaniline failed to give any ring-opened product because of the steric hindrance of the bulky group (Table 2, entry 4).
We then investigated the effect of substituent groups on the aromatic ring of N-methylaniline. In 4-chloro-N-methylaniline, the reaction gave the desired product 2e in high yield with moderate enantioselectivity (Table 2, entry 7). When 3-chloro-N-methylaniline was used, the corresponding product 2d was obtained in slightly lower yield with slightly higher enantioselectivity (Table 2, entry 6). However, the reaction failed to give the expected product when ortho-substituted N-methylanilines were used (Table 2, entry 5). Various para-substituted N-methylanilines were shown to give the expected products in high yields with moderate enantioselectivities (Table 2, entries 8, 9 and 11). From Table 2, it was also found that the electronic property of the N-methylaniline had a significant impact on the reactivity. Electron-rich amines had higher reactivity than electron-deficient amines (Table 2, entries 1–3, 5, and 10).
To evaluate the scope of the reaction, we also examined various aliphatic secondary amines under optimized reaction conditions, and the results are summarized in Table 3. In most cases, aliphatic secondary amines reacted smoothly with 1a to give the corresponding ring-opened products (2i–l) in high yields (up to 90%) with enantioselectivity ranging between 49% ee and 65% ee (Table 3, entries 5–8). We also found that the halide ions might play an important role in transition metal-catalyzed ARO reactions, in which the reactivity and enantioselectivity could be significantly improved by choosing a suitable halide ion. It was found that the reaction yields and enantioselectivities increased in the order of F<Cl<Br<I (Table 3, entries 2–5). But under the optimized reaction conditions, only a low yield (16%) was obtained without halide ion additives (Table 3, entry 1). Therefore, simply changing the halide ligand on the iridium catalyst from chloride to iodide or bromide leads to improvements in the reactivity and enantioselectivity. On the other hand, the difference in enantioselectivity between the fluoro and the iodo complexes is particularly striking with piperidine as the nucleophile. With the Ir–F catalyst, 2i is formed in only 32% yield and 40% ee. Changing to the Ir–I complex gives 2i in 90% yield and 49% ee (Table 3, entries 2 and 5).
Table 3: ARO of oxabenzonorbornadiene 1a with various aliphatic secondary amines.a
|
|
||||||
| Entry | HNR3R4 | Additiveb | Product | Time (h) | Yield (%)c | ee (%)d |
|---|---|---|---|---|---|---|
| 1 |
|
– | 2i | 24 | 16 | 45 |
| 2 | NH4F | 24 | 32 | 40 | ||
| 3 | NH4Cl | 24 | 45 | 43 | ||
| 4 | NH4Br | 24 | 60 | 45 | ||
| 5 | NH4I | 12 | 90 | 49 | ||
| 6 |
|
NH4I | 2j | 20 | 72 | 65 |
| 7 |
|
NH4I | 2k | 8 | 88 | 53 |
| 8 |
|
NH4I | 2l | 15 | 78 | 56 |
aThe reaction was carried out with 1a (0.34 mmol) and 3.0 equiv of aliphatic secondary amine (1.0 mmol) in THF (2.0 mL) at 80 °C in the presence of [Ir(COD)Cl]2 (2.5 mol %) and (S)-p-Tol-BINAP (5.0 mol %). bAdditive is 1.0 equiv relative to 1a. cIsolated yields after silica gel column chromatography. dDetermined by HPLC with a Chiralcel AD column.
Having examined a wide range of secondary amines, it was found that the sterically hindered nucleophiles enhanced the enantioselectivities (up to 89% ee). Inspired by this observation, we then examined more sterically hindered substrate 1b or 1c in the presence of 2.5 mol % [Ir(COD)Cl]2, 5 mol % (S)-p-Tol-BINAP, and 3.0 equiv of secondary amine nucleophiles in THF, at reflux. The results are summarized in Table 4 and Table 5.
The reactions of substrate 1b, which contained methoxy groups on the 5- and 8-positions, with various secondary amines provided ring-opened products 3a–j in yields from 65% to 83% and enantioselectivities from 41% to 89%. We further found that the substituent groups on the substrate did not apparently influence the yields, whereas the ee values increased, in some cases, to as high as 89% ee (Table 4, entry 6).
Table 4: ARO of oxabenzonorbornadiene 1b with various secondary amines.a
|
|
|||||
| Entry | HNR3R4 | Product | Time (h) | Yield (%)b | ee (%)c |
|---|---|---|---|---|---|
| 1 |
|
3a | 12 | 81 | 76 |
| 2 |
|
3b | 20 | 70 | 81 |
| 3 |
|
3c | 8 | 68 | 43 |
| 4 |
|
3d | 12 | 65 | 45 |
| 5 |
|
3e | 12 | 74 | 54 |
| 6 |
|
3f | 12 | 70 | 89 |
| 7 |
|
3g | 6 | 83 | 41 |
| 8 |
|
3h | 12 | 71 | 63 |
| 9 |
|
3i | 12 | 79 | 54 |
| 10 |
|
3j | 12 | 75 | 56 |
aThe reaction was carried out with 1b (0.34 mmol) and 3.0 equiv of secondary amine (1.0 mmol) in THF (2.0 mL) at reflux in the presence of [Ir(COD)Cl]2 (2.5 mol %) and (S)-p-Tol-BINAP (5.0 mol %). The NH4I additive is 1.0 equiv relative to 1b. bIsolated yields after silica gel column chromatography. cDetermined by HPLC with a Chiralcel AD column.
Table 5: Iridium-catalyzed asymmetric ring-opening reaction of substrate 1c.a
|
|
||||||
| Entry | Nucleophile | Ligand | Product | Time (h) | Yield (%)b | ee (%)c |
|---|---|---|---|---|---|---|
| 1 | 2-Piperazin-1-ylbenzonitrile | (S)-p-Tol-BINAP | 4a | 24 | 70 | 37 |
| 2 | 4-Fluorophenylpiperazine | (S)-p-Tol-BINAP | 4b | 24 | 68 | 49 |
| 3 | 2-Fluorophenylpiperazine | (S)-p-Tol-BINAP | 4c | 24 | 73 | 38 |
| 4 | 1-(4-Methoxyphenyl)piperazine | (S)-p-Tol-BINAP | 4d | 24 | 62 | 59 |
| 5 | 3,4-Dichlorophenylpiperazine | (S)-p-Tol-BINAP | 4e | 24 | 27 | 16 |
aConditions: [Ir(COD)Cl]2 (2.5 mol %) and ligand (5.0 mol %) were dissolved in THF and stirred for 10–20 min. Then NH4I was added and the mixture stirred for another 10–20 min. The substrate 1c was added and the mixture heated to reflux. The nucleophiles were added on the first sign of reflux. bIsolated yields after silica gel column chromatography. cDetermined by HPLC with a Chiralcel AD column or AD column.
The absolute configuration of ring-opened product 3f was demonstrated by X-ray crystallography. The single crystal was obtained by evaporation of the solvent from its solution in dichloromethane and petroleum ether. Its configuration was assigned as (1S,2S), and the hydroxyl group and 4-bromo-N-methylaniline group are in a trans relationship (Figure 1).
Based on our studies in this article, the reaction mechanism is proposed as shown in Scheme 2. The chiral dimeric iridium complex A is first formed. The dimer A is cleaved by solvation (THF) to become monomer B. The oxygen atom and the double bond of oxabenzonorbornadiene 1a are then reversibly coordinated to the iridium center of the catalyst to give the intermediate C. Oxidative insertion of C in to the C–O bond forms D. Then, attack of the secondary amine nucleophile along with configurational inversion is proposed to occur in an SN2′ displacement of the iridium catalyst. The trans-1,2-dihydronaphthalenol product 2 is subsequently released and the iridium monomer B is regenerated.
Scheme 2: Proposed mechanism for the ARO of oxabenzonorbornadiene 1a with secondary amine nucleophiles.
Scheme 2: Proposed mechanism for the ARO of oxabenzonorbornadiene 1a with secondary amine nucleophiles.
Conclusion
In summary, we have explored the iridium-catalyzed ARO reaction of oxabicyclic alkenes with N-alkylated anilines or N-substituted piperazine nucleophiles; the reactions gave the desired products in moderate to good yields with good enantioselectivities. The iridium-catalyzed ARO reactions described in this article featured lower cost compared with rhodium-catalyzed ARO reactions, which provided potential applications in asymmetric synthesis of chiral building blocks. The 1,2-trans-configuration of the product was confirmed by X-ray crystallography. The search for an optimized ligand aiming to enhance enantioselectivity and the extension of this reaction to other types of substrates and nucleophiles are currently under investigation in our labs.
Experimental
General procedure (I) for the asymmetric ring-opening reactions of oxabenzonorbornadiene 1a with substituted N-alkylaniline: A 5.0 mL round-bottom flask fitted with a reflux condenser was flame-dried under a stream of nitrogen and cooled to room temperature. [Ir(COD)Cl]2 (5.8 mg, 2.5 mol %) and (S)-p-Tol-BINAP (10.7 mg, 5 mol %) were simultaneously added, followed by an addition of anhydrous tetrahydrofuran (2.0 mL). After they were stirred for about 10 min, oxabenzonorbornadiene 1a (50.0 mg, 0.347 mmol) was added and the resulting mixture was heated to reflux. On the first sign of reflux, nucleophile (3.0 equiv to 1a) was added. The temperature was continuously increased to 80 °C until the reaction was complete as determined by thin layer chromatography. The reaction mixture was then concentrated in vacuo and purified by column chromatography (silica gel: 200–300 mesh) to give the target product.
(1S,2S)-2-[Methyl(phenyl)amino]-1,2-dihydronaphthalen-1-ol (2a): Following the general procedure (I), 2a was obtained as a colorless oil (76.0 mg, 87%). Rf = 0.17 on silica gel (ethyl acetate:petroleum ether = 1:20, v/v). The ee was determined to be 52% using HPLC analysis on a Chiralcel AD column (hexane/2-propanol = 95/5, 0.5 mL/min, λ = 254 nm); Retention times were 32.9 min (major) and 36.5 min (minor). [α]D20 +110.8 (c 1.00, CHCl3). IR (KBr, cm−1): 3514(s), 2980(s), 2869(s), 1645(w), 1599(w), 1500(s), 1381(s), 1297(m), 1136(s), 934(m), 845(m), 794(m). 1H NMR (400 MHz, CDCl3): δ 7.54 (d, J = 4.8 Hz, 1H), 7.28–7.23 (m, 4H), 7.13–7.10 (m, 1H), 6.96 (d, J = 8.4 Hz, 2H), 6.80 (t, J = 7.2 Hz, 1H), 6.58 (d, J = 10.0 Hz, 1H), 5.94–5.90 (m, 1H), 5.10 (d, J = 9.6 Hz, 1H), 4.73 (dd, J = 2.4, 2.4 Hz, 1H), 2.84 (d, J = 2.4 Hz, 3H), 2.38 (br s, 1H). 13C NMR (100 MHz, CDCl3): δ 150.2, 136.5, 131.9, 129.7, 129.2, 128.1, 127.9, 127.7, 126.4, 125.6, 118.1, 114.7, 70.0, 63.5, 33.4. MS (ESI) m/z Calcd for C17H17NO (M+): 251.13; Found: 251.83. Anal. Calcd for C17H17NO: C, 81.24; H, 6.82; N, 5.57. Found: C, 81.52; H, 6.92; N, 5.73.
See Supporting Information for details of the syntheses of the new compounds 2b-l, 3a-j, and 4a-d.
Supporting Information
Experimental procedures and full characterization data for all the new compounds including optical rotations, IR, 1H NMR and 13C NMR, MS and elemental analysis are provided in the Supporting Information. In addition, X-ray structure data for compound 3f are given.
| Supporting Information File 1: Experimental and analytical data | ||
| Format: DOC | Size: 2.1 MB | Download |
References
-
Johnson, B. M.; Chang, P. T. L. Analytical Profiles of Drug Substances and Excipients; 1996; Vol. 24, p 443.
(For reviews.)
Return to citation in text: [1] -
Snyder, S. E.; Aviles-Garay, F. A.; Chakraborti, R.; Nichols, D. E.; Watts, V. J.; Mailman, R. B. J. Med. Chem. 1995, 38, 2395–2409. doi:10.1021/jm00013a015
Return to citation in text: [1] -
Kamal, A.; Gayatri, L. Tetrahedron Lett. 1996, 37, 3359–3362. doi:10.1016/0040-4039(96)00546-1
Return to citation in text: [1] -
Kim, K.; Guo, Y.; Sulikowski, G. A. J. Org. Chem. 1995, 60, 6866–6871. doi:10.1021/jo00126a043
Return to citation in text: [1] -
Perrone, R.; Berardi, F.; Colabufo, N. A.; Leopoldo, M.; Tortorella, V.; Fiorentini, F.; Olgiati, V.; Ghiglieri, A.; Govoni, S. J. Med. Chem. 1995, 38, 942–949. doi:10.1021/jm00006a013
Return to citation in text: [1] -
Caple, R.; Chen, G. M. S.; Nelson, J. D. J. Org. Chem. 1971, 36, 2874–2876. doi:10.1021/jo00818a032
Return to citation in text: [1] -
Lautens, M. Synlett 1993, 177–185. doi:10.1055/s-1993-22393
Return to citation in text: [1] -
Lautens, M.; Colucci, J. T.; Hiebert, S.; Smith, N. D.; Bouchain, G. Org. Lett. 2002, 4, 1879–1882. doi:10.1021/ol025872f
Return to citation in text: [1] -
Lautens, M.; Fagnou, K.; Hiebert, S. Acc. Chem. Res. 2003, 36, 48–58. doi:10.1021/ar010112a
Return to citation in text: [1] -
Bates, C. G.; Gujadhur, R. K.; Venkataraman, D. Org. Lett. 2002, 4, 2803–2806. doi:10.1021/ol0264105
Return to citation in text: [1] -
Lautens, M.; Fagnou, K.; Taylor, M. Org. Lett. 2000, 2, 1677–1679. doi:10.1021/ol005729r
Return to citation in text: [1] [2] -
Lautens, M.; Dockendorff, C.; Fagnou, K.; Malicki, A. Org. Lett. 2002, 4, 1311–1314. doi:10.1021/ol0256062
Return to citation in text: [1] [2] -
Murakami, M.; Igawa, H. Chem. Commun. 2002, 390–391. doi:10.1039/b108808d
Return to citation in text: [1] [2] -
Lautens, M.; Hiebert, S.; Renaud, J.-L. Org. Lett. 2000, 2, 1971–1973. doi:10.1021/ol006052a
Return to citation in text: [1] [2] [3] -
Lautens, M.; Renaud, J.-L.; Hiebert, S. J. Am. Chem. Soc. 2000, 122, 1804–1805. doi:10.1021/ja993427i
Return to citation in text: [1] [2] [3] -
Lautens, M.; Fagnou, K. Tetrahedron 2001, 57, 5067–5072. doi:10.1016/S0040-4020(01)00351-9
Return to citation in text: [1] -
Leong, P.; Lautens, M. J. Org. Chem. 2004, 69, 2194–2196. doi:10.1021/jo035730e
Return to citation in text: [1] -
Lautens, M.; Fagnou, K.; Yang, D.-Q. J. Am. Chem. Soc. 2003, 125, 14884–14892. doi:10.1021/ja034845x
Return to citation in text: [1] -
Webster, R.; Böing, C.; Lautens, M. J. Am. Chem. Soc. 2009, 131, 444–445. doi:10.1021/ja807942m
Return to citation in text: [1] -
López, F.; Harutyunyan, S. R.; Minnaard, A. J.; Feringa, B. L. J. Am. Chem. Soc. 2004, 126, 12784–12785. doi:10.1021/ja046632t
Return to citation in text: [1] -
López, F.; van Zijl, A. W.; Minnaard, A. J.; Feringa, B. L. Chem. Commun. 2006, 409–411. doi:10.1039/b513887f
Return to citation in text: [1] -
Tissot-Croset, K.; Polet, D.; Alexakis, A. Angew. Chem., Int. Ed. 2004, 43, 2426–2428. doi:10.1002/anie.200353744
Return to citation in text: [1] -
Falciola, C. A.; Tissot-Croset, K.; Alexakis, A. Angew. Chem., Int. Ed. 2006, 45, 5995–5998. doi:10.1002/anie.200601855
Return to citation in text: [1] -
Arrayás, R. G.; Cabrera, S.; Carretero, J. C. Org. Lett. 2003, 5, 1333–1336. doi:10.1021/ol034283m
Return to citation in text: [1] -
Bertozzi, F.; Pineschi, M.; Macchia, F.; Arnold, L. A.; Minnaard, A. J.; Feringa, B. L. Org. Lett. 2002, 4, 2703–2705. doi:10.1021/ol026220u
Return to citation in text: [1] -
Lautens, M.; Dockendorff, C. Org. Lett. 2003, 5, 3695–3698. doi:10.1021/ol035369i
Return to citation in text: [1] -
Li, M.; Yan, X.-X.; Hong, W.; Zhu, X.-Z.; Cao, B.-X.; Sun, J.; Hou, X.-L. Org. Lett. 2004, 6, 2833–2835. doi:10.1021/ol048816i
Return to citation in text: [1] -
Zhang, T.-K.; Mo, D.-L.; Dai, L.-X.; Hou, X.-L. Org. Lett. 2008, 10, 3689–3692. doi:10.1021/ol801294b
Return to citation in text: [1] -
Cabrera, S.; Arrayás, R. G.; Carretero, J. C. Angew. Chem. 2004, 116, 4034–4037. doi:10.1002/ange.200460087
Return to citation in text: [1] -
Chen, C.-L.; Martin, S. F. Org. Lett. 2004, 6, 3581–3584. doi:10.1021/ol048517t
Return to citation in text: [1] -
Imamoto, T.; Saitoh, Y.; Koide, A.; Ogura, T.; Yoshida, K. Angew. Chem. 2007, 119, 8790–8793. doi:10.1002/ange.200702513
Return to citation in text: [1] -
Nakamura, M.; Matsuo, K.; Inoue, T.; Nakamura, E. Org. Lett. 2003, 5, 1373–1375. doi:10.1021/ol034375b
Return to citation in text: [1] -
Lautens, M.; Chiu, P.; Ma, S.; Rovis, T. J. Am. Chem. Soc. 1995, 117, 532–533. doi:10.1021/ja00106a062
Return to citation in text: [1] -
Lautens, M.; Ma, S. J. Org. Chem. 1996, 61, 7246–7247. doi:10.1021/jo961615a
Return to citation in text: [1] -
Lautens, M.; Rovis, T. J. Org. Chem. 1997, 62, 5246–5247. doi:10.1021/jo971115x
Return to citation in text: [1] -
Rayabarapu, D. K.; Cheng, C.-H. Chem.–Eur. J. 2003, 9, 3164–3169. doi:10.1002/chem.200204506
Return to citation in text: [1] -
Yang, D.-Q.; Long, Y.-H.; Wang, H.; Zhang, Z.-M. Org. Lett. 2008, 10, 4723–4726. doi:10.1021/ol801768e
Return to citation in text: [1]
| 1. |
Johnson, B. M.; Chang, P. T. L. Analytical Profiles of Drug Substances and Excipients; 1996; Vol. 24, p 443.
(For reviews.) |
| 2. | Snyder, S. E.; Aviles-Garay, F. A.; Chakraborti, R.; Nichols, D. E.; Watts, V. J.; Mailman, R. B. J. Med. Chem. 1995, 38, 2395–2409. doi:10.1021/jm00013a015 |
| 3. | Kamal, A.; Gayatri, L. Tetrahedron Lett. 1996, 37, 3359–3362. doi:10.1016/0040-4039(96)00546-1 |
| 4. | Kim, K.; Guo, Y.; Sulikowski, G. A. J. Org. Chem. 1995, 60, 6866–6871. doi:10.1021/jo00126a043 |
| 5. | Perrone, R.; Berardi, F.; Colabufo, N. A.; Leopoldo, M.; Tortorella, V.; Fiorentini, F.; Olgiati, V.; Ghiglieri, A.; Govoni, S. J. Med. Chem. 1995, 38, 942–949. doi:10.1021/jm00006a013 |
| 11. | Lautens, M.; Fagnou, K.; Taylor, M. Org. Lett. 2000, 2, 1677–1679. doi:10.1021/ol005729r |
| 37. | Yang, D.-Q.; Long, Y.-H.; Wang, H.; Zhang, Z.-M. Org. Lett. 2008, 10, 4723–4726. doi:10.1021/ol801768e |
| 10. | Bates, C. G.; Gujadhur, R. K.; Venkataraman, D. Org. Lett. 2002, 4, 2803–2806. doi:10.1021/ol0264105 |
| 11. | Lautens, M.; Fagnou, K.; Taylor, M. Org. Lett. 2000, 2, 1677–1679. doi:10.1021/ol005729r |
| 12. | Lautens, M.; Dockendorff, C.; Fagnou, K.; Malicki, A. Org. Lett. 2002, 4, 1311–1314. doi:10.1021/ol0256062 |
| 13. | Murakami, M.; Igawa, H. Chem. Commun. 2002, 390–391. doi:10.1039/b108808d |
| 14. | Lautens, M.; Hiebert, S.; Renaud, J.-L. Org. Lett. 2000, 2, 1971–1973. doi:10.1021/ol006052a |
| 15. | Lautens, M.; Renaud, J.-L.; Hiebert, S. J. Am. Chem. Soc. 2000, 122, 1804–1805. doi:10.1021/ja993427i |
| 7. | Lautens, M. Synlett 1993, 177–185. doi:10.1055/s-1993-22393 |
| 8. | Lautens, M.; Colucci, J. T.; Hiebert, S.; Smith, N. D.; Bouchain, G. Org. Lett. 2002, 4, 1879–1882. doi:10.1021/ol025872f |
| 9. | Lautens, M.; Fagnou, K.; Hiebert, S. Acc. Chem. Res. 2003, 36, 48–58. doi:10.1021/ar010112a |
| 32. | Nakamura, M.; Matsuo, K.; Inoue, T.; Nakamura, E. Org. Lett. 2003, 5, 1373–1375. doi:10.1021/ol034375b |
| 6. | Caple, R.; Chen, G. M. S.; Nelson, J. D. J. Org. Chem. 1971, 36, 2874–2876. doi:10.1021/jo00818a032 |
| 33. | Lautens, M.; Chiu, P.; Ma, S.; Rovis, T. J. Am. Chem. Soc. 1995, 117, 532–533. doi:10.1021/ja00106a062 |
| 34. | Lautens, M.; Ma, S. J. Org. Chem. 1996, 61, 7246–7247. doi:10.1021/jo961615a |
| 35. | Lautens, M.; Rovis, T. J. Org. Chem. 1997, 62, 5246–5247. doi:10.1021/jo971115x |
| 36. | Rayabarapu, D. K.; Cheng, C.-H. Chem.–Eur. J. 2003, 9, 3164–3169. doi:10.1002/chem.200204506 |
| 17. | Leong, P.; Lautens, M. J. Org. Chem. 2004, 69, 2194–2196. doi:10.1021/jo035730e |
| 20. | López, F.; Harutyunyan, S. R.; Minnaard, A. J.; Feringa, B. L. J. Am. Chem. Soc. 2004, 126, 12784–12785. doi:10.1021/ja046632t |
| 21. | López, F.; van Zijl, A. W.; Minnaard, A. J.; Feringa, B. L. Chem. Commun. 2006, 409–411. doi:10.1039/b513887f |
| 22. | Tissot-Croset, K.; Polet, D.; Alexakis, A. Angew. Chem., Int. Ed. 2004, 43, 2426–2428. doi:10.1002/anie.200353744 |
| 23. | Falciola, C. A.; Tissot-Croset, K.; Alexakis, A. Angew. Chem., Int. Ed. 2006, 45, 5995–5998. doi:10.1002/anie.200601855 |
| 24. | Arrayás, R. G.; Cabrera, S.; Carretero, J. C. Org. Lett. 2003, 5, 1333–1336. doi:10.1021/ol034283m |
| 25. | Bertozzi, F.; Pineschi, M.; Macchia, F.; Arnold, L. A.; Minnaard, A. J.; Feringa, B. L. Org. Lett. 2002, 4, 2703–2705. doi:10.1021/ol026220u |
| 16. | Lautens, M.; Fagnou, K. Tetrahedron 2001, 57, 5067–5072. doi:10.1016/S0040-4020(01)00351-9 |
| 14. | Lautens, M.; Hiebert, S.; Renaud, J.-L. Org. Lett. 2000, 2, 1971–1973. doi:10.1021/ol006052a |
| 15. | Lautens, M.; Renaud, J.-L.; Hiebert, S. J. Am. Chem. Soc. 2000, 122, 1804–1805. doi:10.1021/ja993427i |
| 26. | Lautens, M.; Dockendorff, C. Org. Lett. 2003, 5, 3695–3698. doi:10.1021/ol035369i |
| 27. | Li, M.; Yan, X.-X.; Hong, W.; Zhu, X.-Z.; Cao, B.-X.; Sun, J.; Hou, X.-L. Org. Lett. 2004, 6, 2833–2835. doi:10.1021/ol048816i |
| 28. | Zhang, T.-K.; Mo, D.-L.; Dai, L.-X.; Hou, X.-L. Org. Lett. 2008, 10, 3689–3692. doi:10.1021/ol801294b |
| 29. | Cabrera, S.; Arrayás, R. G.; Carretero, J. C. Angew. Chem. 2004, 116, 4034–4037. doi:10.1002/ange.200460087 |
| 30. | Chen, C.-L.; Martin, S. F. Org. Lett. 2004, 6, 3581–3584. doi:10.1021/ol048517t |
| 31. | Imamoto, T.; Saitoh, Y.; Koide, A.; Ogura, T.; Yoshida, K. Angew. Chem. 2007, 119, 8790–8793. doi:10.1002/ange.200702513 |
| 14. | Lautens, M.; Hiebert, S.; Renaud, J.-L. Org. Lett. 2000, 2, 1971–1973. doi:10.1021/ol006052a |
| 15. | Lautens, M.; Renaud, J.-L.; Hiebert, S. J. Am. Chem. Soc. 2000, 122, 1804–1805. doi:10.1021/ja993427i |
| 12. | Lautens, M.; Dockendorff, C.; Fagnou, K.; Malicki, A. Org. Lett. 2002, 4, 1311–1314. doi:10.1021/ol0256062 |
| 13. | Murakami, M.; Igawa, H. Chem. Commun. 2002, 390–391. doi:10.1039/b108808d |
| 18. | Lautens, M.; Fagnou, K.; Yang, D.-Q. J. Am. Chem. Soc. 2003, 125, 14884–14892. doi:10.1021/ja034845x |
| 19. | Webster, R.; Böing, C.; Lautens, M. J. Am. Chem. Soc. 2009, 131, 444–445. doi:10.1021/ja807942m |
© 2009 Yang et al; licensee Beilstein-Institut.
This is an Open Access article under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
The license is subject to the Beilstein Journal of Organic Chemistry terms and conditions: (http://www.beilstein-journals.org/bjoc)