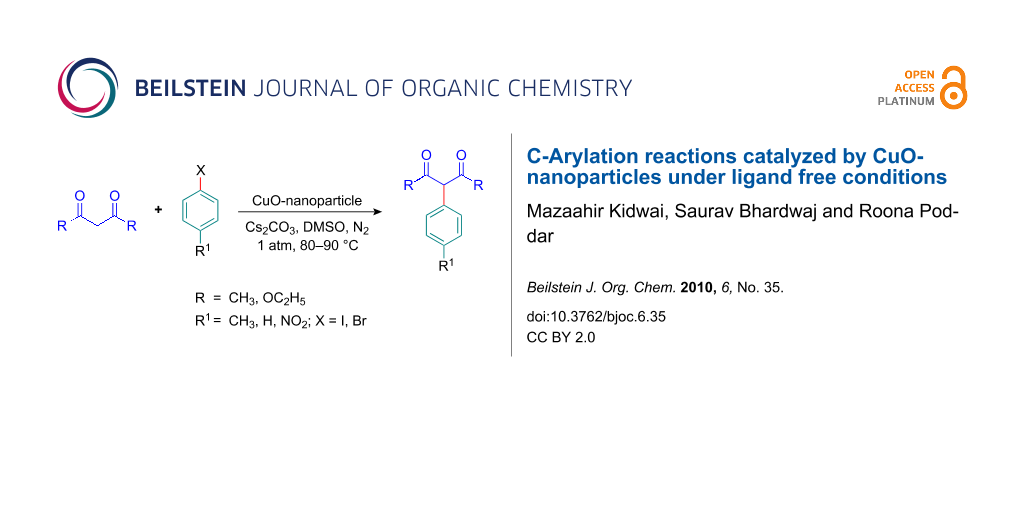
Abstract
CuO-nanoparticles were found to be an excellent heterogeneous catalyst for C-arylation of active methylene compounds using various aryl halides. The products were obtained in good to excellent yield. The catalyst can be recovered and reused for four cycles with almost no loss in activity.
Graphical Abstract
Introduction
Carbon-carbon (C–C) bond formation is one of the most important reactions in organic synthesis [1-3]. The resulting compounds formed from C–C coupling are valuable synthons in organic synthesis [4-7]. However, C-arylation reactions have not been investigated to the same extent as other C–C bond forming reactions.
A great deal of attention has been focused on the development of Pd catalyzed arylation [8-11]. Another important protocol involves arylation of activated methylene compounds mediated by copper salts [12,13]. Recently, proline has also been used along with CuI for C-arylation [14]. But some of the methods suffer from serious drawbacks and limitations, for example, long reaction times [15,16], the high cost of Pd catalysts [17,18] and the need for stoichiometric amounts of copper salts. In addition to these problems, the major drawback is that the majority of catalysts cannot be reused [19]. To overcome from these problems, we have investigated a new catalytic system for C-arylation.
Nanocrystalline metal oxides find numerous applications, e.g., as active adsorbents for gases [20], the destruction of hazardous materials [21] and the oxidation of volatile organic compounds [22]. In addition, metal oxide nanoparticles have been used as heterogeneous catalysts for various organic transformations [23-28]. The high reactivity of CuO-nanoparticles (CuO-np) is due to the high surface area of nanoparticles combined with unusual reactive morphologies. Moreover, heterogeneous catalysts are easy to separate and can be recycled. This is very beneficial for industrial process in the green chemistry domain.
In continuation with our research program to explore different methodologies for the synthesis of organic compounds [29,30] and the role of transition metal nanoparticles as catalysts in organic reactions [31-33], we now report the synthesis of 3-arylpentane-2,4-diones and diethyl 2-aryl-malonates using CuO-nanoparticles as a heterogeneous catalyst.
Results and Discussion
The reaction was carried out several times in order to establish the optimum ratio of reactants. Iodobenzene and acetylacetone were employed as model substrates in a 1:3 ratio. When the iodobenzene (1 mmol) and acetylacetone (3 mmol) were stirred with Cs2CO3 (0.5 mmol) at 80 °C in DMSO, in the presence of CuO-nanoparticles (10 mol %), 3-phenyllpentane-2,4-dione was obtained in 80% yield (Scheme 1).
Scheme 1: Synthesis of 3-phenylpentane-2,4-dione using CuO-nanoparticles.
Scheme 1: Synthesis of 3-phenylpentane-2,4-dione using CuO-nanoparticles.
Other copper salts such as Cu(OAc)2 and ordinary CuO were found to be inferior to CuO-nanoparticles and gave low yields of 3-phenylpentane-2,4-dione. Moreover, Cu-nanoparticles which have been used for O-arylation and S-arylation [34,35], were not effective in the coupling reaction (Table 1).
Table 1: C-arylation reaction catalyzed by different Cu catalystsa.
| Entry | Catalyst | Time (h) | Yieldb (%) |
|---|---|---|---|
| 1 | Cu(OAc)2 | 10 | 20 |
| 2 | Cu-np | 10 | 10 |
| 3 | CuO | 10 | 24 |
| 4 | CuO-np | 8 | 80 |
aReaction conditions: acetylacetone (3 mmol), iodobenzene (1 mmol), 10 mol % of catalyst, Cs2CO3 (0.5 mmol), DMSO; temperature 80 °C; N2; 1 atm.
bIsolated and optimized yield.
In addition, catalytic activity of CuO-nanoparticles was evident since no product was formed in its absence. The increased catalytic activity of CuO-nanoparticles over the commercially available bulk CuO may be attributed to the higher surface area of CuO-nanoparticles. This is thought to be due to morphological differences which have been shown in the TEM image. The number of reactive sites on the surface is small in the case of larger crystallites and considerably greater in the case of smaller crystallites (Figure 1).
Figure 1: Powder X-ray diffraction pattern and TEM image of nano CuO (fresh).
Figure 1: Powder X-ray diffraction pattern and TEM image of nano CuO (fresh).
To investigate further the surface morphology of CuO-nanoparticles, powder XRD and TEM images were taken. Figure 1 shows the XRD pattern of CuO-nanoparticles in which diffraction peaks can be indexed to a monoclinic structure. The intense diffraction peak at an angle 38.4 shows index plane (111) which contains more basic sites with higher density as compared to bulk CuO.
During optimization of reaction conditions, the model reaction was carried out in different solvents. It was found that DMSO was the most effective solvent compared to the other solvents used as shown in Table 2. This is not surprising in view of the fact that the reaction intermediate is a carbanion and therefore will have a greater stability in a polar solvent.
Table 2: CuO-nanoparticles catalyzed coupling reaction of acetylacetone and iodobenzene in various solventsa.
| Entry | Solvent | Time (h) | Yieldb (%) |
|---|---|---|---|
| 1 | DMSO | 8 | 80 |
| 2 | Toluene | 15 | 8 |
| 3 | THF | 15 | 12 |
| 4 | Acetonitrile | 15 | 32 |
aReaction conditions: acetylacetone (3 mmol), iodobenzene (1 mmol), 10 mol % CuO-nanoparticles, Cs2CO3 (0.5 mmol), solvent; temperature 80 °C; N2; 1 atm.
bIsolated and optimized yield.
Moreover, the advantage of the nanoparticles is that, unlike other catalysts, their use is not restricted by their solubility, as the nanoparticles can be dispersed in the desired solvent by agitation or slight sonication.
When an equimolar mixture of chlorobenzene and iodobenzene was treated with acetylacetone under similar reaction conditions, the chlorobenzene was largely unreactive. This shows that the reaction is highly selective towards the halogen present in the aryl halide (Scheme 2).
Scheme 2: Synthesis of 3-phenylpentane-2,4-dione using CuO-nanoparticles.
Scheme 2: Synthesis of 3-phenylpentane-2,4-dione using CuO-nanoparticles.
To study the scope of this procedure, acetylacetone was reacted with various aryl halides and gave the corresponding products in 78–83% yield. It was observed that aryl halides having electron withdrawing groups showed greater reactivity and gave good yield of products compared to aryl halides having electron donating groups. All these results are summarized in Table 3.
Table 3: C-arylation of acetylacetone using different aryl halidesa
| Entry | Aryl halide | Product | Time (h) | Yieldb (%) |
|---|---|---|---|---|
| 1 | iodobenzene | 3-phenylpentane-2,4-dione | 8 | 80 |
| 2 | bromobenzene | 3-phenylpentane-2,4-dione | 10 | 79 |
| 3 | p-nitroiodobenzene | 3-(4-nitrophenyl)-pentane-2,4-dione | 6 | 83 |
| 4 | p-methyliodobenzene | 3-p-tolylpentane-2,4-dione | 10 | 78 |
| 5 | m-trifluoromethyliodobenzene | 3-(3-trifluromethyl-phenyl)-pentane-2,4-dione | 7 | 81 |
| 6 | 1-iodo-2-methylbenzene | 3-o-tolyl-pentane-2,4-dione | 11 | 76 |
| 7 | 1-iodo-4-methoxybenzene | 3-(4-methoxy-phenyl)-pentane-2,4-dione | 12 | 75 |
aReaction conditions: acetylacetone (3 mmol), aryl halide (1 mmol), 10 mol % CuO-nanoparticles, Cs2CO3 (0.5 mmol), DMSO; temperature 80 °C; N2; 1 atm.
bIsolated and optimized yields.
The above results encouraged us to investigate further reactions. Under similar reaction conditions, diethyl malonate was treated with iodobenzene to give the desired product in 78% yield (Scheme 3). The reaction was then repeated with a variety of aryl halides. The results are summarized in Table 4.
Scheme 3: Synthesis of diethyl 2-aryl-malonate using CuO-nanoparticles.
Scheme 3: Synthesis of diethyl 2-aryl-malonate using CuO-nanoparticles.
Table 4: C-arylation of diethyl malonate using different aryl halidesa.
| Entry | Aryl halide | Product | Time (h) | Yieldb (%) |
|---|---|---|---|---|
| 1 | iodobenzene | 2-phenylmalonic acid diethylester | 9 | 78 |
| 2 | bromobenzene | 2-phenylmalonic acid diethylester | 11 | 76 |
| 3 | p-nitroiodobenzene | 2-(4-nitrophenyl)-malonic acid diethylester | 6 | 81 |
| 4 | p-methyliodobenzene | 2-p-tolylmalonic acid diethylester | 12 | 76 |
| 5 | m-trifluoromethyliodobenzene | 2-(3-trifluromethyl-phenyl)-malonic acid diethylester | 7 | 80 |
| 6 | 1-iodo-2-methylbenzene | 2-o-tolylmalonic acid diethylester | 13 | 75 |
| 7 | 1-iodo-4-methoxybenzene | 3-(4-methoxy-phenyl)-malonic acid diethylether | 14 | 74 |
aReaction conditions: diethyl malonate (3 mmol), aryl halide (1 mmol), 10 mol % CuO-nanoparticles, Cs2CO3 (0.5 mmol), DMSO; temperature 80 °C; N2; 1 atm.
bIsolated and optimized yields.
The observed decrease in reactivity in the order p-nitroiodobenzene > iodobenzene > p-methyliodobenzene > 1-iodo-4-methoxybenzene suggests that the reaction proceeds by oxidative addition followed by reductive elimination. In addition to this, the order of reactivity suggests that aryl halides having electron withdrawing groups stabilize the transition state which corresponds to good yields with a short reaction time in comparison to aryl halides having electron donating groups (Figure 2).
Figure 2: Proposed reaction pathway for the CuO-nanoparticles catalyzed C–C coupling reaction.
Figure 2: Proposed reaction pathway for the CuO-nanoparticles catalyzed C–C coupling reaction.
The reaction was then carried out with recycled catalyst. The CuO-nanoparticles were recovered by centrifugation of the reaction mixture and washed thoroughly with ethyl acetate. The resulting nanoparticles could be reused for several cycles without any significant loss of activity. In our study, we used same nanoparticles three times and the results are summarized below (Table 5). The TEM and XRD analysis of recycled CuO-nanoparticles, after the fourth run showed that particles are identical in shape and size, hence CuO-nanoparticles are unchanged during the reaction (Figure 3).
Table 5: Recycle studies of nano-CuO for C-arylation reactiona.
| Run | Time (h) | Yieldb (%) |
|---|---|---|
| 1 | 8 | 80 |
| 2 | 8 | 78 |
| 3 | 8 | 77 |
| 4 | 8 | 76 |
aReaction conditions: acetylacetone (3 mmol), iodobenzene (1 mmol), 10 mol % CuO-nanoparticles, Cs2CO3 (0.5 mmol), DMSO; temperature 80 °C; N2; 1atm.
bIsolated and optimized yields.
Figure 3: Powder X-ray diffraction pattern and TEM image of recycled CuO-nanoparticles.
Figure 3: Powder X-ray diffraction pattern and TEM image of recycled CuO-nanoparticles.
Conclusion
An efficient, facile and economical method for synthesis of 3-arylpentane-2,4-diones (Table 3) and diethyl 2-aryl-malonates (Table 4) has been developed using CuO-nanoparticles as the catalyst. The products were obtained in moderate to good yields and the catalyst can be recycled up to four cycles with almost consistent activity. The present protocol represents a simple and remarkably active catalytic system to catalyze Ullmann-type C–C bond formation, which potentially offers an efficient protocol for accessing a variety of α-arylated dicarbonyl compounds.
Experimental
General
The materials were purchased from Sigma-Aldrich and Merck and were used without further purification. Mass spectra were recorded in a TOF-mass spectrometer model no. KC455. 1H NMR and 13C NMR spectra were recorded on a Bruker spectrospin at 300 MHz and 75 MHz, respectively. All 1H NMR and 13C NMR spectra were run in CDCl3 and chemical shifts are expressed as ppm relative to internal Me4Si. Elemental analyses were performed using Heraeus CHN-Rapid Analyzer. Powder X-ray diffraction measurements were carried out on a Bruker D8 Discover HR-XRD instrument using Cu Kα radiation (λ = 1.54184 Å).
Experimental Section
CuO-nanoparticles (10 mol %) were added to the mixture of iodobenzene (1 mmol), acetylactone (3 mmol) and (0.5 mmol) of Cs2CO3 in DMSO (2 ml) under nitrogen atmosphere. The resulting reaction mixture was stirred at 80 °C for 8 h. Progress of the reaction was continuously monitored by TLC. After completion of reaction, the catalyst was recovered by centrifugation. The nanoparticles, were first washed with distilled methanol to remove some of the Cs2CO3, then several times with ethyl acetate and dried in an oven overnight at 150 °C. The filtrate was poured into 1N HCl and extracted with EtOAc. The combined organic layers were washed with brine, dried over anhydrous Na2SO4 and concentrated at reduced pressure. The residue was chromatographed to afford pure 3-phenyl-2,4-pentadione (Entry 1, Table 3). From the spectral data it was observed that some products show 1,3-keto-enol tautomerism. The structures of all the products were unambiguously established on the basis of their spectral properties (1H NMR, MS and 13C NMR).
Supporting Information
| Supporting Information File 1: Typical procedure and characterization data of prepared compounds | ||
| Format: PDF | Size: 41.7 KB | Download |
References
-
Ren, T. Chem. Rev. 2008, 108, 4185–4207. doi:10.1021/cr8002592
Return to citation in text: [1] -
Hart, D. J. Science 1984, 223, 883–887. doi:10.1126/science.223.4639.883
Return to citation in text: [1] -
Iwama, T.; Rawal, V. H. Org. Lett. 2006, 8, 5725–5728. doi:10.1021/ol062093g
Return to citation in text: [1] -
Ramthoul, Y. K.; Chartrand, A. Org. Lett. 2007, 9, 1029–1032. doi:10.1021/ol063057k
Return to citation in text: [1] -
Masselot, D.; Charmant, J. P. H.; Gallagher, T. J. Am. Chem. Soc. 2006, 128, 694–695. doi:10.1021/ja056964d
Return to citation in text: [1] -
Wu, J. Y.; Nie, L.; Luo, J.; Dai, W.-M. Synlett 2007, 2728–2732. doi:10.1055/s-2007-991053
Return to citation in text: [1] -
Kamikawa, K.; Takemoto, I.; Takemoto, S.; Matsuzaka, H. J. Org. Chem. 2007, 72, 7406–7408. doi:10.1021/jo0711586
Return to citation in text: [1] -
Wright, J. B. J. Org. Chem. 1964, 29, 1905–1909. doi:10.1021/jo01030a059
Return to citation in text: [1] -
Beare, N. A.; Hartwig, J. F. J. Org. Chem. 2002, 67, 541–555. doi:10.1021/jo016226h
Return to citation in text: [1] -
You, J.; Verkade, J. G. Angew. Chem. 2003, 42, 5051–5053. doi:10.1002/anie.200351954
Return to citation in text: [1] -
Thathagar, M. B.; Rothenberg, G. Org. Biomol. Chem. 2006, 4, 111–115. doi:10.1039/b513450a
Return to citation in text: [1] -
Thathagar, M. B.; Beckers, J.; Rothenberg, G. Green Chem. 2004, 6, 215–218. doi:10.1039/b401586j
Return to citation in text: [1] -
Suzuki, H.; Kobayashi, T.; Osuka, A. Chem. Lett. 1983, 12, 589–590. doi:10.1246/cl.1983.589
Return to citation in text: [1] -
Setsune, J.-I.; Matsukawa, K.; Wakemoto, H.; Kitao, T. Chem. Lett. 1981, 10, 367–370. doi:10.1246/cl.1981.367
Return to citation in text: [1] -
Jiang, Y.; Wu, N.; Wu, H.; He, M. Synlett 2005, 2731–2734. doi:10.1055/s-2005-918921
Return to citation in text: [1] -
Yip, S. F.; Cheung, H. Y.; Zhou, Z.; Kwong, F. Y. Org. Lett. 2007, 9, 3469–3472. doi:10.1021/ol701473p
Return to citation in text: [1] -
Fox, J. M.; Huang, X.; Chieffi, A.; Buchwald, S. L. J. Am. Chem. Soc. 2000, 122, 1360–1370. doi:10.1021/ja993912d
Return to citation in text: [1] -
Culkin, D. A.; Hartwig, J. F. J. Am. Chem. Soc. 2001, 123, 5816–5817. doi:10.1021/ja015732l
Return to citation in text: [1] -
Li, P.; Wang, L.; Li, H. Tetrahedron 2005, 61, 8633–8640. doi:10.1016/j.tet.2005.07.013
Return to citation in text: [1] -
Okuro, K.; Furuune, M.; Miura, M.; Nomura, M. J. Org. Chem. 1993, 58, 7606–7607. doi:10.1021/jo00078a053
Return to citation in text: [1] -
Lucas, E.; Decker, S.; Khaleel, A.; Seitz, A.; Fultz, S.; Ponce, A.; Li, W.; Carnes, C.; Klabunde, K. J. Chem.–Eur. J. 2001, 7, 2505–2510. doi:10.1002/1521-3765(20010618)7:12<2505::AID-CHEM25050>3.0.CO;2-R
Return to citation in text: [1] -
Jiang, Y.; Decker, S.; Mohs, C.; Klabunde, K. J. J. Catal. 1998, 180, 24–35. doi:10.1006/jcat.1998.2257
Return to citation in text: [1] -
Larsson, P.-O.; Andersson, A. J. Catal. 1998, 179, 72–89. doi:10.1006/jcat.1998.2198
Return to citation in text: [1] -
Rout, L.; Jammi, S.; Punniyamurthy, T. Org. Lett. 2007, 9, 3397–3399. doi:10.1021/ol0713887
Return to citation in text: [1] -
Kantam, M. L.; Laha, S.; Yadav, J.; Bhargava, S. Tetrahedron Lett. 2008, 49, 3083–3086. doi:10.1016/j.tetlet.2008.03.053
Return to citation in text: [1] -
Chikán, V.; Molnár, Á.; Belázsik, K. J. Catal. 1999, 184, 134–143. doi:10.1006/jcat.1999.2437
Return to citation in text: [1] -
Kantam, M. L.; Yadav, J.; Laha, S.; Sreedhar, B.; Jha, S. Adv. Synth. Catal. 2007, 349, 1938–1942. doi:10.1002/adsc.200600483
Return to citation in text: [1] -
Rout, L.; Sen, T. K.; Punniyamurthy, T. Angew. Chem., Int. Ed. 2007, 46, 5583–5586. doi:10.1002/anie.200701282
Return to citation in text: [1] -
Kidwai, M.; Poddar, R.; Mothsra, P. Beil. J. Org. Chem. 2009, 5, No. 10. doi:10.3762/bjoc.5.10
Return to citation in text: [1] -
Kidwai, M.; Poddar, R.; Diwaniyan, S.; Kuhad, R. C. Adv. Synth. Catal. 2009, 351, 589–595. doi:10.1002/adsc.200800611
Return to citation in text: [1] -
Kidwai, M.; Bhatnagar, D.; Mishra, N. K.; Bansal, V. Catal. Commun. 2008, 9, 2547–2549. doi:10.1016/j.catcom.2008.07.010
Return to citation in text: [1] -
Kidwai, M.; Bhardwaj, S.; Mishra, N. K.; Bansal, V.; Kumar, A.; Mozumdar, S. Catal. Commun. 2009, 10, 1514–1517. doi:10.1016/j.catcom.2009.04.006
Return to citation in text: [1] -
Kidwai, M.; Bansal, V.; Kumar, A.; Mozumdar, S. Green Chem. 2007, 9, 742–745. doi:10.1039/b702287e
Return to citation in text: [1] -
Kidwai, M.; Mishra, N. K.; Bansal, V.; Kumar, A.; Mozumdar, S. Tetrahedron Lett. 2007, 48, 8883–8887. doi:10.1016/j.tetlet.2007.10.050
Return to citation in text: [1] -
Gonzalez-Arellano, C.; Luque, G.; Macquarrie, D. J. Chem. Commun. 2009, 1410–1412. doi:10.1039/b818767c
Return to citation in text: [1]
| 1. | Ren, T. Chem. Rev. 2008, 108, 4185–4207. doi:10.1021/cr8002592 |
| 2. | Hart, D. J. Science 1984, 223, 883–887. doi:10.1126/science.223.4639.883 |
| 3. | Iwama, T.; Rawal, V. H. Org. Lett. 2006, 8, 5725–5728. doi:10.1021/ol062093g |
| 14. | Setsune, J.-I.; Matsukawa, K.; Wakemoto, H.; Kitao, T. Chem. Lett. 1981, 10, 367–370. doi:10.1246/cl.1981.367 |
| 34. | Kidwai, M.; Mishra, N. K.; Bansal, V.; Kumar, A.; Mozumdar, S. Tetrahedron Lett. 2007, 48, 8883–8887. doi:10.1016/j.tetlet.2007.10.050 |
| 35. | Gonzalez-Arellano, C.; Luque, G.; Macquarrie, D. J. Chem. Commun. 2009, 1410–1412. doi:10.1039/b818767c |
| 12. | Thathagar, M. B.; Beckers, J.; Rothenberg, G. Green Chem. 2004, 6, 215–218. doi:10.1039/b401586j |
| 13. | Suzuki, H.; Kobayashi, T.; Osuka, A. Chem. Lett. 1983, 12, 589–590. doi:10.1246/cl.1983.589 |
| 8. | Wright, J. B. J. Org. Chem. 1964, 29, 1905–1909. doi:10.1021/jo01030a059 |
| 9. | Beare, N. A.; Hartwig, J. F. J. Org. Chem. 2002, 67, 541–555. doi:10.1021/jo016226h |
| 10. | You, J.; Verkade, J. G. Angew. Chem. 2003, 42, 5051–5053. doi:10.1002/anie.200351954 |
| 11. | Thathagar, M. B.; Rothenberg, G. Org. Biomol. Chem. 2006, 4, 111–115. doi:10.1039/b513450a |
| 29. | Kidwai, M.; Poddar, R.; Mothsra, P. Beil. J. Org. Chem. 2009, 5, No. 10. doi:10.3762/bjoc.5.10 |
| 30. | Kidwai, M.; Poddar, R.; Diwaniyan, S.; Kuhad, R. C. Adv. Synth. Catal. 2009, 351, 589–595. doi:10.1002/adsc.200800611 |
| 4. | Ramthoul, Y. K.; Chartrand, A. Org. Lett. 2007, 9, 1029–1032. doi:10.1021/ol063057k |
| 5. | Masselot, D.; Charmant, J. P. H.; Gallagher, T. J. Am. Chem. Soc. 2006, 128, 694–695. doi:10.1021/ja056964d |
| 6. | Wu, J. Y.; Nie, L.; Luo, J.; Dai, W.-M. Synlett 2007, 2728–2732. doi:10.1055/s-2007-991053 |
| 7. | Kamikawa, K.; Takemoto, I.; Takemoto, S.; Matsuzaka, H. J. Org. Chem. 2007, 72, 7406–7408. doi:10.1021/jo0711586 |
| 31. | Kidwai, M.; Bhatnagar, D.; Mishra, N. K.; Bansal, V. Catal. Commun. 2008, 9, 2547–2549. doi:10.1016/j.catcom.2008.07.010 |
| 32. | Kidwai, M.; Bhardwaj, S.; Mishra, N. K.; Bansal, V.; Kumar, A.; Mozumdar, S. Catal. Commun. 2009, 10, 1514–1517. doi:10.1016/j.catcom.2009.04.006 |
| 33. | Kidwai, M.; Bansal, V.; Kumar, A.; Mozumdar, S. Green Chem. 2007, 9, 742–745. doi:10.1039/b702287e |
| 20. | Okuro, K.; Furuune, M.; Miura, M.; Nomura, M. J. Org. Chem. 1993, 58, 7606–7607. doi:10.1021/jo00078a053 |
| 22. | Jiang, Y.; Decker, S.; Mohs, C.; Klabunde, K. J. J. Catal. 1998, 180, 24–35. doi:10.1006/jcat.1998.2257 |
| 19. | Li, P.; Wang, L.; Li, H. Tetrahedron 2005, 61, 8633–8640. doi:10.1016/j.tet.2005.07.013 |
| 23. | Larsson, P.-O.; Andersson, A. J. Catal. 1998, 179, 72–89. doi:10.1006/jcat.1998.2198 |
| 24. | Rout, L.; Jammi, S.; Punniyamurthy, T. Org. Lett. 2007, 9, 3397–3399. doi:10.1021/ol0713887 |
| 25. | Kantam, M. L.; Laha, S.; Yadav, J.; Bhargava, S. Tetrahedron Lett. 2008, 49, 3083–3086. doi:10.1016/j.tetlet.2008.03.053 |
| 26. | Chikán, V.; Molnár, Á.; Belázsik, K. J. Catal. 1999, 184, 134–143. doi:10.1006/jcat.1999.2437 |
| 27. | Kantam, M. L.; Yadav, J.; Laha, S.; Sreedhar, B.; Jha, S. Adv. Synth. Catal. 2007, 349, 1938–1942. doi:10.1002/adsc.200600483 |
| 28. | Rout, L.; Sen, T. K.; Punniyamurthy, T. Angew. Chem., Int. Ed. 2007, 46, 5583–5586. doi:10.1002/anie.200701282 |
| 17. | Fox, J. M.; Huang, X.; Chieffi, A.; Buchwald, S. L. J. Am. Chem. Soc. 2000, 122, 1360–1370. doi:10.1021/ja993912d |
| 18. | Culkin, D. A.; Hartwig, J. F. J. Am. Chem. Soc. 2001, 123, 5816–5817. doi:10.1021/ja015732l |
| 15. | Jiang, Y.; Wu, N.; Wu, H.; He, M. Synlett 2005, 2731–2734. doi:10.1055/s-2005-918921 |
| 16. | Yip, S. F.; Cheung, H. Y.; Zhou, Z.; Kwong, F. Y. Org. Lett. 2007, 9, 3469–3472. doi:10.1021/ol701473p |
| 21. | Lucas, E.; Decker, S.; Khaleel, A.; Seitz, A.; Fultz, S.; Ponce, A.; Li, W.; Carnes, C.; Klabunde, K. J. Chem.–Eur. J. 2001, 7, 2505–2510. doi:10.1002/1521-3765(20010618)7:12<2505::AID-CHEM25050>3.0.CO;2-R |
© 2010 Kidwai et al; licensee Beilstein-Institut.
This is an Open Access article under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
The license is subject to the Beilstein Journal of Organic Chemistry terms and conditions: (http://www.beilstein-journals.org/bjoc)