Abstract
A series of pyridine ether PPAR agonists were synthesized through an ADDP and PS-PPh3 modified Mitsunobu protocol, which eliminated significant by-product formation. This method proved to be versatile, efficient and amenable to parallel synthesis.
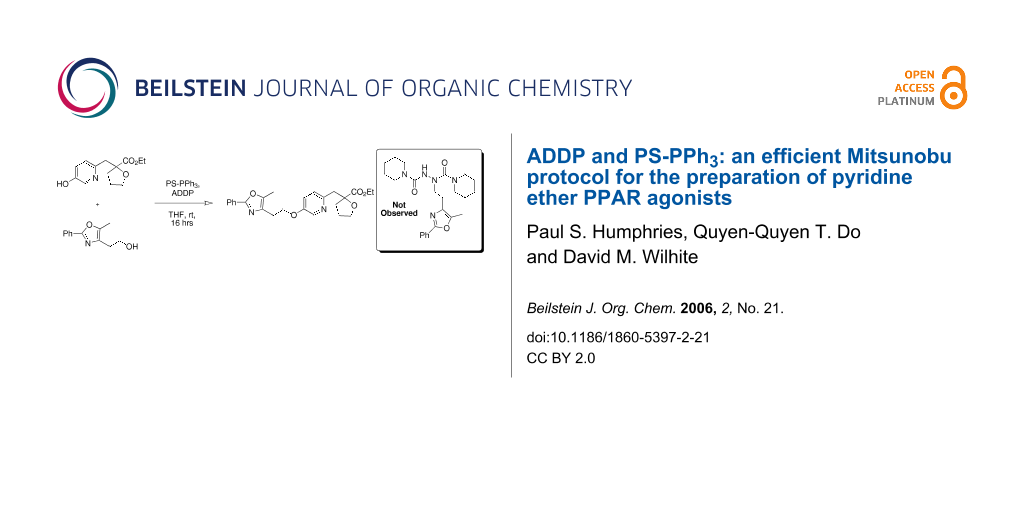
Graphical Abstract
Findings
Peroxisome proliferator-activated receptors (PPARs) are pharmaceutical targets of great importance. Their wide-ranging effects on key transcriptional pathways for lipid handling, insulin sensitivity, inflammation and other functions have led to marketed drugs and vast clinical and preclinical research efforts.[1-11]
In 1991, a series of PPAR analogues were disclosed, which for the first time did not contain a thiazolidine-2,4-dione pharmacophore.[12] These were propanoic acid derivatives with α-substitution to collectively serve as a mimic for the thiazolidine-2,4-dione ring. Based on the above and a knowledge of PPAR ligands publicly disclosed, we wished to synthesize compounds represented by the general structure 1 (Figure 1). Aromatic ethers are structural motifs found in many naturally occurring molecules and compounds of medicinal interest.[13] We envisaged the pyridyl ether moiety of 1 to be efficiently formed via Mitsunobu coupling of the requisite pyridinol and alkyl alcohols.[14-17]
Figure 1: Thiazolidine-2,4-dione mimic & chosen lead scaffold.
Figure 1: Thiazolidine-2,4-dione mimic & chosen lead scaffold.
Our first attempt at the Mitsunobu reaction between pyridinol 2 and alcohol 3, utilizing a modification of the conditions originally reported by Mitsunobu,[18] afforded pyridyl ether 4 in 54% yield (Scheme 1). Interestingly, the reaction did not reach completion and pyridinol 2 was recovered, despite the fact that it was the limiting reagent. Upon closer examination, compound 5 was observed as a major by-product (46% based on 3).
Scheme 1: Reagents and conditions: (a) PS-PPh3, DEAD, THF, rt, 16 h, 54%.
Scheme 1: Reagents and conditions: (a) PS-PPh3, DEAD, THF, rt, 16 h, 54%.
By-products analogous to 5 have been observed in the literature when diethyl azodicarboxylate (DEAD) is used in certain Mitsunobu reactions.[18,19] This by-product formation is believed to be dependent on the pKa of the acidic component (e.g. 2).[18] If the phenol has a pKa > 11, the yield is considerably lower; and with the phenol having pKa > 13, the desired reaction does not occur. The hydrazo anion 6, in these cases, attacks the alkoxyphosphonium directly to afford alkylated hydrazine derivative 7 as the by-product (side reaction in Figure 2),[19] since anion 6 is not efficient in deprotonating the weakly acidic phenol.
Figure 2: Proposed mechanism of the Mitsunobu reaction.
Figure 2: Proposed mechanism of the Mitsunobu reaction.
One way to improve the above redox system is to enhance the basicity of anion 6 by the replacement of the alkoxy group OEt in DEAD with strong electron donating groups such as NR2.[16] Thus, 1,1'-(azodicarbonyl)dipiperidine (ADDP),[20] 4,7-dimethyl-3,5,7-hexahydro-1,2,4,7-tetrazocin-3,8-dione (DHTD),[21] and N,N,N',N'-tetramethylazodicarboxamide (TMAD)[22] have been developed as new reagents in combination with tributyl phosphine (TBP).
We initially chose ADDP, due to its commercial availability and low cost. The original reference utilized ADDP and TBP in benzene,[20] but due to safety and ease of handling we chose to keep PS-PPh3 and THF. Our first attempt was successful and no by-product 8 was observed in the reaction (Scheme 2) [see Supporting Information File 1 and Supporting Information File 2]. With the above result in hand, we then pursued a variety of targets by performing the modified Mitsunobu reaction of 2 and a variety of primary alcohols (Table 1). In general, a variety of diverse alcohols afforded the expected products in excellent yield. As expected, oxazoles, thiazoles, pyrazoles, and pyridines are tolerated in this chemistry. In a limited number of cases, functionality (e.g. basic amines, benzimidazoles, indoles, etc.) caused no reaction to occur and only recovered starting materials were isolated (data not shown).
Scheme 2: Reagents and conditions: (a) PS-PPh3, ADDP, THF, rt, 16 h, 81%.
Scheme 2: Reagents and conditions: (a) PS-PPh3, ADDP, THF, rt, 16 h, 81%.
Table 1: Modified Mitsunobu coupling of pyridinol 2 and a variety of primary alcoholsa
|
|
|||||||
| Entry | Alcohol | Product | Yield (%) | Entry | Alcohol | Product | Yield (%) |
|---|---|---|---|---|---|---|---|
| 1 |
|
9 | 81 | 7 |
|
15 | 79 |
| 2 |
|
10 | 78 | 8 |
|
16 | 85 |
| 3 |
|
11 | 80 | 9 |
|
17 | 77 |
| 4 |
|
12 | 83 | 10 |
|
18 | 80 |
| 5 |
|
13 | 71 | 11 |
|
19 | 72 |
| 6 |
|
14 | 81 | 12 |
|
20 | 76 |
a Reactions were run using 0.5 mmol pyridinol, 0.55 mmol alcohol, 0.75 mmol PS-PPh3, 0.75 mmol ADDP and 5.5 mL tetrahydrofuran.
We then shifted our attention to variation of the pyridinol, whilst holding constant the 2-(5-methyl-2-phenyl-1,3-oxazol-4-yl)ethanol reactant 3 (Table 2). As expected, variation of the 2-substituent of the pyridine ring resulted in equally high yields.
Table 2: Modified Mitsunobu coupling of alcohol 3 and a variety of pyridinolsa
|
|
|||||||
| Entry | Pyridinol | Product | Yield (%) | Entry | Pyridinol | Product | Yield (%) |
|---|---|---|---|---|---|---|---|
| 1 |
|
21 | 86 | 3 |
|
23 | 84 |
| 2 |
|
22 | 95 | 4 |
|
24 | 78 |
a Reactions were run using 0.5 mmol pyridinol, 0.55 mmol alcohol, 0.75 mmol PS-PPh3, 0.75 mmol ADDP and 5.5 mL tetrahydrofuran.
Having efficiently synthesized a diverse set of intermediate esters, we then sought an expedient method for obtaining the final carboxylic acids. We opted for a microwave-assisted procedure for this basic hydrolysis step. As shown in Scheme 3, the carboxylic acids (e.g. 25) could be obtained in a matter of minutes [see Supporting Information File 1 and Supporting Information File 2]. The significant reduction in reaction time resulted in a productivity enhancement due to increased sample processing. A number of the other esters described in this manuscript were also subjected to these conditions and all afforded the pure carboxylic acids in 82–100% yield.
Scheme 3: Reagents and conditions: (a) 1 N aq. NaOH, MeCN, 100°C μW, 10 mins, 96%.
Scheme 3: Reagents and conditions: (a) 1 N aq. NaOH, MeCN, 100°C μW, 10 mins, 96%.
In summary, we have developed a modified Mitsunobu protocol utilizing 1,1'-(azodicarbonyl)dipiperidine (ADDP) and polymer-supported triphenylphosphine (PS-PPh3). Employment of this method resulted in a rapid, convenient, and high-yielding two step protocol for the preparation of PPAR agonists. In particular, the modified Mitsunobu coupling of pyridinols and alcohols proved to be versatile, efficient and amenable to parallel synthesis. A full account of the medicinal chemistry of these compounds will be given elsewhere.
Supporting Information
| Supporting Information File 1: Supporting Information. Experimental procedures and data for all novel compounds described in this manuscript. | ||
| Format: DOC | Size: 522.0 KB | Download |
| Supporting Information File 2: Auxiliary Data. Auxiliary data for all novel compounds described in this manuscript. | ||
| Format: TIFF | Size: 1.2 MB | Download |
References
-
Ram, V. J. Drugs Today 2003, 39, 609–632. doi:10.1358/dot.2003.39.8.799408
Return to citation in text: [1] -
Sternbach, D. D. Annu. Rep. Med. Chem. 2003, 38, 71–80.
Return to citation in text: [1] -
Miller, A. R.; Etgen, G. J. Expert Opin. Invest. Drugs 2003, 12, 1489–1500. doi:10.1517/13543784.12.9.1489
Return to citation in text: [1] -
Willson, T. M.; Brown, P. J.; Sternbach, D. D.; Henke, B. R. J. Med. Chem. 2000, 43, 527–550. doi:10.1021/jm990554g
Return to citation in text: [1] -
van Raalte, D. H.; Li, M.; Pritchard, P. H.; Wasan, K. M. Pharm. Res. 2004, 21, 1531–1538. doi:10.1023/B:PHAM.0000041444.06122.8d
Return to citation in text: [1] -
Miyachi, H. Expert Opin. Ther. Pat. 2004, 14, 607–618.
Return to citation in text: [1] -
Henke, B. R. Prog. Med. Chem. 2004, 42, 1–53.
Return to citation in text: [1] -
Fajas, L.; Auwerx, J. Peroxisome proliferator-activated receptor γ and the transcriptional control of adipogenesis and metabolism. In Handbook of Obesity: Etiology and Pathophysiology; Bray, G. A.; Bouchard, C., Eds.; Marcel Dekker: New York, 2004; pp 559–587.
Return to citation in text: [1] -
Evans, R. M.; Barish, G. D.; Wang, Y.-X. Nat. Med. 2004, 10, 355–361. doi:10.1038/nm1025
Return to citation in text: [1] -
Pershadsingh, H. A. Expert Opin. Invest. Drugs 2004, 13, 215–228.
Return to citation in text: [1] -
Tan, N. S.; Michalik, L.; Desvergne, B.; Wahli, W. Expert Opin. Ther. Targets 2004, 8, 39–48.
Return to citation in text: [1] -
Hulin, B. 3-Aryl-2-hydroxypropionic acid derivatives and analogs as hypoglycemic agents. PCT Int. Appl. WO 91/19702 A1, Dec 26, 1991.
Chem. Abstr., 1992, 117, 26552r.
Return to citation in text: [1] -
Czarnik, A. W. Acc. Chem. Res. 1996, 29, 112–113. doi:10.1021/ar950256n
Return to citation in text: [1] -
Mitsunobu, O. Synthesis 1981, 1–28. doi:10.1055/s-1981-29317
Return to citation in text: [1] -
Hughes, D. L. Org. React. 1992, 42, 335–656.
Return to citation in text: [1] -
Ito, S.; Tsunoda, T. Pure Appl. Chem. 1999, 71, 1053–1057. doi:10.1351/pac199971061053
A brief review.
Return to citation in text: [1] [2] -
Valentine, D. H.; Hillhouse, J. H. Synthesis 2003, 317–334.
Return to citation in text: [1] -
Wada, M.; Mitsunobu, O. Tetrahedron Lett. 1972, 13, 1279–1282. doi:10.1016/S0040-4039(01)84567-6
Return to citation in text: [1] [2] [3] -
von Itzstein, M.; Jenkins, I. D. J. Chem. Soc., Perkin Trans. 1 1986, 437–445. doi:10.1039/p19860000437
Return to citation in text: [1] [2] -
Tsunoda, T.; Yamamiya, Y.; Ito, S. Tetrahedron Lett. 1993, 34, 1639–1642. doi:10.1016/0040-4039(93)85029-V
Return to citation in text: [1] [2] -
Tsunoda, T.; Kawamura, Y.; Uemoto, K.; Ito, S. Heterocycles 1998, 47, 177–179.
Return to citation in text: [1] -
Tsunoda, T.; Otsuka, J.; Yamamiya, Y.; Ito, S. Chem. Lett. 1994, 539–542. doi:10.1246/cl.1994.539
Return to citation in text: [1]
| 1. | Ram, V. J. Drugs Today 2003, 39, 609–632. doi:10.1358/dot.2003.39.8.799408 |
| 2. | Sternbach, D. D. Annu. Rep. Med. Chem. 2003, 38, 71–80. |
| 3. | Miller, A. R.; Etgen, G. J. Expert Opin. Invest. Drugs 2003, 12, 1489–1500. doi:10.1517/13543784.12.9.1489 |
| 4. | Willson, T. M.; Brown, P. J.; Sternbach, D. D.; Henke, B. R. J. Med. Chem. 2000, 43, 527–550. doi:10.1021/jm990554g |
| 5. | van Raalte, D. H.; Li, M.; Pritchard, P. H.; Wasan, K. M. Pharm. Res. 2004, 21, 1531–1538. doi:10.1023/B:PHAM.0000041444.06122.8d |
| 6. | Miyachi, H. Expert Opin. Ther. Pat. 2004, 14, 607–618. |
| 7. | Henke, B. R. Prog. Med. Chem. 2004, 42, 1–53. |
| 8. | Fajas, L.; Auwerx, J. Peroxisome proliferator-activated receptor γ and the transcriptional control of adipogenesis and metabolism. In Handbook of Obesity: Etiology and Pathophysiology; Bray, G. A.; Bouchard, C., Eds.; Marcel Dekker: New York, 2004; pp 559–587. |
| 9. | Evans, R. M.; Barish, G. D.; Wang, Y.-X. Nat. Med. 2004, 10, 355–361. doi:10.1038/nm1025 |
| 10. | Pershadsingh, H. A. Expert Opin. Invest. Drugs 2004, 13, 215–228. |
| 11. | Tan, N. S.; Michalik, L.; Desvergne, B.; Wahli, W. Expert Opin. Ther. Targets 2004, 8, 39–48. |
| 18. | Wada, M.; Mitsunobu, O. Tetrahedron Lett. 1972, 13, 1279–1282. doi:10.1016/S0040-4039(01)84567-6 |
| 14. | Mitsunobu, O. Synthesis 1981, 1–28. doi:10.1055/s-1981-29317 |
| 15. | Hughes, D. L. Org. React. 1992, 42, 335–656. |
| 16. |
Ito, S.; Tsunoda, T. Pure Appl. Chem. 1999, 71, 1053–1057. doi:10.1351/pac199971061053
A brief review. |
| 17. | Valentine, D. H.; Hillhouse, J. H. Synthesis 2003, 317–334. |
| 20. | Tsunoda, T.; Yamamiya, Y.; Ito, S. Tetrahedron Lett. 1993, 34, 1639–1642. doi:10.1016/0040-4039(93)85029-V |
| 12. |
Hulin, B. 3-Aryl-2-hydroxypropionic acid derivatives and analogs as hypoglycemic agents. PCT Int. Appl. WO 91/19702 A1, Dec 26, 1991.
Chem. Abstr., 1992, 117, 26552r. |
| 16. |
Ito, S.; Tsunoda, T. Pure Appl. Chem. 1999, 71, 1053–1057. doi:10.1351/pac199971061053
A brief review. |
| 21. | Tsunoda, T.; Kawamura, Y.; Uemoto, K.; Ito, S. Heterocycles 1998, 47, 177–179. |
| 19. | von Itzstein, M.; Jenkins, I. D. J. Chem. Soc., Perkin Trans. 1 1986, 437–445. doi:10.1039/p19860000437 |
| 22. | Tsunoda, T.; Otsuka, J.; Yamamiya, Y.; Ito, S. Chem. Lett. 1994, 539–542. doi:10.1246/cl.1994.539 |
| 18. | Wada, M.; Mitsunobu, O. Tetrahedron Lett. 1972, 13, 1279–1282. doi:10.1016/S0040-4039(01)84567-6 |
| 18. | Wada, M.; Mitsunobu, O. Tetrahedron Lett. 1972, 13, 1279–1282. doi:10.1016/S0040-4039(01)84567-6 |
| 19. | von Itzstein, M.; Jenkins, I. D. J. Chem. Soc., Perkin Trans. 1 1986, 437–445. doi:10.1039/p19860000437 |
| 20. | Tsunoda, T.; Yamamiya, Y.; Ito, S. Tetrahedron Lett. 1993, 34, 1639–1642. doi:10.1016/0040-4039(93)85029-V |
© 2006 Humphries et al; licensee Beilstein-Institut.
This is an Open Access article under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
The license is subject to the Beilstein Journal of Organic Chemistry terms and conditions: (http://www.beilstein-journals.org/bjoc)