Abstract
Potassium thiocyanate acts as an efficient sulfur surrogate in C–S cross-coupling reactions mediated by recyclable copper oxide nanoparticles under ligand free conditions. This protocol avoids foul smelling thiols, for the synthesis of a variety of symmetrical diaryl sulfides, via the cross-coupling of different aryl halides with potassium thiocyanate, affording corresponding products in moderate to excellent yields.
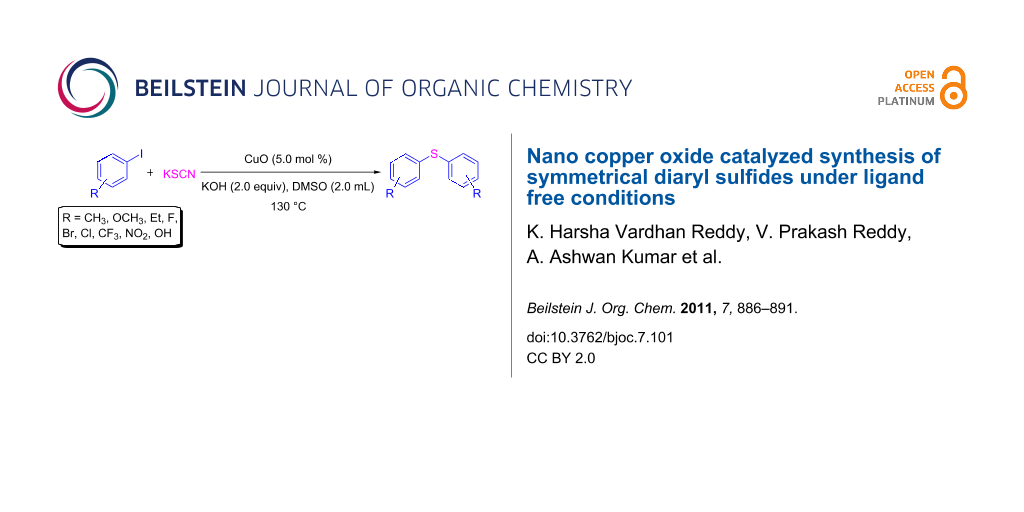
Graphical Abstract
Introduction
After the discovery of copper-promoted Ullmann reaction [1-3] for the construction of carbon-hetero atom bonds, several protocols have been reported over the years to establish C–N, C–O, and C–S linkages. Carbon–sulfur bonds are widespread, occurring in numerous pharmaceutically and biologically active compounds [4-8]. A large variety of aryl sulfides are in use for diverse clinical applications in the treatment of cancer [9], HIV [10,11], Alzheimer’s and Parkinson’s diseases [12].
During the last decades, transition and boron group metals, such as palladium [13-18], nickel [19,20], copper [21-37], iron [38], indium [39,40], lanthanum [41] and cobalt [42] have been used in cross-coupling reactions, developed for the formation of carbon–sulfur bonds. Most of these coupling protocols involve the reaction between thiols and aryl halides, resulting in the formation of C–S bonds. Most of these metal-catalyzed reactions involve volatile and foul-smelling thiols as the main drawback, which leads to environmental and safety problems. To overcome these problems, Zhou [43] and coworkers recently reported an efficient C–S bond formation by the reaction of potassium thiocyanate and aryl halides in the presence of a copper catalyst and a ligand in aqueous medium at 130 °C for 48 h. Tao et al. described the synthesis of diaryl sulfides catalyzed by CuI via cross-coupling between aryl halides and thioacetamide using Cs2CO3 as the base and DMSO–H2O as the solvent at 120 °C [44].
These recently developed protocols involve expensive catalytic systems and ligands [43], long reaction times [43,44], metal contamination of the final product, and non recyclability of the catalyst. This leads to increased costs as well as limiting the scope of the reaction. From the synthetic point of view, it is desirable to find novel recyclable catalytic systems, especially under ligand-free conditions, for the synthesis of such highly useful organic compounds.
In the past few years, considerable efforts have been made in the area of heterogeneous catalysis for various organic transformations. In general, heterogeneous catalysts offer higher surface area and lower coordinating sites, which are responsible for their higher catalytic activity [45-47]. Furthermore, heterogeneous catalysis has the advantage of high atom efficiency, easy product purification, and reusability of the catalyst. However, up until now, the investigation of nanoparticles as catalysts has been limited and will be widely studied in future.
Results and Discussion
In continuation of our investigations on metal-catalyzed cross-coupling reactions, we have explored the CuO-catalyzed synthesis of diaryl sulfides under ligand-free conditions (Scheme 1). To the best of our knowledge, this is the first recyclable copper oxide nanoparticle-catalyzed cross-coupling of aryl halides with potassium thiocyanate [46-51].
Scheme 1: Synthesis of symmetrical aryl sulfides catalyzed by copper oxide nanoparticles.
Scheme 1: Synthesis of symmetrical aryl sulfides catalyzed by copper oxide nanoparticles.
Initially, the reaction between potassium thiocyanate and iodobenzene was selected as the model reaction for optimizing the reaction conditions involving various copper sources, bases, solvents, and temperature (Table 1).
Table 1: Screening of copper sources for the cross-coupling reaction between iodo benzene and potassium thiocyanate.a
| Entry | Catalyst | Solvent | Base | T (°) | Yield (%)b |
|---|---|---|---|---|---|
| 1 | CuO | DMSO | K2CO3 | rt | 0 |
| 2 | CuO | DMSO | K2CO3 | 80 | 46 |
| 3 | CuO | DMSO | K2CO3 | 130 | 51 |
| 4 | CuO | DMSO | Cs2CO3 | 130 | 79 |
| 5 | CuO | DMSO | KOH | 130 | 94 |
| 6 | CuO | DMSO | K3PO4 | 130 | 42 |
| 7 | CuO | Toluene | KOH | 130 | trace |
| 8 | CuO | H2O | KOH | 130 | trace |
| 9 | CuO | PEG | KOH | 130 | 60 |
| 10 | CuO | DMF | KOH | 130 | 70 |
| 11 | – | DMSO | KOH | 130 | 0c |
| 12 | CuO | DMSO | – | 130 | 0d |
| 13 | CuCl2·2H2O | DMSO | KOH | 130 | 83 |
| 14 | CuSO4·5H2O | DMSO | KOH | 130 | 71 |
| 15 | Cu(OAc)·H2O | DMSO | KOH | 130 | 79 |
aReaction conditions: Iodobenzene (2.0 mmol), potassium thiocyanate (1.5 mmol), nano CuO (5.0 mol %), solvent (2.0 mL), base (2.0 equiv), 130 °C, 20 h. bIsolated yield. cIn the absence of catalyst. dIn the absence of base.
First, several copper catalysts were screened (Table 1), and CuO was found to be promising for this tandem reaction (Table 1, entry 5). Amongst various bases screened, Cs2CO3 and KOH afforded the symmetrical aryl sulfides in excellent yields (Table 1, entries 4, 5). Other bases such as K2CO3 and K3PO4 gave lesser amounts of the desired product (Table 1, entries 3, 6). Among the solvents, DMSO yielded the best results (Table 1, entry 5) whereas PEG and DMF gave the products in moderate yields (Table 1, entries 9 and 10), whilst solvents such as toluene and water were ineffective (Table 1, entries 7 and 8). The coupling reaction did not occur in the absence of the catalyst (Table 1, entry 11) or base (Table 1, entry 12). When the reaction was conducted either at room temperature and 80 °C, no product was obtained or the yield was very low (Table 1, entries 1 and 2). The ideal temperature for the reaction was found to be 130 °C.
A study was conducted on C–S cross-coupling reaction using various sulfur sources under these conditions (Table 2). Among these sulfur surrogates potassium thiocyanate gave good yields in this C–S cross-coupling reaction.
Table 2: Nano CuO catalyzed C–S cross-coupling of iodobenzene with different sulfur sources.a
| Entry | Aryl halide | Sulfur source | Yield (%)b |
|---|---|---|---|
| 1 |
|
KSCN | 94 |
| 2 | NH4SCN | 91 | |
| 3 | Na2S2O3 | 59 | |
| 4 |
|
KSCN | 91 |
| 5 | NH4SCN | 85 | |
| 6 | Na2S2O3 | 54 | |
| 7 |
|
KSCN | 92 |
| 8 | NH4SCN | 81 | |
| 9 | Na2S2O3 | 58 | |
aReaction conditions: Aryl halides (2.0 mmol), sulfur sources (1.5 mmol), nano CuO (5.0 mol %), DMSO (2.0 mL), KOH (2.0 equiv), 130 °C, 20 h, bIsolated yield.
While expanding the scope of this nano CuO catalyzed tandem cross-coupling, the reaction of potassium thiocyanate with various aryl halides was examined under the optimized conditions. In general, all the reactions were very clean, and the symmetrical aryl sulfides were obtained in moderate to good yields. This protocol efficiently coupled iodobenzenes with electron donating groups (e.g., Me, OMe and Et) with potassium thiocyanate to produce the corresponding products in excellent yields (Table 3, entries 4, 6 and 8), whereas in the presence of an electron withdrawing group (CF3, NO2) a slight decrease in the yield of the diaryl sulfide (Table 3, entries 12 and 13) was observed. Under these reaction conditions, various hetero aromatic iodides were reacted with potassium thiocyanate and gave the corresponding diaryl sulfides in appreciable yields (Table 3, entries 15, 16 and 17). In case of the reactions of aromatic and hetero aromatic bromides with potassium thiocyanate, longer reaction times were required in order to obtain reasonable yields of diaryl sulfides (Table 3, entry 2, 5, 7 and 18). Iodobenzene was found to be a more reactive substrate than bromo, and chloro benzenes (Table 3, entries 1, 2 and 3).
Table 3: Copper oxide nanoparticles catalyzed synthesis of diaryl sulfidesa.
|
|
|||
| Entry | Aryl halide | Product | Yield (%)b |
|---|---|---|---|
| 1 |
|
|
94 |
| 2 |
|
|
63c |
| 3 |
|
|
trace |
| 4 |
|
|
91 |
| 5 |
|
|
68c |
| 6 |
|
|
92 |
| 7 |
|
|
70c |
| 8 |
|
|
87 |
| 9 |
|
|
72 |
| 10 |
|
|
78 |
| 11 |
|
|
76 |
| 12 |
|
|
79 |
| 13 |
|
|
78 |
| 14 |
|
|
75 |
| 15 |
|
|
66 |
| 16 |
|
|
62 |
| 17 |
|
|
69 |
| 18 |
|
|
72c |
aReaction conditions: Aryl halides (2.0 mmol), potassium thiocyanate (1.5 mmol), nano CuO (5.0 mol %), DMSO (2.0 mL), KOH (2.0 equiv), 130 °C, 20 h. b Isolated yield. cAfter 34 h.
Conclusion
We have developed a CuO nanoparticles catalyzed synthesis of symmetrical diaryl sulfides via cascade reaction of aryl halides with potassium thiocyanate under ligand free conditions. The reaction avoids foul smelling thiols and the catalyst is economical, air stable, functions under ligand free conditions and is recyclable for up to four cycles without loss of catalytic activity [20,39-41] (Table 4).
Experimental
General procedure for the synthesis of diaryl sulfides: A mixture of aryl iodide (2.0 mmol), potassium thiocyanate (1.5 mmol), nano CuO (5.0 mol %), and KOH (2.0 equiv) was stirred at 130 °C under a N2 atmosphere in DMSO (2.0 mL). The progress of the reaction was monitored by TLC. When the reaction was complete, the reaction mixture was allowed to cool, a 1:1 mixture ethyl acetate and water (20 mL) was added and the CuO was removed by centrifugation. The organic layer was washed successively with brine and water, and dried with Na2SO4. The solvent and volatiles were completely removed under vacuum to give the crude product, which was purified by column chromatography on silica gel to yield the analytically pure product in up to 94% yield. The identity and purity of the product was confirmed by 1H and 13C NMR spectroscopy.
Supporting Information
| Supporting Information File 1: Experimental details and spectroscopic data for new compounds. | ||
| Format: PDF | Size: 2.8 MB | Download |
References
-
Ullmann, F. Ber. Dtsch. Chem. Ges. 1903, 36, 2382.
Return to citation in text: [1] -
Lindley, J. Tetrahedron 1984, 40, 1433. doi:10.1016/S0040-4020(01)91791-0
Return to citation in text: [1] -
Hassan, J.; Sévignon, M.; Gozzi, C.; Schulz, E.; Lemaire, M. Chem. Rev. 2002, 102, 1359. doi:10.1021/cr000664r
Return to citation in text: [1] -
Johannesson, P.; Lindeberg, G.; Johansson, A.; Nikiforovich, G. V.; Gogoll, A.; Synnergren, B.; Le Grèves, M.; Nyberg, F.; Karlén, A.; Hallberg, A. J. Med. Chem. 2002, 45, 1767. doi:10.1021/jm011063a
Return to citation in text: [1] -
Mugesh, G.; Du Mont, W.-W.; Sies, H. Chem. Rev. 2001, 101, 2125. doi:10.1021/cr000426w
Return to citation in text: [1] -
Morimoto, K.; Tsuji, K.; Iio, T.; Miyata, N.; Uchida, A.; Osawa, R.; Kitsutaka, H.; Takahashi, A. Carcinogenesis 1991, 12, 703. doi:10.1093/carcin/12.4.703
Return to citation in text: [1] -
Sader, H. S.; Johnson, D. M.; Jones, R. N. Antimicrob. Agents Chemother. 2004, 48, 53. doi:10.1128/AAC.48.1.53-62.2004
Return to citation in text: [1] -
Ceruti, M.; Balliano, G.; Rocco, F.; Milla, P.; Arpicco, S.; Cattel, L.; Viola, F. Lipids 2001, 36, 629. doi:10.1007/s11745-001-0767-8
Return to citation in text: [1] -
Kaldor, S. W.; Kalish, V. J.; Davies, J. F., II; Shetty, B. V.; Fritz, J. E.; Appelt, K.; Burgess, J. A.; Campanale, K. M.; Chirgadze, N. Y.; Clawson, D. K.; Dressman, B. A.; Hatch, S. D.; Khalil, D. A.; Kosa, M. B.; Lubbehusen, P. P.; Muesing, M. A.; Patick, A. K.; Reich, S. H.; Su, K. S.; Tatlock, J. H. J. Med. Chem. 1997, 40, 3979. doi:10.1021/jm9704098
Return to citation in text: [1] -
Tsutsui, H.; Hayashi, Y.; Narasaka, K. Chem. Lett. 1997, 26, 317. doi:10.1246/cl.1997.317
Return to citation in text: [1] -
Uchiyama, K.; Yoshida, M.; Hayashi, Y.; Narasaka, K. Chem. Lett. 1998, 27, 607. doi:10.1246/cl.1998.607
Return to citation in text: [1] -
De Martino, G.; Edler, M. C.; La Regina, G.; Coluccia, A.; Barbera, M. C.; Barrow, D.; Nicholson, R. I.; Chiosis, G.; Brancale, A.; Hamel, E.; Artico, M.; Silvestri, R. J. Med. Chem. 2006, 49, 947. doi:10.1021/jm050809s
Return to citation in text: [1] -
Murata, M.; Buchwald, S. L. Tetrahedron 2004, 60, 7397. doi:10.1016/j.tet.2004.05.044
Return to citation in text: [1] -
Mispelaere-Canivet, C.; Spindler, J.-F.; Perrio, S.; Beslin, P. Tetrahedron 2005, 61, 5253. doi:10.1016/j.tet.2005.03.078
Return to citation in text: [1] -
Itoh, T.; Mase, T. Org. Lett. 2004, 6, 4587. doi:10.1021/ol047996t
Return to citation in text: [1] -
Fernández-Rodríguez, M. A.; Shen, Q.; Hartwig, J. F. J. Am. Chem. Soc. 2006, 128, 2180. doi:10.1021/ja0580340
Return to citation in text: [1] -
Lee, J.-Y.; Lee, P. H. J. Org. Chem. 2008, 73, 7413. doi:10.1021/jo801169h
Return to citation in text: [1] -
Jiang, Z.; She, J.; Lina, X. Adv. Synth. Catal. 2009, 351, 2558. doi:10.1002/adsc.200900501
Return to citation in text: [1] -
Taniguchi, N. J. Org. Chem. 2004, 69, 6904. doi:10.1021/jo040184q
Return to citation in text: [1] -
Jammi, S.; Barua, P.; Rout, L.; Saha, P.; Punniyamurthy, T. Tetrahedron Lett. 2008, 49, 1484. doi:10.1016/j.tetlet.2007.12.118
Return to citation in text: [1] [2] -
Kwong, F. Y.; Buchwald, S. L. Org. Lett. 2002, 4, 3517. doi:10.1021/ol0266673
Return to citation in text: [1] -
Chen, Y.-J.; Chen, H.-H. Org. Lett. 2006, 8, 5609. doi:10.1021/ol062339h
Return to citation in text: [1] -
Taniguchi, N. J. Org. Chem. 2007, 72, 1241. doi:10.1021/jo062131+
Return to citation in text: [1] -
Sperotto, E.; Van Klink, G. P. M.; De Vries, J. G.; Van Koten, G. J. Org. Chem. 2008, 73, 5625. doi:10.1021/jo800491k
Return to citation in text: [1] -
Rout, L.; Saha, P.; Jammi, S.; Punniyamurthy, T. Eur. J. Org. Chem. 2008, 640. doi:10.1002/ejoc.200700978
Return to citation in text: [1] -
Herrero, M. T.; SanMartin, R.; Domínguez, E. Tetrahedron 2009, 65, 1500. doi:10.1016/j.tet.2008.11.062
Return to citation in text: [1] -
Prasad, D. J. C.; Naidu, A. B.; Sekar, G. Tetrahedron Lett. 2009, 50, 1411. doi:10.1016/j.tetlet.2009.01.022
Return to citation in text: [1] -
Wang, H.; Jiang, L.; Chen, T.; Li, Y. Eur. J. Org. Chem. 2010, 12, 2324. doi:10.1002/ejoc.201000003
Return to citation in text: [1] -
She, J.; Jiang, Z.; Wang, Y. Tetrahedron Lett. 2009, 50, 593. doi:10.1016/j.tetlet.2008.11.082
Return to citation in text: [1] -
Basu, B.; Mandal, B.; Das, S.; Kundu, S. Tetrahedron Lett. 2009, 50, 5523. doi:10.1016/j.tetlet.2009.07.076
Return to citation in text: [1] -
Feng, Y.; Wang, H.; Sun, F.; Li, Y.; Fu, X.; Jin, K. Tetrahedron 2009, 65, 9737. doi:10.1016/j.tet.2009.09.085
Return to citation in text: [1] -
Jogdand, N. R.; Shingate, B. B.; Shingare, M. S. Tetrahedron Lett. 2009, 50, 6092. doi:10.1016/j.tetlet.2009.08.064
Return to citation in text: [1] -
Feng, Y.-S.; Li, Y.-Y.; Tang, L.; Wu, W.; Xu., H.-J. 2010, 51, 2489. doi:10.1016/j.tetlet.2010.02.155
Return to citation in text: [1] -
Chen, C.-K.; Chen, Y.-W.; Lin, C.-H.; Lin, H.-P.; Lee, C.-F. Chem. Commun. 2010, 46, 282. doi:10.1039/b918117b
Return to citation in text: [1] -
Jammi, S.; Sakthivel, S.; Rout, L.; Mukherjee, T.; Mandal, S.; Mitra, R.; Saha, P.; Punniyamurthy, T. J. Org. Chem. 2009, 74, 1971. doi:10.1021/jo8024253
Return to citation in text: [1] -
Reddy, K. H. V.; Reddy, V. P.; Shankar, J.; Madhav, B.; Kumar, B. S. P. A.; Nageswar, Y. V. D. Tetrahedron Lett. 2011, 52, 2679. doi:10.1016/j.tetlet.2011.03.070
Return to citation in text: [1] -
Firouzabadi, H.; Iranpoor, N.; Gholinejad, M. Adv. Synth. Catal. 2010, 352, 119–124. doi:10.1002/adsc.200900671
Return to citation in text: [1] -
Correa, A.; Carril, M.; Bolm, C. Angew. Chem., Int. Ed. 2008, 47, 2880. doi:10.1002/anie.200705668
Return to citation in text: [1] -
Reddy, V. P.; Swapna, K.; Kumar, A. V.; Rao, K. R. J. Org. Chem. 2009, 74, 3189. doi:10.1021/jo802731j
Return to citation in text: [1] [2] -
Reddy, V. P.; Kumar, A. V.; Swapna, K.; Rao, K. R. Org. Lett. 2009, 11, 1697. doi:10.1021/ol900009a
Return to citation in text: [1] [2] -
Murthy, S. N.; Madhav, B.; Reddy, V. P.; Nageswar, Y. V. D. Eur. J. Org. Chem. 2009, 34, 5902. doi:10.1002/ejoc.200900989
Return to citation in text: [1] [2] -
Wong, Y.-C.; Jayanth, T. T.; Cheng, C.-H. Org. Lett. 2006, 8, 5613. doi:10.1021/ol062344l
Return to citation in text: [1] -
Ke, F.; Qu, Y.; Jiang, Z.; Li, Z.; Wu, D.; Zhou, X. Org. Lett. 2011, 13, 454. doi:10.1021/ol102784c
Return to citation in text: [1] [2] [3] -
Tao, C.; Lv, A.; Zhao, N.; Yang, S.; Liu, X.; Zhou, J.; Liu, W.; Zhao, J. Synlett 2011, 1, 134. doi:10.1055/s-0030-1259079
Return to citation in text: [1] [2] -
Pacchioni, G. Surf. Rev. Lett. 2000, 7, 277. doi:10.1142/S0218625X00000336
Return to citation in text: [1] -
Knight, W. D.; Clemenger, K.; De Heer, W. A.; Saunders, W. A.; Chou, M. Y.; Cohen, M. L. Phys. Rev. Lett. 1984, 52, 2141. doi:10.1103/PhysRevLett.52.2141
Return to citation in text: [1] [2] -
Kaldor, A.; Cox, D.; Zakin, M. R. Molecular Surface Chemistry: Reactions of Gas-Phase Metal Clusters. In Adv. Chem. Phys.; Prigogine, I.; Rice, S. A., Eds.; Part 2, Vol. 70; John Wiley & Sons, Inc.: Hoboken, NJ, 1988; pp 211 ff. doi:10.1002/9780470122693.ch6
Return to citation in text: [1] [2] -
Siegel, J. R.; Rosenblatt, D. H. J. Am. Chem. Soc. 1958, 80, 1753. doi:10.1021/ja01540a062
Return to citation in text: [1] -
Tarbell, D. S.; Harnish, D. P. Chem. Rev. 1951, 49, 1. doi:10.1021/cr60152a001
Return to citation in text: [1] -
Hoggarth, E.; Sexton, W. A. J. Chem. Soc. 1947, 815. doi:10.1039/JR9470000815
Return to citation in text: [1] -
Jiang, Y.; Qin, Y.; Xie, S.; Zhang, X.; Dong, J.; Ma, D. Org. Lett. 2009, 11, 5250. doi:10.1021/ol902186d
Return to citation in text: [1]
| 20. | Jammi, S.; Barua, P.; Rout, L.; Saha, P.; Punniyamurthy, T. Tetrahedron Lett. 2008, 49, 1484. doi:10.1016/j.tetlet.2007.12.118 |
| 39. | Reddy, V. P.; Swapna, K.; Kumar, A. V.; Rao, K. R. J. Org. Chem. 2009, 74, 3189. doi:10.1021/jo802731j |
| 40. | Reddy, V. P.; Kumar, A. V.; Swapna, K.; Rao, K. R. Org. Lett. 2009, 11, 1697. doi:10.1021/ol900009a |
| 41. | Murthy, S. N.; Madhav, B.; Reddy, V. P.; Nageswar, Y. V. D. Eur. J. Org. Chem. 2009, 34, 5902. doi:10.1002/ejoc.200900989 |
| 45. | Pacchioni, G. Surf. Rev. Lett. 2000, 7, 277. doi:10.1142/S0218625X00000336 |
| 46. | Knight, W. D.; Clemenger, K.; De Heer, W. A.; Saunders, W. A.; Chou, M. Y.; Cohen, M. L. Phys. Rev. Lett. 1984, 52, 2141. doi:10.1103/PhysRevLett.52.2141 |
| 47. | Kaldor, A.; Cox, D.; Zakin, M. R. Molecular Surface Chemistry: Reactions of Gas-Phase Metal Clusters. In Adv. Chem. Phys.; Prigogine, I.; Rice, S. A., Eds.; Part 2, Vol. 70; John Wiley & Sons, Inc.: Hoboken, NJ, 1988; pp 211 ff. doi:10.1002/9780470122693.ch6 |
| 46. | Knight, W. D.; Clemenger, K.; De Heer, W. A.; Saunders, W. A.; Chou, M. Y.; Cohen, M. L. Phys. Rev. Lett. 1984, 52, 2141. doi:10.1103/PhysRevLett.52.2141 |
| 47. | Kaldor, A.; Cox, D.; Zakin, M. R. Molecular Surface Chemistry: Reactions of Gas-Phase Metal Clusters. In Adv. Chem. Phys.; Prigogine, I.; Rice, S. A., Eds.; Part 2, Vol. 70; John Wiley & Sons, Inc.: Hoboken, NJ, 1988; pp 211 ff. doi:10.1002/9780470122693.ch6 |
| 48. | Siegel, J. R.; Rosenblatt, D. H. J. Am. Chem. Soc. 1958, 80, 1753. doi:10.1021/ja01540a062 |
| 49. | Tarbell, D. S.; Harnish, D. P. Chem. Rev. 1951, 49, 1. doi:10.1021/cr60152a001 |
| 50. | Hoggarth, E.; Sexton, W. A. J. Chem. Soc. 1947, 815. doi:10.1039/JR9470000815 |
| 51. | Jiang, Y.; Qin, Y.; Xie, S.; Zhang, X.; Dong, J.; Ma, D. Org. Lett. 2009, 11, 5250. doi:10.1021/ol902186d |
| 1. | Ullmann, F. Ber. Dtsch. Chem. Ges. 1903, 36, 2382. |
| 2. | Lindley, J. Tetrahedron 1984, 40, 1433. doi:10.1016/S0040-4020(01)91791-0 |
| 3. | Hassan, J.; Sévignon, M.; Gozzi, C.; Schulz, E.; Lemaire, M. Chem. Rev. 2002, 102, 1359. doi:10.1021/cr000664r |
| 12. | De Martino, G.; Edler, M. C.; La Regina, G.; Coluccia, A.; Barbera, M. C.; Barrow, D.; Nicholson, R. I.; Chiosis, G.; Brancale, A.; Hamel, E.; Artico, M.; Silvestri, R. J. Med. Chem. 2006, 49, 947. doi:10.1021/jm050809s |
| 43. | Ke, F.; Qu, Y.; Jiang, Z.; Li, Z.; Wu, D.; Zhou, X. Org. Lett. 2011, 13, 454. doi:10.1021/ol102784c |
| 10. | Tsutsui, H.; Hayashi, Y.; Narasaka, K. Chem. Lett. 1997, 26, 317. doi:10.1246/cl.1997.317 |
| 11. | Uchiyama, K.; Yoshida, M.; Hayashi, Y.; Narasaka, K. Chem. Lett. 1998, 27, 607. doi:10.1246/cl.1998.607 |
| 43. | Ke, F.; Qu, Y.; Jiang, Z.; Li, Z.; Wu, D.; Zhou, X. Org. Lett. 2011, 13, 454. doi:10.1021/ol102784c |
| 44. | Tao, C.; Lv, A.; Zhao, N.; Yang, S.; Liu, X.; Zhou, J.; Liu, W.; Zhao, J. Synlett 2011, 1, 134. doi:10.1055/s-0030-1259079 |
| 9. | Kaldor, S. W.; Kalish, V. J.; Davies, J. F., II; Shetty, B. V.; Fritz, J. E.; Appelt, K.; Burgess, J. A.; Campanale, K. M.; Chirgadze, N. Y.; Clawson, D. K.; Dressman, B. A.; Hatch, S. D.; Khalil, D. A.; Kosa, M. B.; Lubbehusen, P. P.; Muesing, M. A.; Patick, A. K.; Reich, S. H.; Su, K. S.; Tatlock, J. H. J. Med. Chem. 1997, 40, 3979. doi:10.1021/jm9704098 |
| 43. | Ke, F.; Qu, Y.; Jiang, Z.; Li, Z.; Wu, D.; Zhou, X. Org. Lett. 2011, 13, 454. doi:10.1021/ol102784c |
| 4. | Johannesson, P.; Lindeberg, G.; Johansson, A.; Nikiforovich, G. V.; Gogoll, A.; Synnergren, B.; Le Grèves, M.; Nyberg, F.; Karlén, A.; Hallberg, A. J. Med. Chem. 2002, 45, 1767. doi:10.1021/jm011063a |
| 5. | Mugesh, G.; Du Mont, W.-W.; Sies, H. Chem. Rev. 2001, 101, 2125. doi:10.1021/cr000426w |
| 6. | Morimoto, K.; Tsuji, K.; Iio, T.; Miyata, N.; Uchida, A.; Osawa, R.; Kitsutaka, H.; Takahashi, A. Carcinogenesis 1991, 12, 703. doi:10.1093/carcin/12.4.703 |
| 7. | Sader, H. S.; Johnson, D. M.; Jones, R. N. Antimicrob. Agents Chemother. 2004, 48, 53. doi:10.1128/AAC.48.1.53-62.2004 |
| 8. | Ceruti, M.; Balliano, G.; Rocco, F.; Milla, P.; Arpicco, S.; Cattel, L.; Viola, F. Lipids 2001, 36, 629. doi:10.1007/s11745-001-0767-8 |
| 44. | Tao, C.; Lv, A.; Zhao, N.; Yang, S.; Liu, X.; Zhou, J.; Liu, W.; Zhao, J. Synlett 2011, 1, 134. doi:10.1055/s-0030-1259079 |
| 38. | Correa, A.; Carril, M.; Bolm, C. Angew. Chem., Int. Ed. 2008, 47, 2880. doi:10.1002/anie.200705668 |
| 41. | Murthy, S. N.; Madhav, B.; Reddy, V. P.; Nageswar, Y. V. D. Eur. J. Org. Chem. 2009, 34, 5902. doi:10.1002/ejoc.200900989 |
| 21. | Kwong, F. Y.; Buchwald, S. L. Org. Lett. 2002, 4, 3517. doi:10.1021/ol0266673 |
| 22. | Chen, Y.-J.; Chen, H.-H. Org. Lett. 2006, 8, 5609. doi:10.1021/ol062339h |
| 23. | Taniguchi, N. J. Org. Chem. 2007, 72, 1241. doi:10.1021/jo062131+ |
| 24. | Sperotto, E.; Van Klink, G. P. M.; De Vries, J. G.; Van Koten, G. J. Org. Chem. 2008, 73, 5625. doi:10.1021/jo800491k |
| 25. | Rout, L.; Saha, P.; Jammi, S.; Punniyamurthy, T. Eur. J. Org. Chem. 2008, 640. doi:10.1002/ejoc.200700978 |
| 26. | Herrero, M. T.; SanMartin, R.; Domínguez, E. Tetrahedron 2009, 65, 1500. doi:10.1016/j.tet.2008.11.062 |
| 27. | Prasad, D. J. C.; Naidu, A. B.; Sekar, G. Tetrahedron Lett. 2009, 50, 1411. doi:10.1016/j.tetlet.2009.01.022 |
| 28. | Wang, H.; Jiang, L.; Chen, T.; Li, Y. Eur. J. Org. Chem. 2010, 12, 2324. doi:10.1002/ejoc.201000003 |
| 29. | She, J.; Jiang, Z.; Wang, Y. Tetrahedron Lett. 2009, 50, 593. doi:10.1016/j.tetlet.2008.11.082 |
| 30. | Basu, B.; Mandal, B.; Das, S.; Kundu, S. Tetrahedron Lett. 2009, 50, 5523. doi:10.1016/j.tetlet.2009.07.076 |
| 31. | Feng, Y.; Wang, H.; Sun, F.; Li, Y.; Fu, X.; Jin, K. Tetrahedron 2009, 65, 9737. doi:10.1016/j.tet.2009.09.085 |
| 32. | Jogdand, N. R.; Shingate, B. B.; Shingare, M. S. Tetrahedron Lett. 2009, 50, 6092. doi:10.1016/j.tetlet.2009.08.064 |
| 33. | Feng, Y.-S.; Li, Y.-Y.; Tang, L.; Wu, W.; Xu., H.-J. 2010, 51, 2489. doi:10.1016/j.tetlet.2010.02.155 |
| 34. | Chen, C.-K.; Chen, Y.-W.; Lin, C.-H.; Lin, H.-P.; Lee, C.-F. Chem. Commun. 2010, 46, 282. doi:10.1039/b918117b |
| 35. | Jammi, S.; Sakthivel, S.; Rout, L.; Mukherjee, T.; Mandal, S.; Mitra, R.; Saha, P.; Punniyamurthy, T. J. Org. Chem. 2009, 74, 1971. doi:10.1021/jo8024253 |
| 36. | Reddy, K. H. V.; Reddy, V. P.; Shankar, J.; Madhav, B.; Kumar, B. S. P. A.; Nageswar, Y. V. D. Tetrahedron Lett. 2011, 52, 2679. doi:10.1016/j.tetlet.2011.03.070 |
| 37. | Firouzabadi, H.; Iranpoor, N.; Gholinejad, M. Adv. Synth. Catal. 2010, 352, 119–124. doi:10.1002/adsc.200900671 |
| 42. | Wong, Y.-C.; Jayanth, T. T.; Cheng, C.-H. Org. Lett. 2006, 8, 5613. doi:10.1021/ol062344l |
| 19. | Taniguchi, N. J. Org. Chem. 2004, 69, 6904. doi:10.1021/jo040184q |
| 20. | Jammi, S.; Barua, P.; Rout, L.; Saha, P.; Punniyamurthy, T. Tetrahedron Lett. 2008, 49, 1484. doi:10.1016/j.tetlet.2007.12.118 |
| 13. | Murata, M.; Buchwald, S. L. Tetrahedron 2004, 60, 7397. doi:10.1016/j.tet.2004.05.044 |
| 14. | Mispelaere-Canivet, C.; Spindler, J.-F.; Perrio, S.; Beslin, P. Tetrahedron 2005, 61, 5253. doi:10.1016/j.tet.2005.03.078 |
| 15. | Itoh, T.; Mase, T. Org. Lett. 2004, 6, 4587. doi:10.1021/ol047996t |
| 16. | Fernández-Rodríguez, M. A.; Shen, Q.; Hartwig, J. F. J. Am. Chem. Soc. 2006, 128, 2180. doi:10.1021/ja0580340 |
| 17. | Lee, J.-Y.; Lee, P. H. J. Org. Chem. 2008, 73, 7413. doi:10.1021/jo801169h |
| 18. | Jiang, Z.; She, J.; Lina, X. Adv. Synth. Catal. 2009, 351, 2558. doi:10.1002/adsc.200900501 |
| 39. | Reddy, V. P.; Swapna, K.; Kumar, A. V.; Rao, K. R. J. Org. Chem. 2009, 74, 3189. doi:10.1021/jo802731j |
| 40. | Reddy, V. P.; Kumar, A. V.; Swapna, K.; Rao, K. R. Org. Lett. 2009, 11, 1697. doi:10.1021/ol900009a |
© 2011 Reddy et al; licensee Beilstein-Institut.
This is an Open Access article under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
The license is subject to the Beilstein Journal of Organic Chemistry terms and conditions: (http://www.beilstein-journals.org/bjoc)