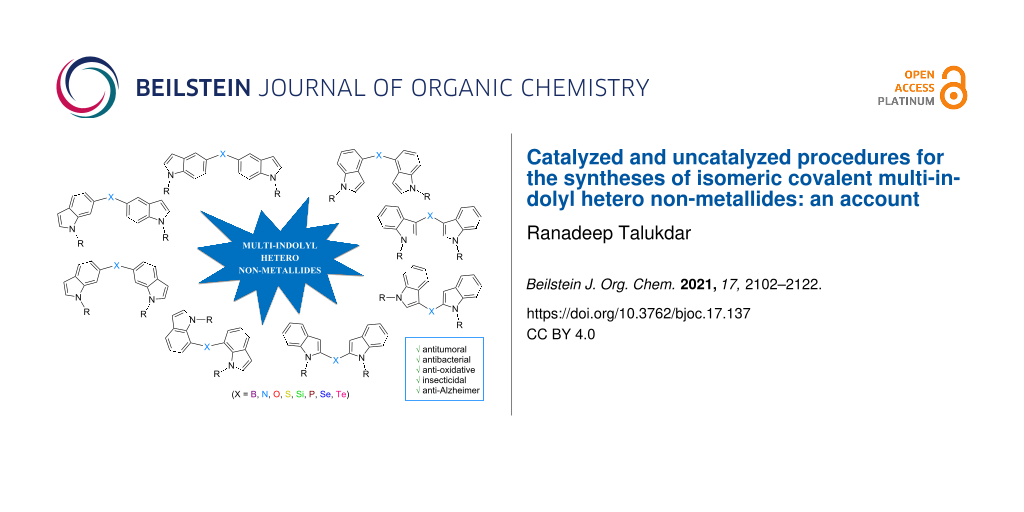
Abstract
Two or more indole molecules tailored to a single non-metal central atom, through any of their C2–7 positions are not only structurally engaging but also constitute a class of important pharmacophores. Although the body of such multi-indolyl non-metallide molecules are largely shared to the anticancer agent bis(indolyl)methane, other heteroatomic analogs also possess similar medicinal properties. This concise review will discuss various catalytic and uncatalytic synthetic strategies adopted for the synthesis of the non-ionic (non-metallic) versions of these important molecules till date.
Graphical Abstract
Introduction
Indole can be considered as a “prodigy” in the family of nitrogen-based heterocycles, because of its diverse presence in bioactive molecules [1-8], coupled with the distinct nucleophilic chemistry revolving its aromatic benzo-fused pyrrole system as encountered throughout the bibliography [9-15]. It is therefore obvious that a non-metal hydride will become exceptionally crucial when its hydrogen atoms are replaced by this special heterocycle, forming a multi-indolyl hetero non-metallide. In contemporary period, the said molecules have earned extensive importance in pharmacology to prevent cancer of a number of human organs, certified by the recent flooding of scientific literature related to bis(indolyl)methanes, which shows the usefulness of this class of molecules for prevention of this terminal disease [16-23]. Related molecules consisting of heteroatoms at the central tethering position have also appeared in the spotlight of anticancer research recently. In line with this high importance associated with the molecules of current topic, i.e., more than one indole molecule flanked by a central atom, conglomeration of the available synthetic methods will have a high scientific value. This review will give a concise account of the same, although preparations of ionic bis(indolyl) metal salts will not be considered [24-33].
Review
The pyrrole C2 and C3 linkages
By virtue of the two available sites in its pyrrole substructure, two indoles can be attached to a central atom via their C-2 or C-3 positions in a symmetric way. The non-symmetric variety may connect them with C-2 of one with the C-3 of another, via the central atom. Below described are such synthetic strategies which are classified depending on the central tethering atom, largely with boron, carbon, nitrogen, oxygen, silicon, phosphorus, sulfur, selenium, and tellurium. This review will skip the reports on the corresponding carbon-centered analogs.
Boranes
First discovered in 1894 [34], 2,2’-bisarylborinates are used for treating prostate cancers utilizing their property of inhibiting the transient receptor potential channels such as TRPM-7 [35]. In 2015, Murakami synthesized the novel indole C-2 borinic acid derivative 3 by reacting N-methylindole (1) with triisopropyl borate (2) in a strongly basic medium (Scheme 1). The product formation proceeds through the indole C-2 deprotonation mechanism [36].
Scheme 1: Synthesis of 2,2’-bis(indole)borinic ester 3.
Scheme 1: Synthesis of 2,2’-bis(indole)borinic ester 3.
The reason behind the C-2 attachment of the boron atom rather than at the C-3 position of the indole ring was explained by McGough et al. [37]. They performed a base-free catalytic I2-assisted indole C–H functionalization (electrophilic borylation) using the N-protected indole 1 and NHC·borane 4a that gave a mixture of the mono and bis isomers (5 and 6, respectively) in fair to excellent yields (Scheme 2a). Increasing the amount of iodine led to less unreacted starting material 1, and increased formation of the bisindole product 6. An almost quantitative conversion of 1 was observed with a high excess of the indole reactant.
Scheme 2: Synthesis of 2,2’-bisindole NHC·boranes by an SEAr mechanism.
Scheme 2: Synthesis of 2,2’-bisindole NHC·boranes by an SEAr mechanism.
It is seen that in the presence of a base the C-2 deprotonation becomes very fast in 9 (for regaining aromaticity) so the boron at the initial C-3-borylated intermediate 8 (formed via SEAr) cannot migrate fast enough, leading to a C-3 borylation product 10a (unlike Pd) [38-40]. Here the absence of the base resulted in a slow or no C-2 deprotonation of 9, which in turn forces the boron to migrate to C-2 from C-3 (8, Scheme 2b) to result in the C-2 borylation (10b).
Amines
Bis(indolyl)amines have recently become important as organic electroluminescent materials [41]. Hongtao and co-workers reported the synthesis of tetrakisindole species 13 through the coupling of aniline (12) and indole-2-boronic acid pinacol ester 11 using the Buchwald–Hartwig method (Scheme 3a) [42]. In a similar fashion, Han reported the syntheses of the symmetric and unsymmetric triaryl-substituted amines 15, 18, and 20 [43]. Taking aniline as the pivotal moiety, it was coupled with isomeric bromoindoles 14 and 16 for the synthesis of the targeted products (Scheme 3b).
Scheme 3: Syntheses of indolyl amines through Buchwald–Hartwig cross coupling.
Scheme 3: Syntheses of indolyl amines through Buchwald–Hartwig cross coupling.
Ethers
Hongtao and co-workers also studied the electroluminescence properties of the 3,3’-bis(indolyl) ether derivatives 23, 26, and 28. The materials were prepared by the Pd(0)-mediated coupling of lithium N-arylindole-3-alkoxide 21 with 3-bromo-N-arylindole 22, followed by a further C-2 bromination (24) and subsequent Suzuki reaction with boronic acids 27 or 25 (Scheme 4) [42]. A similar class of molecules have found broad applications in organic electroluminescent devices [44].
Scheme 4: Synthesis of 3,3’-bis(indolyl) ethers.
Scheme 4: Synthesis of 3,3’-bis(indolyl) ethers.
Silanes
Heteroaryl compounds containing silicon, an earth abundant and non-toxic element, are important in organic electronics or photonics and in the field of drug discovery and nuclear medicine [45-50].
The first property could be attributed to the facile orbital interactions of the σ* orbital of silicon and the π* orbital of the butadiene unit, which overall lowers the energy of the LUMO [51,52]. Known previously with expensive transition-metal catalyst (Ru) [53], Grubbs demonstrated the first KOt-Bu-catalyzed C2–H silylation of N-methylindole (1) with observed H2 evolution [54]. Here the di(indol-2-yl)silane (31) was found as a minor product though (Scheme 5a). The reaction has a high turnover number of 92 and it was halted in the presence of radical scavengers. However, the mechanism was unidentified, although it was proved to not going via a Minisci-type silyl radical addition [55], as the reaction with pyridine did not afford any product.
Bell studied the properties of such molecules which are similar to those used in OLED devices (organic light emitting diodes) in 2017. The molecule 34 was synthesized by base-mediated reaction of bisindole derivative 32 with Ph2SiCl2 (33, Scheme 5b) [56]. The dissociation of the indole C-2–Si bond upon UV light excitation generates a hole transport layer (HTL) in these materials, facilitating the optical activity [57].
In 1996, Frenzel reported the synthesis of bis(indol-3-yl)silane 38 that involved n-BuLi as the base [58]. The strategy was later adopted by Ohshita in 2004 (40a, Scheme 6) [59].
Scheme 6: n-BuLi-mediated syntheses of bis(indol-3-yl)silanes.
Scheme 6: n-BuLi-mediated syntheses of bis(indol-3-yl)silanes.
Between 2016 and 2018, some acid-catalyzed syntheses of bis(indol-3-yl)silanes appeared [60-63]. Chen and co-workers demonstrated a Brønsted acid-catalyzed Friedel–Crafts process, where hydrosilanes 41 were treated with an excess amount of indoles (Scheme 7a and Scheme 7b) [60]. Brookhart’s acid [H(OEt2)2]+[BArF4]− (42) was used to generate ether-stabilized silicon cations of type 46 and norbornene was added as a proton scavenger [64]. Following this procedure, Yonekura synthesized the similar compound 40, using a catalytic Lewis acid Zn(NTf2)2 and stoichiometric Lewis base γ-picoline combination in n-butyronitrile as solvent (Scheme 7c) [61]. This electron-donating solvent and toluene in the former reaction acted as stabilizers to the electron-deficient silicon species in the similar mechanisms. First, the Brønsted or Lewis acid coordinates with silane 51 leading to a solvent-stabilized electron-deficient silane complex 57, where N-protected indole attacks in a Friedel–Crafts fashion to give the 3-silylindoles 60 along with molecular hydrogen (Scheme 7b and Scheme 7d). A repetition of the processes leads to the bis(indol-3-yl)silanes 40.
Scheme 7: Acid-catalyzed syntheses of bis(indol-3-yl)silanes and mechanisms.
Scheme 7: Acid-catalyzed syntheses of bis(indol-3-yl)silanes and mechanisms.
Han described a Lewis acid-promoted C3-silylation of N-protected substituted indoles by a disproportionation mechanism of the latter. He used both B(C6F5)3 and Al(C6F5)3 in the reactions (Scheme 8a and Scheme 8c) which followed a similar mechanism (Scheme 8b) [62,63]. The reduced form of indole, i.e., indoline 50 coordinates with the Lewis acid to form a complex which activates PhSiH3 (frustrated Lewis pair) for silylation (69, Scheme 8b).
Scheme 8: B(C6F5)3 and Al(C6F5)3-catalyzed syntheses of bis(indol-3-yl)silanes reported by Han.
Scheme 8: B(C6F5)3 and Al(C6F5)3-catalyzed syntheses of bis(indol-3-yl)silanes reported by Han.
Phosphines
The base-mediated syntheses of bis(indol-2-yl)phosphines 76 and 78 were demonstrated by Yu. A suitable halophosphine 75 was reacted with C2-deprotonated C3-tethered (77) or untethered (74) N-protected indoles for that purpose (Scheme 9a) [65]. Later, Wassenaar reported a similar strategy with trichlorophosphine as the electrophile for attaching three indole moieties to a single P-atom (80, Scheme 9b) [66]. A similar protocol was adopted by van de Watering in their recent syntheses [67,68].
Scheme 9: Base-mediated syntheses of bis and tris(indol-2-yl)phosphines.
Scheme 9: Base-mediated syntheses of bis and tris(indol-2-yl)phosphines.
Sulfides
The C2 tethering of indoles with sulfur can be achieved in neutral medium by treatment with various SL2 (L is a leaving group) moieties [69,70]. This is a common method for the synthesis of bis(indol-2-yl)sulfides which are the precursors of potent bioactive molecules [71-73].
The simple synthetic strategies for the molecular units 82 were first reported by Barbier in 1989. The condensation of tryptamine monoacetate (81a) or indole oxime (81b) with sulfur dichloride in a Friedel–Crafts fashion (Scheme 10a) gave 82 with moderate to good product yields, respectively [69,70]. A similar work by Janosik involved strongly basic conditions at low temperature with bis(phenylsulfonyl)sulfide (83) as the sulfur donor (Scheme 10b) [73,74].
Scheme 10: Synthesis of bis(indol-2-yl)sulfides using SL2-type reagents.
Scheme 10: Synthesis of bis(indol-2-yl)sulfides using SL2-type reagents.
Disulfides are also important reagents for accessing bis(indolyl)sulfides. To synthesize the unsymmetrical bis(indolyl)sulfide 88, Janosik reacted the indole disulfide 87 with free indole and obtained the product 88 in 81% yield, where the sulfur linkages were 2,3’- with respect to the two indole nuclei (Scheme 11a) [73-76]. Hall and Dockendorf prepared the corresponding 2,2’-sulfur-substituted compounds 90 by reacting tryptophan amines 89 and 90 with S2Cl2 under neutral and acidic conditions, respectively (Scheme 11b and Scheme 11c) [77,78].
Scheme 11: Synthesis of 2,3’- and 2,2’-bis(indolyl)sulfides using disulfides as substrates.
Scheme 11: Synthesis of 2,3’- and 2,2’-bis(indolyl)sulfides using disulfides as substrates.
Kamal took a different approach using a CuO nanoparticle-supported graphene-oxide (denoted as CuO@GO, 0.38 mol %) catalyzed S-arylation (C–S coupling) of 2-iodoindole (92) to synthesize diindol-2-ylsulfide (84) in 75% yield (Scheme 12) [79]. Here 1.5 equivalents of thiourea acted as the sulfur source.
Scheme 12: Synthesis of diindol-2-ylsulfide (84) from 2-iodoindole (92) and thiourea.
Scheme 12: Synthesis of diindol-2-ylsulfide (84) from 2-iodoindole (92) and thiourea.
Bis(indol-3-yl)sulfides are also present as structural motifs in important organic compounds having semiconductor properties [80]. The syntheses of these compounds were studied by Janosik in 2006. The N-silyl-protected 3-bromoindole 93 was subjected to strong basic medium (t-BuLi) at low temperature and then quenched with either bis(phenylsulfonyl)sulfide (83) or indole disulfide 94 (Scheme 13) to afford the products 95 or 96, respectively [76].
Scheme 13: Synthesis of bis(indol-3-yl)sulfides using N-silylated 3-bromoindole 93.
Scheme 13: Synthesis of bis(indol-3-yl)sulfides using N-silylated 3-bromoindole 93.
Manishankar and co-workers dealt with a facile Fischer indole process to convert thiodiketones 97 to bis(indol-3-yl)sulfides 98 by refluxing them with phenylhydrazine hydrochloride salt in ethanol [81]. Interestingly, changing the solvent to THF switched the product to thioketone 99 (Scheme 14). Refluxing the thioketones 99 again with phenylhydrazine hydrochloride in ethanol resulted in the desired bis(indol-3-yl)sulfides 98. On the other hand, the treatment of thioketones 99 with phenylhydrazine afforded the corresponding hydrazones 100 only, thus stating the requirement of acid for this Fischer indole synthesis.
Scheme 14: Fischer indole synthesis of bis(indol-3-yl)sulfides using thio diketones.
Scheme 14: Fischer indole synthesis of bis(indol-3-yl)sulfides using thio diketones.
Elemental sulfur has also been utilized in preparing bis(indol-3-yl)sulfides under transition-metal compound catalyzed spontaneous oxidation of the central chalcogen atom. Such reactions were carried out by Shibahara (2014) and Yang (2016) [82,83]. Both reactions used aerial oxygen as the oxidizing agent for sulfur (Scheme 15). Shibahara utilized 20 mol % copper(I) thiophene-2-carboxylate (CuTC) as the catalyst, where heating N-methylindole (1) with elemental sulfur in DMSO as solvent at 90 °C under aerial oxygen led to the desired product 101 in 49% yield [82]. Other copper catalysts such as CuCl or CuBr gave low yields, even when used with 2,2’-bipyridyl as the ligand. First, oxidation of copper(I) takes place, which interacts with elemental sulfur to “activate” it. A nucleophilic attack from N-methylindole (1) to the sulfur species 102 takes place to generate copper sulfide complex 103. An oxidative homocoupling gives the bis(indol-3-yl)sulfide 101. Simultaneously, an oxidative homocoupling of the copper sulfide complex can take place to afford disulfide 104, that reacts with N-methylindole again under oxidative conditions, catalyzed by CuTC to give the desired product 101 (Scheme 15a).
Scheme 15: Oxidative synthesis of bis(indol-3-yl)sulfides using indoles and elemental sulfur.
Scheme 15: Oxidative synthesis of bis(indol-3-yl)sulfides using indoles and elemental sulfur.
On the other hand, Yang synthesized bis(indol-3-yl)sulfides 105 through the reaction of indole with elemental sulfur, catalyzed by iron(II) sulfate in the presence of stoichiometric amounts of KI in air [83]. The I− from KI formed ferrous iodide, which reacts with indole to form the iron bis-indolide 107, followed by reaction with N,N-dimethylmethanethioamide to get the S atom inserted (108). A reductive elimination then generated the bis(indol-3-yl)sulfides 105 along with Fe0, which was re-oxidized by aerial oxygen to re-participate in the reaction (Scheme 15b).
There are several uses of sulfoxides as a thiol-free sulfur source for introducing sulfur at the indole C3 position [84-86]. In 2013, Hamashima reported a synthesis of di(indol-3-yl)sulfide (105a) by reacting indole with DMSO in the presence of trifluoroacetic anhydride (TFAA) in total 6 steps (Scheme 16a) [84]. Already used by Hartke in 1988, this reagent combination (109) is a source for MeS+, so its use does not lead to any formation of disulfides [87]. First, 109 is attacked by indole and a demethylation of sulfur occurs leading to 3-(methylthio)indole (111). As the sulfur in 111 is methyl-protected, no dimerization occurs. Oxidation of sulfur by oxone followed by repetition of the previous steps afford the diindol-3-ylsulfonium salt 114, which in the presence of a base gives product 105a.
Scheme 16: Synthesis of bis(indol-3-yl)sulfides using sulfoxides as sulfur source.
Scheme 16: Synthesis of bis(indol-3-yl)sulfides using sulfoxides as sulfur source.
Li et al. used 2-(fluorosulfonyl)difluoroacetic acid (115) as the “S” source to synthesize bis(indol-3-yl)sulfides 116 from N-protected indoles 1 or 61 [85]. The products 116 were formed within a few seconds in the presence of a moderate base at high temperature (Scheme 16b), tolerating groups having both electron-donating and withdrawing nature on 1. Here the base assisted the condensation of 2-(fluorosulfonyl)difluoroacetic acid (115) with 1 followed by decarboxylation to give difluorocarbene and sulfinate 119, that combine to produce sulfanol 121, which in the presence of acid and reaction with another molecule of indole affords 105.
In 2018, Procter used a similar strategy to that reported by Hamashima for the synthesis of similar molecules 125 with good to moderate yields using electron-donating groups at the indole ring. The yields decreased with indoles having electron-withdrawing groups (Scheme 16c) [86]. Here diallyl sulfoxide (123) was used with TFAA to obtain diallyl intermediate 127. The latter undergoes a [3,3]-sigmatropic reaction to afford allyl (2-allylindol-3-yl)sulfide 128, which is oxidized by m-CPBA to sulfine 124. Repetition of the steps along with indole addition led to the desired products. Here the absence of a β-hydrogen in the diallylsulfoxide (123) did not allow any Pummerer rearrangement [88,89].
Selenides
In 1997, Showalter synthesized bis(indol-2-yl)selanes (or selenides) 130 having potential tyrosine kinase inhibitor activities [90,91]. The synthesis was achieved by reacting diselenium dichloride with (R)-tryptophan amide 129 (Scheme 17a) [92]. Bis(indol-2-yl)selane 130 was found as a byproduct having very low such bioactivity. The polyselanes formed were separated by treating them with NaBH4, which did not affect the monoselane 130.
Scheme 17: Syntheses of bis(indol-2-yl)selanes.
Scheme 17: Syntheses of bis(indol-2-yl)selanes.
On the other hand, selenopyrans structurally resemble indolocarbazoles, which possess AhR affinity [93]. Janosik presented a synthesis of such selenopyrans 132 via the bis(indol-2-yl)selanes 131 [73]. Treating these compounds with orthoformate esters in the presence of the Brønsted acid MeSO3H led to the target selenopyrans (Scheme 17b). The methylated analogs of 132 displayed high efficiency for activating AhR.
Bis(indol-3-yl)selanes possess antioxidant properties. Pioneered by Wilshire [94], their syntheses were studied by Abele [95], Naidu [96], Yang [83], Thurow [97], and Talukdar [98]. The work of Abele in 2004 involved refluxing SeO2 with N-unprotected indole in benzene which resulted in low yields of the products 134 (Scheme 18a) [44]. Using different N-protected substituted indoles 135, Naidu observed improved yields of 136 when catalytic oxidant I2 was added in 1,4-dioxane as solvent (Scheme 18b) [96]. Using aerial oxygen as the oxidant, Yang used Se0 in the presence of stoichiometric KI and catalytic amounts of FeII for the synthesis of similar bis(indol-3-yl)selanes (Scheme 18c) [83].
Scheme 18: Syntheses of bis(indol-3-yl)selanes.
Scheme 18: Syntheses of bis(indol-3-yl)selanes.
In 2018 Thurow reported a method using stoichiometric SeO2 along with sub-stoichiometric PhSSPh (138) to obtain a mixture of the desired diindol-3-ylselane (137a) along with mono- and di(phenylthio)-substituted indoles 139 and 140 (Scheme 18d) [97]. Catalytic iodine was used to oxidize PhSSPh (138) to PhSI (141), to which indole adds to give (phenylthio)indole 139 along with HI. HI reduces SeO2 to Se. Se interacts with two molecules of indole in the presence of air to give the desired product 137a. In a parallel pathway the product decomposes to selenone 144, 3-(phenylthio)indole (139) and regenerates Se.
In a recent effort by Talukdar, the cheap and non-anhydrous solvent ethanol was used to prepare the desired bis(indol-3-yl)selanes 136 in moderate yields [98]. Following the assumption (formation of triselenide 145) made by Wilshire [94] together with the detection of the oxidized products isatins in the reaction mixture, a disproportionation mechanism of SeO2 can be drawn giving bis(indol-3-yl)triselenide 145 and SeVI (Scheme 18e). The triselenide 145 converts into bis(indol-3-yl)selane 146 with liberation of Se0. SeVI can generate SeII or SeIV by either oxidizing indoles to isatins, or by a comproportionation reaction with Se0 to give 136.
Tellurides
Engman claimed a synthesis of the titular compounds 147 and 148 in the year 1994 by reacting the C2 anion 149 of the N-sulfonyl-protected indole 1o with metallic Te in four steps including desulfonylation (Scheme 19) [99]. The treatment with base followed by the addition of elemental tellurium to N-protected indole 1o generates lithium telluride 150. Telluride 150 is then oxidized to ditelluride 151 by treatment with ferrocyanide. A Cu powder-mediated reduction gives the N-protected bis(indol-2-yl)tellane 147. The final desulfonated product 148 is a potent thiol peroxidase reducing agent [100].
Scheme 19: Synthesis of bis(indol-2-yl)tellane 147.
Scheme 19: Synthesis of bis(indol-2-yl)tellane 147.
The benzenoid C4 and C7 linkages
The syntheses of bisindolyl non-metallides connected through benzenoid rings of the indoles are less studied compared to the same through their pyrrole counterpart. The corresponding compounds are investigated for boron, nitrogen, oxygen, sulfur, and selenium as the central connecting atom.
Boranes
The indole alkaloid dragmacidin D is a marine secondary metabolite which was recently found active against Parkinson’s and Alzheimer’s diseases [101-103]. In 2002, Jiang, while studying its synthesis, found the tris(indolyl)borane 154 instead of the desired chiral indole alcohol 155 while reacting the N-silylated 4-bromoindole 152 with n-BuLi in a failed regioselective ring opening attempt of chiral oxirane 153 in the presence of BF3.Et2O (Scheme 20) [104]. The synthetic route to the desired product was smoothly brought to its course by employing CuCN in the medium.
Scheme 20: Synthesis of tris(indolyl)borane 154.
Scheme 20: Synthesis of tris(indolyl)borane 154.
Amines
The enzymes indoleamine 2,3-dioxygenase 1 (IDO1) and tryptophan 2,3-dioxygenase (TDO) are responsible for tryptophan metabolism in the human body. Thus, the inhibition of these enzymes may help in tumor immunotherapy [105-107]. Xu recently found indole-2-carboxylic acid derivatives as IDO1/TDO dual inhibitors. In their effort to synthesize the following bis(indol-4-yl)amine derivatives via a Buchwald amination led to the 4-amino-substituted compounds 158 or acids 159 after basic hydrolysis (Scheme 21) [108]. Compound 159c had the maximum potency against IDO1 and TDO with IC50 values of 2.72 mM and 3.48 mM, respectively compared to 159a and 159b, which is 15 and 28.5 times higher than that of hit compound 160.
Scheme 21: Synthesis of bis(indol-4-yl)amines 159.
Scheme 21: Synthesis of bis(indol-4-yl)amines 159.
As discussed earlier, bis(indolyl)amines possess electroluminescent properties [41,109]. In 2009, Yagi and co-workers synthesized a large library of bis(indol-5-yl)amines 163 for studying their efficiency in organic electroluminescent devices, where 5-bromoindoles and 5-aminoindoles were taken as partners in a Buchwald coupling (Scheme 22a) [44]. On the other hand, in 2015, Organ’s group performed a phosphine-ligand free Buchwald amination of 5-chloroindole (164) with amine 165 to give the desired product 167, where the use of the Pd-PEPPSI-IPentCl precatalyst 166 in presence of the strong base led to the formation of the over-aminated product 168 (Scheme 22b) [110].
Scheme 22: Synthesis of bis(indol-5-yl)amines.
Scheme 22: Synthesis of bis(indol-5-yl)amines.
Alzheimer’s disease is caused by the β-amyloid-42 aggregation in brain tissue [111,112]. In 2017, Sreenivasachary synthesized a library of 6,5’- and 6,6’-bis(indolyl)amines and other similar 7-azaindole derivatives as potent anti-Alzheimer agents (171, 172) by a Buchwald coupling of the corresponding C3-substituted amines 170 and indole 5/6-bromides 169 (Scheme 23) [113]. Cyano, 4-piperidinyl and N-methylpiperidinyl substitutions at the indole and 7-azaindoles were necessary to improve the brain penetration ability of the products.
Scheme 23: Synthesis of 6,5’/6,6’-bis(indolyl)amines.
Scheme 23: Synthesis of 6,5’/6,6’-bis(indolyl)amines.
Up to >80% inhibition of the amyloid-β peptide aggregates were achieved with these compounds, with the highest activity found for the 4-N-methylpiperidyl derivative.
Ethers
The synthesis of the bis(indol-6-yl) ether 175 was performed by Chai in 2017. Their protocol used a Cu(OAc)2-mediated coupling of N-silylated 6-hydroxyindole 174 with the corresponding boronic acid 173 (Scheme 24) [114]. For further synthetic transformations of 175, N-protection with bromo esters 176 followed by hydrolysis towards acids 177a and 177b were performed. The products 177a and 177b are potent anti-HIV agents.
Scheme 24: Synthesis of potent HIV-inhibitors 6,6’-bis(indolyl) ethers.
Scheme 24: Synthesis of potent HIV-inhibitors 6,6’-bis(indolyl) ethers.
Although the synthesis of 7,7’-bis-indolyl ether was known prior to Chai’s report [114]. In 1989, Black found the 7,7’-dimerised product 179 of the indole derivative 178 as a hindered biphenyl analog via its prompt oxidation in the presence of quinones. The bis(indol-7-yl) ether 180 was found in 10% yield when chloranil was used as the oxidant (Scheme 25) [115]. The high electrophilicity of 178 at the C7 position resulted in this product formation. The reaction proceeds through the radical intermediate 181.
Scheme 25: Synthesis of bis(indol-7-yl) ether.
Scheme 25: Synthesis of bis(indol-7-yl) ether.
Sulfides
Reddy synthesized the di(indol-5-yl)sulfide (183) via a cascade strategy with 5-iodoindole (182) in the presence of thiourea and a recyclable CuO nanoparticle catalyst (Scheme 26) [116]. This heterogeneous catalysis strategy bypasses the use of unpleasant aryl thiols, which are generally coupled with other aryl halides in the presence of transition-metal catalysts for obtaining diaryl sulfides [117].
Scheme 26: Synthesis of di(indol-5-yl)sulfide (183).
Scheme 26: Synthesis of di(indol-5-yl)sulfide (183).
Selenides
Along with the oxygen insertion, Black et al. also performed the oxidative selenium insertion into the C-7 position of highly electrophilic 2-methylindole derivative 184. The dual role of selenium dioxide consists of activation of the C-7 position giving the dimerized 7,7’-bis(indolyl) products 185 with the 2-methyl group transformed to the aldehyde in the same step (Scheme 27) [118,119]. The less electronically activated N-acyl substrate gave a slightly better yield. Selenation occurs at C-3 instead of C-7 for the C-3 unsubstituted substrates.
Scheme 27: Syntheses of 2,2’-diformyl-7,7’-bis(indolyl)selenides.
Scheme 27: Syntheses of 2,2’-diformyl-7,7’-bis(indolyl)selenides.
Conclusion
This review summarizes the various (un)catalytic synthetic techniques of the symmetric and unsymmetric bis/tris(indolyl)-containing non-metallides consisting of multiple indole molecules covalently connected via C2, C3 (pyrrole ring) and C4–C7 (benzenoid ring) by different central atoms. Like the bis(indolyl)methanes (anticancer substances), these products are important potential pharmaceutically active ingredients as well. As a result, they have gathered much attention in the current decade as suggested by the number of contemporary publications associated. The described schemes involve both simple and challenging strategies depending on the central tethering atom involved. As time progresses, research on the synthesis and application of this class of molecules will be more broadened.
References
-
Rahman, A.; Basha, A. Indole Alkaloids, 1st ed.; Frontiers in Natural Product Research Series, Vol. 2; Taylor & Francis, 1998.
Return to citation in text: [1] -
Higuchi, K.; Kawasaki, T. Nat. Prod. Rep. 2007, 24, 843–868. doi:10.1039/b516351j
Return to citation in text: [1] -
Ruiz-Sanchis, P.; Savina, S. A.; Albericio, F.; Álvarez, M. Chem. – Eur. J. 2011, 17, 1388–1408. doi:10.1002/chem.201001451
Return to citation in text: [1] -
Kaushik, N. K.; Kaushik, N.; Attri, P.; Kumar, N.; Kim, C. H.; Verma, A. K.; Choi, E. H. Molecules 2013, 18, 6620–6662. doi:10.3390/molecules18066620
Return to citation in text: [1] -
Yanagihara, M.; Sasaki-Takahashi, N.; Sugahara, T.; Yamamoto, S.; Shinomi, M.; Yamashita, I.; Hayashida, M.; Yamanoha, B.; Numata, A.; Yamori, T.; Andoh, T. Cancer Sci. 2005, 96, 816–824. doi:10.1111/j.1349-7006.2005.00117.x
Return to citation in text: [1] -
Barden, T. C. Indoles: Industrial, Agricultural and Over-the-Counter Uses. In Heterocyclic Scaffolds II; Gribble, G., Ed.; Topics in Heterocyclic Chemistry, Vol. 26; Springer: Berlin, Heidelberg, 2011; pp 31–46. doi:10.1007/7081_2010_48
Return to citation in text: [1] -
Zhao, S.; Andrade, R. B. J. Am. Chem. Soc. 2013, 135, 13334–13337. doi:10.1021/ja408114u
Return to citation in text: [1] -
Teng, M.; Zi, W.; Ma, D. Angew. Chem., Int. Ed. 2014, 53, 1814–1817. doi:10.1002/anie.201310928
Return to citation in text: [1] -
Cacchi, S.; Fabrizi, G. Chem. Rev. 2011, 111, PR215–PR283. doi:10.1021/cr100403z
Return to citation in text: [1] -
Sundberg, R. J. The Chemistry of Indoles; Academic Press: New York, NY, USA, 1970.
Return to citation in text: [1] -
Lakhdar, S.; Westermaier, M.; Terrier, F.; Goumont, R.; Boubaker, T.; Ofial, A. R.; Mayr, H. J. Org. Chem. 2006, 71, 9088–9095. doi:10.1021/jo0614339
Return to citation in text: [1] -
Cooper, M. M.; Hignett, G. J.; Newton, R. F.; Joule, J. A.; Harris, M.; Hinchley, J. D. J. Chem. Soc., Chem. Commun. 1977, 432–434. doi:10.1039/c39770000432
Return to citation in text: [1] -
Kishbaugh, T. L. S. Reactions of Indole with Nucleophiles. In Heterocyclic Scaffolds II; Gribble, G., Ed.; Topics in Heterocyclic Chemistry, Vol. 26; Springer: Berlin, Heidelberg, 2010; pp 117–140. doi:10.1007/7081_2010_35
Return to citation in text: [1] -
Talukdar, R.; Tiwari, D. P.; Saha, A.; Ghorai, M. K. Org. Lett. 2014, 16, 3954–3957. doi:10.1021/ol501763n
Return to citation in text: [1] -
Talukdar, R. Org. Biomol. Chem. 2020, 18, 8876–8880. doi:10.1039/d0ob01977a
Return to citation in text: [1] -
Wu, H.; Liu, B.; Yang, K.; Winston-McPherson, G. N.; Leisten, E. D.; Vezina, C. M.; Ricke, W. A.; Peterson, R. E.; Tang, W. Bioorg. Med. Chem. Lett. 2020, 30, 126959. doi:10.1016/j.bmcl.2020.126959
Return to citation in text: [1] -
Winston-McPherson, G. N.; Xie, H.; Yang, K.; Li, X.; Shu, D.; Tang, W. Bioorg. Med. Chem. Lett. 2019, 29, 2345–2348. doi:10.1016/j.bmcl.2019.06.014
Return to citation in text: [1] -
Lucarini, S.; Antonietti, F.; Tontini, A.; Mestichelli, P.; Magnani, M.; Duranti, A. Tetrahedron Lett. 2011, 52, 2812–2814. doi:10.1016/j.tetlet.2011.03.117
Return to citation in text: [1] -
Noguchi-Yachide, T.; Tetsuhashi, M.; Aoyama, H.; Hashimoto, Y. Chem. Pharm. Bull. 2009, 57, 536–540. doi:10.1248/cpb.57.536
Return to citation in text: [1] -
Nazari, P.; Noroozi, M.; Papan, A. M.; Ghanavati, S. P. M. Int. J. Mod. Pharm. Res. 2019, 3 (6), 11–17.
Return to citation in text: [1] -
Gao, N.; Cheng, S.; Budhraja, A.; Liu, E.-H.; Chen, J.; Chen, D.; Yang, Z.; Luo, J.; Shi, X.; Zhang, Z. PLoS One 2012, 7, e31783. doi:10.1371/journal.pone.0031783
Return to citation in text: [1] -
Thomson, C. A.; Ho, E.; Strom, M. B. Nutr. Rev. 2016, 74, 432–443. doi:10.1093/nutrit/nuw010
Return to citation in text: [1] -
Shiri, M.; Zolfigol, M. A.; Kruger, H. G.; Tanbakouchian, Z. Chem. Rev. 2010, 110, 2250–2293. doi:10.1021/cr900195a
Return to citation in text: [1] -
Reck, C. E.; Bretschneider-Hurley, A.; Heeg, M. J.; Winter, C. H. Organometallics 1998, 17, 2906–2911. doi:10.1021/om980101u
Return to citation in text: [1] -
Miyoshi, T.; Takeda, N.; Fukami, M.; Sato, S.; Ueda, M.; Miyata, O. Chem. Pharm. Bull. 2014, 62, 927–932. doi:10.1248/cpb.c14-00404
Return to citation in text: [1] -
Boche, G.; Marsch, M.; Harbach, J.; Harms, K.; Ledig, B.; Schubert, F.; Lohrenz, J. C. W.; Ahlbrecht, H. Chem. Ber. 1993, 126, 1887–1894. doi:10.1002/cber.19931260820
Return to citation in text: [1] -
Guo, L.; Wang, S.; Wei, Y.; Zhou, S.; Zhu, X.; Mu, X. Inorg. Chem. 2017, 56, 6197–6207. doi:10.1021/acs.inorgchem.7b00179
Return to citation in text: [1] -
Chen, S.; Li, B.; Wang, X.; Huang, Y.; Li, J.; Zhu, H.; Zhao, L.; Frenking, G.; Roesky, H. W. Chem. – Eur. J. 2017, 23, 13633–13637. doi:10.1002/chem.201703804
Return to citation in text: [1] -
Zhang, G.; Deng, B.; Wang, S.; Wei, Y.; Zhou, S.; Zhu, X.; Huang, Z.; Mu, X. Dalton Trans. 2016, 45, 15445–15456. doi:10.1039/c6dt02922a
Return to citation in text: [1] -
Langer, J.; Krieck, S.; Görls, H.; Kreisel, G.; Seidel, W.; Westerhausen, M. New J. Chem. 2010, 34, 1667–1677. doi:10.1039/c0nj00136h
Return to citation in text: [1] -
Zhu, X.; Zhou, S.; Wang, S.; Wei, Y.; Zhang, L.; Wang, F.; Wang, S.; Feng, Z. Chem. Commun. 2012, 48, 12020–12022. doi:10.1039/c2cc36045d
Return to citation in text: [1] -
Zhu, X.; Wang, S.; Zhou, S.; Wei, Y.; Zhang, L.; Wang, F.; Feng, Z.; Guo, L.; Mu, X. Inorg. Chem. 2012, 51, 7134–7143. doi:10.1021/ic300137r
Return to citation in text: [1] -
Kamnev, A. A.; Shchelochkov, A. G.; Tarantilis, P. A.; Polissiou, M. G.; Perfiliev, Y. D. Monatsh. Chem. 2001, 132, 675–681. doi:10.1007/s007060170081
Return to citation in text: [1] -
Michaelis, A. Ber. Dtsch. Chem. Ges. 1894, 27, 244–262. doi:10.1002/cber.18940270150
Return to citation in text: [1] -
Chokshi, R.; Fruasaha, P.; Kozak, J. A. Channels 2012, 6, 362–369. doi:10.4161/chan.21628
Return to citation in text: [1] -
Murakami, S.; Suzuki, T. Method for producing boronic acid derivative, and novel boronic acid derivative. Eur. Pat. Appl. EP2886548A1, June 24, 2015.
Return to citation in text: [1] -
McGough, J. S.; Cid, J.; Ingleson, M. J. Chem. – Eur. J. 2017, 23, 8180–8184. doi:10.1002/chem.201702060
Return to citation in text: [1] -
Li, Y.; Wang, W.-H.; He, K.-H.; Shi, Z.-J. Organometallics 2012, 31, 4397–4400. doi:10.1021/om300284t
Return to citation in text: [1] -
Campeau, L.-C.; Parisien, M.; Jean, A.; Fagnou, K. J. Am. Chem. Soc. 2006, 128, 581–590. doi:10.1021/ja055819x
Return to citation in text: [1] -
Potavathri, S.; Pereira, K. C.; Gorelsky, S. I.; Pike, A.; LeBris, A. P.; DeBoef, B. J. Am. Chem. Soc. 2010, 132, 14676–14681. doi:10.1021/ja107159b
Return to citation in text: [1] -
Yen, F.-W.; Chang, C. H.; Teng, C.-M. Indenotriphenylene-based derivative for organic electroluminescent device. Eur. Pat. Appl. EP3059773A1, Jan 22, 2018.
Return to citation in text: [1] [2] -
Hongtao, F.; Yanrui, L.; Xing, W. Indole derivative an application thereof to organic electroluminescence. Chin. Pat. Appl. CN104725296A, June 24, 2015.
Return to citation in text: [1] [2] -
Han, S. I.; Kim, Y. B.; Kim, H. M. Preparation of fused heterocyclic compounds for organic electroluminescent devices. Kor. Patent KR2016077940A, July 4, 2016.
Return to citation in text: [1] -
Igarashi, T.; Yagi, K. Organic electroluminescent device, and new indole derivative. Jap. Patent JP2009076834, April 9, 2009.
Return to citation in text: [1] [2] [3] -
Zhang, F.; Wu, D.; Xu, Y.; Feng, X. J. Mater. Chem. 2011, 21, 17590–17600. doi:10.1039/c1jm12801a
Return to citation in text: [1] -
Showell, G. A.; Mills, J. S. Drug Discovery Today 2003, 8, 551–556. doi:10.1016/s1359-6446(03)02726-0
Return to citation in text: [1] -
Franz, A. K.; Wilson, S. O. J. Med. Chem. 2013, 56, 388–405. doi:10.1021/jm3010114
Return to citation in text: [1] -
Ball, L. T.; Lloyd-Jones, G. C.; Russell, C. A. Science 2012, 337, 1644–1648. doi:10.1126/science.1225709
Return to citation in text: [1] -
Denmark, S. E.; Baird, J. D. Chem. – Eur. J. 2006, 12, 4954–4963. doi:10.1002/chem.200600034
Return to citation in text: [1] -
Langkopf, E.; Schinzer, D. Chem. Rev. 1995, 95, 1375–1408. doi:10.1021/cr00037a011
Return to citation in text: [1] -
Cai, Y.; Qin, A.; Tang, B. Z. J. Mater. Chem. C 2017, 5, 7375–7389. doi:10.1039/c7tc02511d
Return to citation in text: [1] -
Tibbelin, J.; Wallner, A.; Emanuelsson, R.; Heijkenskjöld, F.; Rosenberg, M.; Yamazaki, K.; Nauroozi, D.; Karlsson, L.; Feifel, R.; Pettersson, R.; Baumgartner, J.; Ott, S.; Ottosson, H. Chem. Sci. 2014, 5, 360–371. doi:10.1039/c3sc52389f
Return to citation in text: [1] -
Klare, H. F. T.; Oestreich, M.; Ito, J.-i.; Nishiyama, H.; Ohki, Y.; Tatsumi, K. J. Am. Chem. Soc. 2011, 133, 3312–3315. doi:10.1021/ja111483r
Return to citation in text: [1] -
Toutov, A. A.; Liu, W.-B.; Betz, K. N.; Fedorov, A.; Stoltz, B. M.; Grubbs, R. H. Nature 2015, 518, 80–84. doi:10.1038/nature14126
Return to citation in text: [1] -
Du, W.; Kaskar, B.; Blumbergs, P.; Subramanian, P.-K.; Curran, D. P. Bioorg. Med. Chem. 2003, 11, 451–458. doi:10.1016/s0968-0896(02)00437-6
Return to citation in text: [1] -
Bell, B. M.; Clark, M. B., Jr.; Devore, D. D.; De Vries, T. S.; Froese, R. D.; Gray, K. C.; Jackson, D. H. K.; Kuech, T. F.; Na, H.-Y.; Kearns, K. L.; Lee, K.-J.; Mukhopadhyay, S.; Rachford, A. A.; Spencer, L. P.; Woodward, W. H. H. ACS Appl. Mater. Interfaces 2017, 9, 13369–13379. doi:10.1021/acsami.7b00208
Return to citation in text: [1] -
Cho, I.; Jeon, N. J.; Kwon, O. K.; Kim, D. W.; Jung, E. H.; Noh, J. H.; Seo, J.; Seok, S. I.; Park, S. Y. Chem. Sci. 2017, 8, 734–741. doi:10.1039/c6sc02832b
Return to citation in text: [1] -
Frenzel, A.; Herbst-Irmer, R.; Klingebiel, U.; Noltemeyer, M.; Rudolph, S. Main Group Chem. 1996, 1, 399–408. doi:10.1080/13583149612331338727
Return to citation in text: [1] -
Ohshita, J.; Lee, K.-H.; Kimura, K.; Kunai, A. Organometallics 2004, 23, 5622–5625. doi:10.1021/om049656h
Return to citation in text: [1] -
Chen, Q.-A.; Klare, H. F. T.; Oestreich, M. J. Am. Chem. Soc. 2016, 138, 7868–7871. doi:10.1021/jacs.6b04878
Return to citation in text: [1] [2] -
Yonekura, K.; Iketani, Y.; Sekine, M.; Tani, T.; Matsui, F.; Kamakura, D.; Tsuchimoto, T. Organometallics 2017, 36, 3234–3249. doi:10.1021/acs.organomet.7b00382
Return to citation in text: [1] [2] -
Han, Y.; Zhang, S.; He, J.; Zhang, Y. J. Am. Chem. Soc. 2017, 139, 7399–7407. doi:10.1021/jacs.7b03534
Return to citation in text: [1] [2] -
Han, Y.; Zhang, S.; He, J.; Zhang, Y. ACS Catal. 2018, 8, 8765–8773. doi:10.1021/acscatal.8b01847
Return to citation in text: [1] [2] -
Brookhart, M.; Grant, B.; Volpe, A. F., Jr. Organometallics 1992, 11, 3920–3922. doi:10.1021/om00059a071
Return to citation in text: [1] -
Yu, J. O.; Lam, E.; Sereda, J. L.; Rampersad, N. C.; Lough, A. J.; Browning, C. S.; Farrar, D. H. Organometallics 2005, 24, 37–47. doi:10.1021/om0401004
Return to citation in text: [1] -
Wassenaar, J.; de Bruin, B.; Siegler, M. A.; Spek, A. L.; Reek, J. N. H.; van der Vlugt, J. I. Chem. Commun. 2010, 46, 1232–1234. doi:10.1039/b917632b
Return to citation in text: [1] -
van de Watering, F. F.; van der Vlugt, J. I.; Dzik, W. I.; de Bruin, B.; Reek, J. N. H. Chem. – Eur. J. 2017, 23, 12709–12713. doi:10.1002/chem.201702727
Return to citation in text: [1] -
van de Watering, F. F.; Stroek, W.; van der Vlugt, J. I.; de Bruin, B.; Dzik, W. I.; Reek, J. N. H. Eur. J. Inorg. Chem. 2018, 1254–1265. doi:10.1002/ejic.201701209
Return to citation in text: [1] -
Barbier, M.; Devys, M. J. Heterocycl. Chem. 1989, 26, 265–267. doi:10.1002/jhet.5570260148
Return to citation in text: [1] [2] -
Devys, M.; Barbier, M. Synthesis 1990, 214–215. doi:10.1055/s-1990-26834
Return to citation in text: [1] [2] -
Torreilles, J.; Guérin, M.-C.; Previero, A. Biochimie 1985, 67, 929–947. doi:10.1016/s0300-9084(85)80289-3
Return to citation in text: [1] -
Devys, M.; Barbier, M.; Loiselet, I.; Rouxel, T.; Sarniguet, A.; Kollmann, A.; Bousquet, J.-F. Tetrahedron Lett. 1988, 29, 6447–6448. doi:10.1016/s0040-4039(00)82369-2
Return to citation in text: [1] -
Wincent, E.; Shirani, H.; Bergman, J.; Rannug, U.; Janosik, T. Bioorg. Med. Chem. 2009, 17, 1648–1653. doi:10.1016/j.bmc.2008.12.072
Return to citation in text: [1] [2] [3] [4] -
Shirani, H.; Janosik, T. J. Org. Chem. 2007, 72, 8984–8986. doi:10.1021/jo701627g
Return to citation in text: [1] [2] -
Janosik, T.; Bergman, J. Heterocycles 2002, 57, 1273–1278. doi:10.3987/com-02-9477
Return to citation in text: [1] -
Shirani, H.; Stensland, B.; Bergman, J.; Janosik, T. Synlett 2006, 2459–2463. doi:10.1055/s-2006-950427
Return to citation in text: [1] [2] -
Gao, D.; Sand, R.; Fu, H.; Sharmin, N.; Gallin, W. J.; Hall, D. G. Bioorg. Med. Chem. Lett. 2013, 23, 5503–5506. doi:10.1016/j.bmcl.2013.08.070
Return to citation in text: [1] -
Dockendorff, C.; Gandhi, D. M.; Kimball, I. H.; Eum, K. S.; Rusinova, R.; Ingólfsson, H. I.; Kapoor, R.; Peyear, T.; Dodge, M. W.; Martin, S. F.; Aldrich, R. W.; Andersen, O. S.; Sack, J. T. Biochemistry 2018, 57, 2733–2743. doi:10.1021/acs.biochem.8b00292
Return to citation in text: [1] -
Kamal, A.; Srinivasulu, V.; Murty, J. N. S. R. C.; Shankaraiah, N.; Nagesh, N.; Reddy, T. S.; Rao, A. V. S. Adv. Synth. Catal. 2013, 355, 2297–2307. doi:10.1002/adsc.201300416
Return to citation in text: [1] -
Hung, T. Q.; Dang, T. T.; Villinger, A.; Sung, T. V.; Langer, P. Org. Biomol. Chem. 2012, 10, 9041–9044. doi:10.1039/c2ob26489g
Return to citation in text: [1] -
Chitra, S.; Paul, N.; Muthusubramanian, S.; Manisankar, P. RSC Adv. 2012, 2, 1432–1438. doi:10.1039/c1ra00878a
Return to citation in text: [1] -
Shibahara, F.; Kanai, T.; Yamaguchi, E.; Kamei, A.; Yamauchi, T.; Murai, T. Chem. – Asian J. 2014, 9, 237–244. doi:10.1002/asia.201300882
Return to citation in text: [1] [2] -
Yang, Y.; Li, W.; Ying, B.; Liao, H.; Shen, C.; Zhang, P. ChemCatChem 2016, 8, 2916–2919. doi:10.1002/cctc.201600589
Return to citation in text: [1] [2] [3] [4] -
Hamashima, T.; Mori, Y.; Sawada, K.; Kasahara, Y.; Murayama, D.; Kamei, Y.; Okuno, H.; Yokoyama, Y.; Suzuki, H. Chem. Pharm. Bull. 2013, 61, 292–303. doi:10.1248/cpb.c12-00882
Return to citation in text: [1] [2] -
Li, Y.; Shi, L.-T.; Zhu, W.-Q.; Li, H.; Zhang, Q. Synlett 2018, 29, 1847–1850. doi:10.1055/s-0037-1609573
Return to citation in text: [1] [2] -
Šiaučiulis, M.; Sapmaz, S.; Pulis, A. P.; Procter, D. J. Chem. Sci. 2018, 9, 754–759. doi:10.1039/c7sc04723a
Return to citation in text: [1] [2] -
Hartke, K.; Teuber, D.; Gerber, H.-D. Tetrahedron 1988, 44, 3261–3270. doi:10.1016/s0040-4020(01)85959-7
Return to citation in text: [1] -
Kaiser, D.; Klose, I.; Oost, R.; Neuhaus, J.; Maulide, N. Chem. Rev. 2019, 119, 8701–8780. doi:10.1021/acs.chemrev.9b00111
Return to citation in text: [1] -
Fedorov, N. V.; Shevchenko, M. V.; Anisimov, A. V.; Viktorova, E. A. Chem. Heterocycl. Compd. 1985, 21, 624–626. doi:10.1007/bf00515059
Return to citation in text: [1] -
Pietras, R. J.; Arboleda, J.; Reese, D. M.; Wongvipat, N.; Pegram, M. D.; Ramos, L.; Gorman, C. M.; Parker, M. G.; Sliwkowski, M. X.; Slamon, D. J. Oncogene 1995, 10, 2435–2446.
Return to citation in text: [1] -
Ethier, S. P. J. Natl. Cancer Inst. 1995, 87, 964–973. doi:10.1093/jnci/87.13.964
Return to citation in text: [1] -
Showalter, H. D. H.; Sercel, A. D.; Leja, B. M.; Wolfangel, C. D.; Ambroso, L. A.; Elliott, W. L.; Fry, D. W.; Kraker, A. J.; Howard, C. T.; Lu, G. H.; Moore, C. W.; Nelson, J. M.; Roberts, B. J.; Vincent, P. W.; Denny, W. A.; Thompson, A. M. J. Med. Chem. 1997, 40, 413–426. doi:10.1021/jm960689b
Return to citation in text: [1] -
Nguyen, L. P.; Bradfield, C. A. Chem. Res. Toxicol. 2008, 21, 102–116. doi:10.1021/tx7001965
Return to citation in text: [1] -
Wilshire, J. F. K. Aust. J. Chem. 1967, 20, 359–364. doi:10.1071/ch9670359
Return to citation in text: [1] [2] -
Abele, E.; Popelis, J.; Shestakova, I.; Domracheva, I.; Arsenyan, P.; Lukevics, E. Chem. Heterocycl. Compd. 2004, 40, 742–746. doi:10.1023/b:cohc.0000040769.55088.e3
Return to citation in text: [1] -
Naidu, P. S.; Majumder, S.; Bhuyan, P. J. Mol. Diversity 2015, 19, 685–693. doi:10.1007/s11030-015-9605-3
Return to citation in text: [1] [2] -
Thurow, S.; Penteado, F.; Perin, G.; Alves, D.; Santi, C.; Monti, B.; Schiesser, C. H.; Barcellos, T.; Lenardão, E. J. Org. Chem. Front. 2018, 5, 1983–1991. doi:10.1039/c8qo00360b
Return to citation in text: [1] [2] -
Talukdar, R. Asian J. Org. Chem. 2019, 8, 88–92. doi:10.1002/ajoc.201800584
Return to citation in text: [1] [2] -
Engman, L.; Stern, D.; Pelcman, M.; Andersson, C. M. J. Org. Chem. 1994, 59, 1973–1979. doi:10.1021/jo00087a008
Return to citation in text: [1] -
Wendel, A. Phosphorus, Sulfur Silicon Relat. Elem. 1992, 67, 405–415. doi:10.1080/10426509208045863
Return to citation in text: [1] -
Marletta, M. A. J. Med. Chem. 1994, 37, 1899–1907. doi:10.1021/jm00039a001
Return to citation in text: [1] -
Molina, J. A.; Jiménez-Jiménez, F. J.; Ortí-Pareja, M.; Navarro, J. A. Drugs Aging 1998, 12, 251–259. doi:10.2165/00002512-199812040-00001
Return to citation in text: [1] -
Thorns, V.; Hansen, L.; Masliah, E. Exp. Neurol. 1998, 150, 14–20. doi:10.1006/exnr.1997.6751
Return to citation in text: [1] -
Yang, C.-G.; Wang, J.; Jiang, B. Tetrahedron Lett. 2002, 43, 1063–1066. doi:10.1016/s0040-4039(01)02331-0
Return to citation in text: [1] -
van Baren, N.; Van den Eynde, B. J. Front. Immunol. 2015, 6, 34. doi:10.3389/fimmu.2015.00034
Return to citation in text: [1] -
Ye, Z.; Yue, L.; Shi, J.; Shao, M.; Wu, T. J. Cancer (Wyoming, Aust.) 2019, 10, 2771–2782. doi:10.7150/jca.31727
Return to citation in text: [1] -
Abdel-Magid, A. F. ACS Med. Chem. Lett. 2017, 8, 11–13. doi:10.1021/acsmedchemlett.6b00458
Return to citation in text: [1] -
Cui, G.; Lai, F.; Wang, X.; Chen, X.; Xu, B. Eur. J. Med. Chem. 2020, 188, 111985. doi:10.1016/j.ejmech.2019.111985
Return to citation in text: [1] -
Nakayama, M.; Tsubaki, T. Indole derivative and application thereof. Jap. Pat. Appl. 2008133225A, June 12, 2008.
Return to citation in text: [1] -
Sharif, S.; Rucker, R. P.; Chandrasoma, N.; Mitchell, D.; Rodriguez, M. J.; Froese, R. D. J.; Organ, M. G. Angew. Chem., Int. Ed. 2015, 54, 9507–9511. doi:10.1002/anie.201502822
Return to citation in text: [1] -
Bharadwaj, P. R.; Dubey, A. K.; Masters, C. L.; Martins, R. N.; Macreadie, I. G. J. Cell. Mol. Med. 2009, 13, 412–421. doi:10.1111/j.1582-4934.2009.00609.x
Return to citation in text: [1] -
Murphy, M. P.; LeVine, H., III. J. Alzheimer's Dis. 2010, 19, 311–323. doi:10.3233/jad-2010-1221
Return to citation in text: [1] -
Sreenivasachary, N.; Kroth, H.; Benderitter, P.; Hamel, A.; Varisco, Y.; Hickman, D. T.; Froestl, W.; Pfeifer, A.; Muhs, A. Bioorg. Med. Chem. Lett. 2017, 27, 1405–1411. doi:10.1016/j.bmcl.2017.02.001
Return to citation in text: [1] -
Chai, Y.; Zou, M.; Zhu, M. New small molecule compound, its preparation method and the method using the compounds for treating/prevention HIV-1 aids infections. Chin. Pat. Appl. CN107089936A, Aug 25, 2017.
Return to citation in text: [1] [2] -
Black, D. StC.; Choy, A.; Craig, D. C.; Ivory, A. J.; Kumar, N. J. Chem. Soc., Chem. Commun. 1989, 111–112. doi:10.1039/c39890000111
Return to citation in text: [1] -
Reddy, K. H. V.; Reddy, V. P.; Shankar, J.; Madhav, B.; Kumar, B. S. P. A.; Nageswar, Y. V. D. Tetrahedron Lett. 2011, 52, 2679–2682. doi:10.1016/j.tetlet.2011.03.070
Return to citation in text: [1] -
Chen, L.; Fajer, A. N.; Yessimbekov, Z.; Kazemi, M.; Mohammadi, M. J. Sulfur Chem. 2019, 40, 451–468. doi:10.1080/17415993.2019.1596268
Return to citation in text: [1] -
Jones, A. W.; Wahyuningsih, T. D.; Pchalek, K.; Kumar, N.; Black, D. StC. Tetrahedron 2005, 61, 10490–10500. doi:10.1016/j.tet.2005.08.048
Return to citation in text: [1] -
Sokai, S.-I.; Kubo, A.; Katsuura, K.; Mochinaya, K.; Ezak, M. Chem. Pharm. Bull. 1972, 20, 76–81. doi:10.1248/cpb.20.76
Return to citation in text: [1]
| 62. | Han, Y.; Zhang, S.; He, J.; Zhang, Y. J. Am. Chem. Soc. 2017, 139, 7399–7407. doi:10.1021/jacs.7b03534 |
| 63. | Han, Y.; Zhang, S.; He, J.; Zhang, Y. ACS Catal. 2018, 8, 8765–8773. doi:10.1021/acscatal.8b01847 |
| 65. | Yu, J. O.; Lam, E.; Sereda, J. L.; Rampersad, N. C.; Lough, A. J.; Browning, C. S.; Farrar, D. H. Organometallics 2005, 24, 37–47. doi:10.1021/om0401004 |
| 41. | Yen, F.-W.; Chang, C. H.; Teng, C.-M. Indenotriphenylene-based derivative for organic electroluminescent device. Eur. Pat. Appl. EP3059773A1, Jan 22, 2018. |
| 109. | Nakayama, M.; Tsubaki, T. Indole derivative and application thereof. Jap. Pat. Appl. 2008133225A, June 12, 2008. |
| 66. | Wassenaar, J.; de Bruin, B.; Siegler, M. A.; Spek, A. L.; Reek, J. N. H.; van der Vlugt, J. I. Chem. Commun. 2010, 46, 1232–1234. doi:10.1039/b917632b |
| 44. | Igarashi, T.; Yagi, K. Organic electroluminescent device, and new indole derivative. Jap. Patent JP2009076834, April 9, 2009. |
| 105. | van Baren, N.; Van den Eynde, B. J. Front. Immunol. 2015, 6, 34. doi:10.3389/fimmu.2015.00034 |
| 106. | Ye, Z.; Yue, L.; Shi, J.; Shao, M.; Wu, T. J. Cancer (Wyoming, Aust.) 2019, 10, 2771–2782. doi:10.7150/jca.31727 |
| 107. | Abdel-Magid, A. F. ACS Med. Chem. Lett. 2017, 8, 11–13. doi:10.1021/acsmedchemlett.6b00458 |
| 108. | Cui, G.; Lai, F.; Wang, X.; Chen, X.; Xu, B. Eur. J. Med. Chem. 2020, 188, 111985. doi:10.1016/j.ejmech.2019.111985 |
| 104. | Yang, C.-G.; Wang, J.; Jiang, B. Tetrahedron Lett. 2002, 43, 1063–1066. doi:10.1016/s0040-4039(01)02331-0 |
| 77. | Gao, D.; Sand, R.; Fu, H.; Sharmin, N.; Gallin, W. J.; Hall, D. G. Bioorg. Med. Chem. Lett. 2013, 23, 5503–5506. doi:10.1016/j.bmcl.2013.08.070 |
| 78. | Dockendorff, C.; Gandhi, D. M.; Kimball, I. H.; Eum, K. S.; Rusinova, R.; Ingólfsson, H. I.; Kapoor, R.; Peyear, T.; Dodge, M. W.; Martin, S. F.; Aldrich, R. W.; Andersen, O. S.; Sack, J. T. Biochemistry 2018, 57, 2733–2743. doi:10.1021/acs.biochem.8b00292 |
| 79. | Kamal, A.; Srinivasulu, V.; Murty, J. N. S. R. C.; Shankaraiah, N.; Nagesh, N.; Reddy, T. S.; Rao, A. V. S. Adv. Synth. Catal. 2013, 355, 2297–2307. doi:10.1002/adsc.201300416 |
| 73. | Wincent, E.; Shirani, H.; Bergman, J.; Rannug, U.; Janosik, T. Bioorg. Med. Chem. 2009, 17, 1648–1653. doi:10.1016/j.bmc.2008.12.072 |
| 74. | Shirani, H.; Janosik, T. J. Org. Chem. 2007, 72, 8984–8986. doi:10.1021/jo701627g |
| 114. | Chai, Y.; Zou, M.; Zhu, M. New small molecule compound, its preparation method and the method using the compounds for treating/prevention HIV-1 aids infections. Chin. Pat. Appl. CN107089936A, Aug 25, 2017. |
| 73. | Wincent, E.; Shirani, H.; Bergman, J.; Rannug, U.; Janosik, T. Bioorg. Med. Chem. 2009, 17, 1648–1653. doi:10.1016/j.bmc.2008.12.072 |
| 74. | Shirani, H.; Janosik, T. J. Org. Chem. 2007, 72, 8984–8986. doi:10.1021/jo701627g |
| 75. | Janosik, T.; Bergman, J. Heterocycles 2002, 57, 1273–1278. doi:10.3987/com-02-9477 |
| 76. | Shirani, H.; Stensland, B.; Bergman, J.; Janosik, T. Synlett 2006, 2459–2463. doi:10.1055/s-2006-950427 |
| 71. | Torreilles, J.; Guérin, M.-C.; Previero, A. Biochimie 1985, 67, 929–947. doi:10.1016/s0300-9084(85)80289-3 |
| 72. | Devys, M.; Barbier, M.; Loiselet, I.; Rouxel, T.; Sarniguet, A.; Kollmann, A.; Bousquet, J.-F. Tetrahedron Lett. 1988, 29, 6447–6448. doi:10.1016/s0040-4039(00)82369-2 |
| 73. | Wincent, E.; Shirani, H.; Bergman, J.; Rannug, U.; Janosik, T. Bioorg. Med. Chem. 2009, 17, 1648–1653. doi:10.1016/j.bmc.2008.12.072 |
| 113. | Sreenivasachary, N.; Kroth, H.; Benderitter, P.; Hamel, A.; Varisco, Y.; Hickman, D. T.; Froestl, W.; Pfeifer, A.; Muhs, A. Bioorg. Med. Chem. Lett. 2017, 27, 1405–1411. doi:10.1016/j.bmcl.2017.02.001 |
| 69. | Barbier, M.; Devys, M. J. Heterocycl. Chem. 1989, 26, 265–267. doi:10.1002/jhet.5570260148 |
| 70. | Devys, M.; Barbier, M. Synthesis 1990, 214–215. doi:10.1055/s-1990-26834 |
| 114. | Chai, Y.; Zou, M.; Zhu, M. New small molecule compound, its preparation method and the method using the compounds for treating/prevention HIV-1 aids infections. Chin. Pat. Appl. CN107089936A, Aug 25, 2017. |
| 67. | van de Watering, F. F.; van der Vlugt, J. I.; Dzik, W. I.; de Bruin, B.; Reek, J. N. H. Chem. – Eur. J. 2017, 23, 12709–12713. doi:10.1002/chem.201702727 |
| 68. | van de Watering, F. F.; Stroek, W.; van der Vlugt, J. I.; de Bruin, B.; Dzik, W. I.; Reek, J. N. H. Eur. J. Inorg. Chem. 2018, 1254–1265. doi:10.1002/ejic.201701209 |
| 110. | Sharif, S.; Rucker, R. P.; Chandrasoma, N.; Mitchell, D.; Rodriguez, M. J.; Froese, R. D. J.; Organ, M. G. Angew. Chem., Int. Ed. 2015, 54, 9507–9511. doi:10.1002/anie.201502822 |
| 69. | Barbier, M.; Devys, M. J. Heterocycl. Chem. 1989, 26, 265–267. doi:10.1002/jhet.5570260148 |
| 70. | Devys, M.; Barbier, M. Synthesis 1990, 214–215. doi:10.1055/s-1990-26834 |
| 111. | Bharadwaj, P. R.; Dubey, A. K.; Masters, C. L.; Martins, R. N.; Macreadie, I. G. J. Cell. Mol. Med. 2009, 13, 412–421. doi:10.1111/j.1582-4934.2009.00609.x |
| 112. | Murphy, M. P.; LeVine, H., III. J. Alzheimer's Dis. 2010, 19, 311–323. doi:10.3233/jad-2010-1221 |
| 80. | Hung, T. Q.; Dang, T. T.; Villinger, A.; Sung, T. V.; Langer, P. Org. Biomol. Chem. 2012, 10, 9041–9044. doi:10.1039/c2ob26489g |
| 76. | Shirani, H.; Stensland, B.; Bergman, J.; Janosik, T. Synlett 2006, 2459–2463. doi:10.1055/s-2006-950427 |
| 81. | Chitra, S.; Paul, N.; Muthusubramanian, S.; Manisankar, P. RSC Adv. 2012, 2, 1432–1438. doi:10.1039/c1ra00878a |
| 117. | Chen, L.; Fajer, A. N.; Yessimbekov, Z.; Kazemi, M.; Mohammadi, M. J. Sulfur Chem. 2019, 40, 451–468. doi:10.1080/17415993.2019.1596268 |
| 118. | Jones, A. W.; Wahyuningsih, T. D.; Pchalek, K.; Kumar, N.; Black, D. StC. Tetrahedron 2005, 61, 10490–10500. doi:10.1016/j.tet.2005.08.048 |
| 119. | Sokai, S.-I.; Kubo, A.; Katsuura, K.; Mochinaya, K.; Ezak, M. Chem. Pharm. Bull. 1972, 20, 76–81. doi:10.1248/cpb.20.76 |
| 115. | Black, D. StC.; Choy, A.; Craig, D. C.; Ivory, A. J.; Kumar, N. J. Chem. Soc., Chem. Commun. 1989, 111–112. doi:10.1039/c39890000111 |
| 116. | Reddy, K. H. V.; Reddy, V. P.; Shankar, J.; Madhav, B.; Kumar, B. S. P. A.; Nageswar, Y. V. D. Tetrahedron Lett. 2011, 52, 2679–2682. doi:10.1016/j.tetlet.2011.03.070 |
| 85. | Li, Y.; Shi, L.-T.; Zhu, W.-Q.; Li, H.; Zhang, Q. Synlett 2018, 29, 1847–1850. doi:10.1055/s-0037-1609573 |
| 86. | Šiaučiulis, M.; Sapmaz, S.; Pulis, A. P.; Procter, D. J. Chem. Sci. 2018, 9, 754–759. doi:10.1039/c7sc04723a |
| 84. | Hamashima, T.; Mori, Y.; Sawada, K.; Kasahara, Y.; Murayama, D.; Kamei, Y.; Okuno, H.; Yokoyama, Y.; Suzuki, H. Chem. Pharm. Bull. 2013, 61, 292–303. doi:10.1248/cpb.c12-00882 |
| 87. | Hartke, K.; Teuber, D.; Gerber, H.-D. Tetrahedron 1988, 44, 3261–3270. doi:10.1016/s0040-4020(01)85959-7 |
| 83. | Yang, Y.; Li, W.; Ying, B.; Liao, H.; Shen, C.; Zhang, P. ChemCatChem 2016, 8, 2916–2919. doi:10.1002/cctc.201600589 |
| 84. | Hamashima, T.; Mori, Y.; Sawada, K.; Kasahara, Y.; Murayama, D.; Kamei, Y.; Okuno, H.; Yokoyama, Y.; Suzuki, H. Chem. Pharm. Bull. 2013, 61, 292–303. doi:10.1248/cpb.c12-00882 |
| 85. | Li, Y.; Shi, L.-T.; Zhu, W.-Q.; Li, H.; Zhang, Q. Synlett 2018, 29, 1847–1850. doi:10.1055/s-0037-1609573 |
| 86. | Šiaučiulis, M.; Sapmaz, S.; Pulis, A. P.; Procter, D. J. Chem. Sci. 2018, 9, 754–759. doi:10.1039/c7sc04723a |
| 82. | Shibahara, F.; Kanai, T.; Yamaguchi, E.; Kamei, A.; Yamauchi, T.; Murai, T. Chem. – Asian J. 2014, 9, 237–244. doi:10.1002/asia.201300882 |
| 83. | Yang, Y.; Li, W.; Ying, B.; Liao, H.; Shen, C.; Zhang, P. ChemCatChem 2016, 8, 2916–2919. doi:10.1002/cctc.201600589 |
| 82. | Shibahara, F.; Kanai, T.; Yamaguchi, E.; Kamei, A.; Yamauchi, T.; Murai, T. Chem. – Asian J. 2014, 9, 237–244. doi:10.1002/asia.201300882 |
| 90. | Pietras, R. J.; Arboleda, J.; Reese, D. M.; Wongvipat, N.; Pegram, M. D.; Ramos, L.; Gorman, C. M.; Parker, M. G.; Sliwkowski, M. X.; Slamon, D. J. Oncogene 1995, 10, 2435–2446. |
| 91. | Ethier, S. P. J. Natl. Cancer Inst. 1995, 87, 964–973. doi:10.1093/jnci/87.13.964 |
| 92. | Showalter, H. D. H.; Sercel, A. D.; Leja, B. M.; Wolfangel, C. D.; Ambroso, L. A.; Elliott, W. L.; Fry, D. W.; Kraker, A. J.; Howard, C. T.; Lu, G. H.; Moore, C. W.; Nelson, J. M.; Roberts, B. J.; Vincent, P. W.; Denny, W. A.; Thompson, A. M. J. Med. Chem. 1997, 40, 413–426. doi:10.1021/jm960689b |
| 88. | Kaiser, D.; Klose, I.; Oost, R.; Neuhaus, J.; Maulide, N. Chem. Rev. 2019, 119, 8701–8780. doi:10.1021/acs.chemrev.9b00111 |
| 89. | Fedorov, N. V.; Shevchenko, M. V.; Anisimov, A. V.; Viktorova, E. A. Chem. Heterocycl. Compd. 1985, 21, 624–626. doi:10.1007/bf00515059 |
| 1. | Rahman, A.; Basha, A. Indole Alkaloids, 1st ed.; Frontiers in Natural Product Research Series, Vol. 2; Taylor & Francis, 1998. |
| 2. | Higuchi, K.; Kawasaki, T. Nat. Prod. Rep. 2007, 24, 843–868. doi:10.1039/b516351j |
| 3. | Ruiz-Sanchis, P.; Savina, S. A.; Albericio, F.; Álvarez, M. Chem. – Eur. J. 2011, 17, 1388–1408. doi:10.1002/chem.201001451 |
| 4. | Kaushik, N. K.; Kaushik, N.; Attri, P.; Kumar, N.; Kim, C. H.; Verma, A. K.; Choi, E. H. Molecules 2013, 18, 6620–6662. doi:10.3390/molecules18066620 |
| 5. | Yanagihara, M.; Sasaki-Takahashi, N.; Sugahara, T.; Yamamoto, S.; Shinomi, M.; Yamashita, I.; Hayashida, M.; Yamanoha, B.; Numata, A.; Yamori, T.; Andoh, T. Cancer Sci. 2005, 96, 816–824. doi:10.1111/j.1349-7006.2005.00117.x |
| 6. | Barden, T. C. Indoles: Industrial, Agricultural and Over-the-Counter Uses. In Heterocyclic Scaffolds II; Gribble, G., Ed.; Topics in Heterocyclic Chemistry, Vol. 26; Springer: Berlin, Heidelberg, 2011; pp 31–46. doi:10.1007/7081_2010_48 |
| 7. | Zhao, S.; Andrade, R. B. J. Am. Chem. Soc. 2013, 135, 13334–13337. doi:10.1021/ja408114u |
| 8. | Teng, M.; Zi, W.; Ma, D. Angew. Chem., Int. Ed. 2014, 53, 1814–1817. doi:10.1002/anie.201310928 |
| 34. | Michaelis, A. Ber. Dtsch. Chem. Ges. 1894, 27, 244–262. doi:10.1002/cber.18940270150 |
| 45. | Zhang, F.; Wu, D.; Xu, Y.; Feng, X. J. Mater. Chem. 2011, 21, 17590–17600. doi:10.1039/c1jm12801a |
| 46. | Showell, G. A.; Mills, J. S. Drug Discovery Today 2003, 8, 551–556. doi:10.1016/s1359-6446(03)02726-0 |
| 47. | Franz, A. K.; Wilson, S. O. J. Med. Chem. 2013, 56, 388–405. doi:10.1021/jm3010114 |
| 48. | Ball, L. T.; Lloyd-Jones, G. C.; Russell, C. A. Science 2012, 337, 1644–1648. doi:10.1126/science.1225709 |
| 49. | Denmark, S. E.; Baird, J. D. Chem. – Eur. J. 2006, 12, 4954–4963. doi:10.1002/chem.200600034 |
| 50. | Langkopf, E.; Schinzer, D. Chem. Rev. 1995, 95, 1375–1408. doi:10.1021/cr00037a011 |
| 97. | Thurow, S.; Penteado, F.; Perin, G.; Alves, D.; Santi, C.; Monti, B.; Schiesser, C. H.; Barcellos, T.; Lenardão, E. J. Org. Chem. Front. 2018, 5, 1983–1991. doi:10.1039/c8qo00360b |
| 24. | Reck, C. E.; Bretschneider-Hurley, A.; Heeg, M. J.; Winter, C. H. Organometallics 1998, 17, 2906–2911. doi:10.1021/om980101u |
| 25. | Miyoshi, T.; Takeda, N.; Fukami, M.; Sato, S.; Ueda, M.; Miyata, O. Chem. Pharm. Bull. 2014, 62, 927–932. doi:10.1248/cpb.c14-00404 |
| 26. | Boche, G.; Marsch, M.; Harbach, J.; Harms, K.; Ledig, B.; Schubert, F.; Lohrenz, J. C. W.; Ahlbrecht, H. Chem. Ber. 1993, 126, 1887–1894. doi:10.1002/cber.19931260820 |
| 27. | Guo, L.; Wang, S.; Wei, Y.; Zhou, S.; Zhu, X.; Mu, X. Inorg. Chem. 2017, 56, 6197–6207. doi:10.1021/acs.inorgchem.7b00179 |
| 28. | Chen, S.; Li, B.; Wang, X.; Huang, Y.; Li, J.; Zhu, H.; Zhao, L.; Frenking, G.; Roesky, H. W. Chem. – Eur. J. 2017, 23, 13633–13637. doi:10.1002/chem.201703804 |
| 29. | Zhang, G.; Deng, B.; Wang, S.; Wei, Y.; Zhou, S.; Zhu, X.; Huang, Z.; Mu, X. Dalton Trans. 2016, 45, 15445–15456. doi:10.1039/c6dt02922a |
| 30. | Langer, J.; Krieck, S.; Görls, H.; Kreisel, G.; Seidel, W.; Westerhausen, M. New J. Chem. 2010, 34, 1667–1677. doi:10.1039/c0nj00136h |
| 31. | Zhu, X.; Zhou, S.; Wang, S.; Wei, Y.; Zhang, L.; Wang, F.; Wang, S.; Feng, Z. Chem. Commun. 2012, 48, 12020–12022. doi:10.1039/c2cc36045d |
| 32. | Zhu, X.; Wang, S.; Zhou, S.; Wei, Y.; Zhang, L.; Wang, F.; Feng, Z.; Guo, L.; Mu, X. Inorg. Chem. 2012, 51, 7134–7143. doi:10.1021/ic300137r |
| 33. | Kamnev, A. A.; Shchelochkov, A. G.; Tarantilis, P. A.; Polissiou, M. G.; Perfiliev, Y. D. Monatsh. Chem. 2001, 132, 675–681. doi:10.1007/s007060170081 |
| 51. | Cai, Y.; Qin, A.; Tang, B. Z. J. Mater. Chem. C 2017, 5, 7375–7389. doi:10.1039/c7tc02511d |
| 52. | Tibbelin, J.; Wallner, A.; Emanuelsson, R.; Heijkenskjöld, F.; Rosenberg, M.; Yamazaki, K.; Nauroozi, D.; Karlsson, L.; Feifel, R.; Pettersson, R.; Baumgartner, J.; Ott, S.; Ottosson, H. Chem. Sci. 2014, 5, 360–371. doi:10.1039/c3sc52389f |
| 16. | Wu, H.; Liu, B.; Yang, K.; Winston-McPherson, G. N.; Leisten, E. D.; Vezina, C. M.; Ricke, W. A.; Peterson, R. E.; Tang, W. Bioorg. Med. Chem. Lett. 2020, 30, 126959. doi:10.1016/j.bmcl.2020.126959 |
| 17. | Winston-McPherson, G. N.; Xie, H.; Yang, K.; Li, X.; Shu, D.; Tang, W. Bioorg. Med. Chem. Lett. 2019, 29, 2345–2348. doi:10.1016/j.bmcl.2019.06.014 |
| 18. | Lucarini, S.; Antonietti, F.; Tontini, A.; Mestichelli, P.; Magnani, M.; Duranti, A. Tetrahedron Lett. 2011, 52, 2812–2814. doi:10.1016/j.tetlet.2011.03.117 |
| 19. | Noguchi-Yachide, T.; Tetsuhashi, M.; Aoyama, H.; Hashimoto, Y. Chem. Pharm. Bull. 2009, 57, 536–540. doi:10.1248/cpb.57.536 |
| 20. | Nazari, P.; Noroozi, M.; Papan, A. M.; Ghanavati, S. P. M. Int. J. Mod. Pharm. Res. 2019, 3 (6), 11–17. |
| 21. | Gao, N.; Cheng, S.; Budhraja, A.; Liu, E.-H.; Chen, J.; Chen, D.; Yang, Z.; Luo, J.; Shi, X.; Zhang, Z. PLoS One 2012, 7, e31783. doi:10.1371/journal.pone.0031783 |
| 22. | Thomson, C. A.; Ho, E.; Strom, M. B. Nutr. Rev. 2016, 74, 432–443. doi:10.1093/nutrit/nuw010 |
| 23. | Shiri, M.; Zolfigol, M. A.; Kruger, H. G.; Tanbakouchian, Z. Chem. Rev. 2010, 110, 2250–2293. doi:10.1021/cr900195a |
| 42. | Hongtao, F.; Yanrui, L.; Xing, W. Indole derivative an application thereof to organic electroluminescence. Chin. Pat. Appl. CN104725296A, June 24, 2015. |
| 96. | Naidu, P. S.; Majumder, S.; Bhuyan, P. J. Mol. Diversity 2015, 19, 685–693. doi:10.1007/s11030-015-9605-3 |
| 9. | Cacchi, S.; Fabrizi, G. Chem. Rev. 2011, 111, PR215–PR283. doi:10.1021/cr100403z |
| 10. | Sundberg, R. J. The Chemistry of Indoles; Academic Press: New York, NY, USA, 1970. |
| 11. | Lakhdar, S.; Westermaier, M.; Terrier, F.; Goumont, R.; Boubaker, T.; Ofial, A. R.; Mayr, H. J. Org. Chem. 2006, 71, 9088–9095. doi:10.1021/jo0614339 |
| 12. | Cooper, M. M.; Hignett, G. J.; Newton, R. F.; Joule, J. A.; Harris, M.; Hinchley, J. D. J. Chem. Soc., Chem. Commun. 1977, 432–434. doi:10.1039/c39770000432 |
| 13. | Kishbaugh, T. L. S. Reactions of Indole with Nucleophiles. In Heterocyclic Scaffolds II; Gribble, G., Ed.; Topics in Heterocyclic Chemistry, Vol. 26; Springer: Berlin, Heidelberg, 2010; pp 117–140. doi:10.1007/7081_2010_35 |
| 14. | Talukdar, R.; Tiwari, D. P.; Saha, A.; Ghorai, M. K. Org. Lett. 2014, 16, 3954–3957. doi:10.1021/ol501763n |
| 15. | Talukdar, R. Org. Biomol. Chem. 2020, 18, 8876–8880. doi:10.1039/d0ob01977a |
| 44. | Igarashi, T.; Yagi, K. Organic electroluminescent device, and new indole derivative. Jap. Patent JP2009076834, April 9, 2009. |
| 83. | Yang, Y.; Li, W.; Ying, B.; Liao, H.; Shen, C.; Zhang, P. ChemCatChem 2016, 8, 2916–2919. doi:10.1002/cctc.201600589 |
| 38. | Li, Y.; Wang, W.-H.; He, K.-H.; Shi, Z.-J. Organometallics 2012, 31, 4397–4400. doi:10.1021/om300284t |
| 39. | Campeau, L.-C.; Parisien, M.; Jean, A.; Fagnou, K. J. Am. Chem. Soc. 2006, 128, 581–590. doi:10.1021/ja055819x |
| 40. | Potavathri, S.; Pereira, K. C.; Gorelsky, S. I.; Pike, A.; LeBris, A. P.; DeBoef, B. J. Am. Chem. Soc. 2010, 132, 14676–14681. doi:10.1021/ja107159b |
| 42. | Hongtao, F.; Yanrui, L.; Xing, W. Indole derivative an application thereof to organic electroluminescence. Chin. Pat. Appl. CN104725296A, June 24, 2015. |
| 37. | McGough, J. S.; Cid, J.; Ingleson, M. J. Chem. – Eur. J. 2017, 23, 8180–8184. doi:10.1002/chem.201702060 |
| 43. | Han, S. I.; Kim, Y. B.; Kim, H. M. Preparation of fused heterocyclic compounds for organic electroluminescent devices. Kor. Patent KR2016077940A, July 4, 2016. |
| 95. | Abele, E.; Popelis, J.; Shestakova, I.; Domracheva, I.; Arsenyan, P.; Lukevics, E. Chem. Heterocycl. Compd. 2004, 40, 742–746. doi:10.1023/b:cohc.0000040769.55088.e3 |
| 36. | Murakami, S.; Suzuki, T. Method for producing boronic acid derivative, and novel boronic acid derivative. Eur. Pat. Appl. EP2886548A1, June 24, 2015. |
| 93. | Nguyen, L. P.; Bradfield, C. A. Chem. Res. Toxicol. 2008, 21, 102–116. doi:10.1021/tx7001965 |
| 35. | Chokshi, R.; Fruasaha, P.; Kozak, J. A. Channels 2012, 6, 362–369. doi:10.4161/chan.21628 |
| 41. | Yen, F.-W.; Chang, C. H.; Teng, C.-M. Indenotriphenylene-based derivative for organic electroluminescent device. Eur. Pat. Appl. EP3059773A1, Jan 22, 2018. |
| 73. | Wincent, E.; Shirani, H.; Bergman, J.; Rannug, U.; Janosik, T. Bioorg. Med. Chem. 2009, 17, 1648–1653. doi:10.1016/j.bmc.2008.12.072 |
| 55. | Du, W.; Kaskar, B.; Blumbergs, P.; Subramanian, P.-K.; Curran, D. P. Bioorg. Med. Chem. 2003, 11, 451–458. doi:10.1016/s0968-0896(02)00437-6 |
| 53. | Klare, H. F. T.; Oestreich, M.; Ito, J.-i.; Nishiyama, H.; Ohki, Y.; Tatsumi, K. J. Am. Chem. Soc. 2011, 133, 3312–3315. doi:10.1021/ja111483r |
| 54. | Toutov, A. A.; Liu, W.-B.; Betz, K. N.; Fedorov, A.; Stoltz, B. M.; Grubbs, R. H. Nature 2015, 518, 80–84. doi:10.1038/nature14126 |
| 96. | Naidu, P. S.; Majumder, S.; Bhuyan, P. J. Mol. Diversity 2015, 19, 685–693. doi:10.1007/s11030-015-9605-3 |
| 83. | Yang, Y.; Li, W.; Ying, B.; Liao, H.; Shen, C.; Zhang, P. ChemCatChem 2016, 8, 2916–2919. doi:10.1002/cctc.201600589 |
| 44. | Igarashi, T.; Yagi, K. Organic electroluminescent device, and new indole derivative. Jap. Patent JP2009076834, April 9, 2009. |
| 64. | Brookhart, M.; Grant, B.; Volpe, A. F., Jr. Organometallics 1992, 11, 3920–3922. doi:10.1021/om00059a071 |
| 61. | Yonekura, K.; Iketani, Y.; Sekine, M.; Tani, T.; Matsui, F.; Kamakura, D.; Tsuchimoto, T. Organometallics 2017, 36, 3234–3249. doi:10.1021/acs.organomet.7b00382 |
| 60. | Chen, Q.-A.; Klare, H. F. T.; Oestreich, M. J. Am. Chem. Soc. 2016, 138, 7868–7871. doi:10.1021/jacs.6b04878 |
| 61. | Yonekura, K.; Iketani, Y.; Sekine, M.; Tani, T.; Matsui, F.; Kamakura, D.; Tsuchimoto, T. Organometallics 2017, 36, 3234–3249. doi:10.1021/acs.organomet.7b00382 |
| 62. | Han, Y.; Zhang, S.; He, J.; Zhang, Y. J. Am. Chem. Soc. 2017, 139, 7399–7407. doi:10.1021/jacs.7b03534 |
| 63. | Han, Y.; Zhang, S.; He, J.; Zhang, Y. ACS Catal. 2018, 8, 8765–8773. doi:10.1021/acscatal.8b01847 |
| 100. | Wendel, A. Phosphorus, Sulfur Silicon Relat. Elem. 1992, 67, 405–415. doi:10.1080/10426509208045863 |
| 60. | Chen, Q.-A.; Klare, H. F. T.; Oestreich, M. J. Am. Chem. Soc. 2016, 138, 7868–7871. doi:10.1021/jacs.6b04878 |
| 101. | Marletta, M. A. J. Med. Chem. 1994, 37, 1899–1907. doi:10.1021/jm00039a001 |
| 102. | Molina, J. A.; Jiménez-Jiménez, F. J.; Ortí-Pareja, M.; Navarro, J. A. Drugs Aging 1998, 12, 251–259. doi:10.2165/00002512-199812040-00001 |
| 103. | Thorns, V.; Hansen, L.; Masliah, E. Exp. Neurol. 1998, 150, 14–20. doi:10.1006/exnr.1997.6751 |
| 58. | Frenzel, A.; Herbst-Irmer, R.; Klingebiel, U.; Noltemeyer, M.; Rudolph, S. Main Group Chem. 1996, 1, 399–408. doi:10.1080/13583149612331338727 |
| 59. | Ohshita, J.; Lee, K.-H.; Kimura, K.; Kunai, A. Organometallics 2004, 23, 5622–5625. doi:10.1021/om049656h |
| 99. | Engman, L.; Stern, D.; Pelcman, M.; Andersson, C. M. J. Org. Chem. 1994, 59, 1973–1979. doi:10.1021/jo00087a008 |
| 56. | Bell, B. M.; Clark, M. B., Jr.; Devore, D. D.; De Vries, T. S.; Froese, R. D.; Gray, K. C.; Jackson, D. H. K.; Kuech, T. F.; Na, H.-Y.; Kearns, K. L.; Lee, K.-J.; Mukhopadhyay, S.; Rachford, A. A.; Spencer, L. P.; Woodward, W. H. H. ACS Appl. Mater. Interfaces 2017, 9, 13369–13379. doi:10.1021/acsami.7b00208 |
| 97. | Thurow, S.; Penteado, F.; Perin, G.; Alves, D.; Santi, C.; Monti, B.; Schiesser, C. H.; Barcellos, T.; Lenardão, E. J. Org. Chem. Front. 2018, 5, 1983–1991. doi:10.1039/c8qo00360b |
| 57. | Cho, I.; Jeon, N. J.; Kwon, O. K.; Kim, D. W.; Jung, E. H.; Noh, J. H.; Seo, J.; Seok, S. I.; Park, S. Y. Chem. Sci. 2017, 8, 734–741. doi:10.1039/c6sc02832b |
© 2021 Talukdar; licensee Beilstein-Institut.
This is an Open Access article under the terms of the Creative Commons Attribution License (https://creativecommons.org/licenses/by/4.0). Please note that the reuse, redistribution and reproduction in particular requires that the author(s) and source are credited and that individual graphics may be subject to special legal provisions.
The license is subject to the Beilstein Journal of Organic Chemistry terms and conditions: (https://www.beilstein-journals.org/bjoc/terms)