Abstract
Trifluoromethyl propargylamines react with various azide derivatives to afford 1,4-disubstituted 1,2,3-triazoles through a Huisgen 1,3-dipolar cycloaddition. The reaction is catalyzed by a Cu(I) species in acetonitrile, and the corresponding products are obtained in good yields. This process thus offers an entry to new trifluoromethyl peptidomimetics as interesting scaffolds.
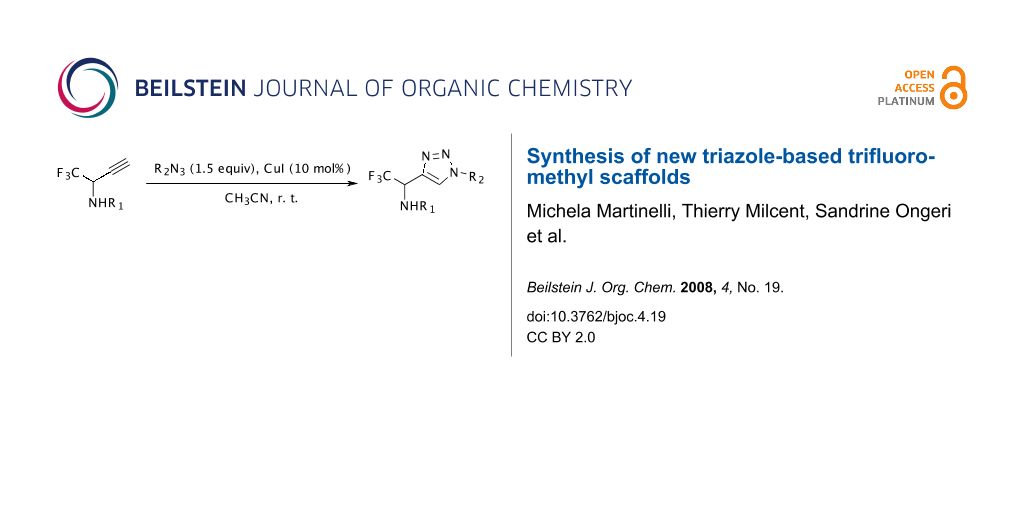
Graphical Abstract
Background
The 1,2,3-triazole system has widespread uses, and it has been considered as an interesting component in terms of biological activity [1-5]. Although the use of heterocyclic moieties in peptidomimetics has been widely reported [6], the application of 1,2,3-triazoles in the field of conformational studies has occurred only recently [7-13]. In particular, Angelo and co-workers [8,12] reported the synthesis of triazole foldamers able to adopt specific protein-like conformations. On the other hand, it is well known that the introduction of fluorine atoms or a fluoroalkyl group can greatly modify the physico-chemical features and thus the biological properties of a molecule (resistance to metabolic oxidation and hydrolysis, modification of pKa, hydrophobicity,...) [14-17]. Furthermore, the development of CF3-containing scaffolds has gained a real interest especially in the peptidomimetic area [18-21]. In continuation of our interest in the synthesis of original trifluoromethyl compounds [22-26], and in order to study the influence of trifluoromethyl groups on the conformation of peptidomimetics, we decided to explore the preparation of trifluoromethyl triazole derivatives. Herein we turn our attention to the synthesis of new triazoles from trifluoromethyl propargylamines using the Huisgen 1,3-dipolar cycloaddition [27-29].
Results and Discussion
The synthetic approach depicted in Scheme 1 shows that the desired compounds could be easily obtained via a 1,3-dipolar cycloaddition from the corresponding propargylamines which are obtained using an efficient procedure from the trifluoromethyl imines previously described by our group [30-32].
Scheme 1: Synthetic approach to the trifluoromethyl triazoles.
Scheme 1: Synthetic approach to the trifluoromethyl triazoles.
The copper(I)-catalyzed 1,3-dipolar cycloaddition [33-38] of organic azides and alkynes (also called “click chemistry”) resulting in the formation of 1,2,3-triazoles has become an increasingly attractive area [39]. According to the literature [33-38], the Cu(I) species can be used directly (e.g. CuI), or generated by oxidation of a Cu(0) or reduction of a Cu(II) species. Catalysis by the CuI is known to yield exclusively the 1,4-disubstituted regioisomer [33,34]. First, the N-(p-methoxyphenyl)-1-(trifluoromethyl)propargylamine was reacted with benzyl azide in the presence of CuI (10 mol%) and showed good reactivity with completion of the reaction within 24 h, whereas the use of CuSO4/Na ascorbate afforded the cycloadduct in low yield. The reaction was then carried out with different propargylamines (N-(p-methoxyphenyl) and N-benzyl) and various azides at room temperature in acetonitrile within 24 h which afforded the compounds 2a-i with good yields (63-92%) after purification by column chromatography. The results are summarized in Table 1.
Table 1: Copper(I)-catalyzed synthesis of 1,4-disubstituted triazoles
|
|
||||
| Entry | R1 | R2 | Product | Yield (%)b |
|---|---|---|---|---|
| 1 | -PMPa | -Bn | 2a | 82 |
| 2 | -PMP | -CH2COPh | 2b | 76 |
| 3 | -PMP | -CH2OCOC(CH3)3 | 2c | 73 |
| 4 | -PMP | -CH2CO2CH3 | 2d | 83 |
| 5 | -PMP | -CH2CH2OH | 2e | 87 |
| 6 | -Bna | -Bn | 2f | 75 |
| 7 | -Bn | -CH2COPh | 2g | 63 |
| 8 | -Bn | -CH2CO2CH3 | 2h | 92 |
| 9 | -Bn | -(CH2)2OH | 2i | 73 |
aPMP: p-methoxyphenyl, Bn: benzyl. bYield after flash purification.
As expected the new triazoles were formed in a fully regioselective manner affording the 1,4-regioisomer as highlighted from NOE experiments on compound 2c (Figure 1). A strong correlation was observed between the hydrogen Ha and Hb respectively. The structure of the other compounds 2a-i was assigned by analogy with 2c.
Figure 1: Experimentally found NOE correlation for compound 2c.
Figure 1: Experimentally found NOE correlation for compound 2c.
In our goal to study the influence of the CF3 group on the conformation of peptidomimetics, we applied our strategy to the enantiopure trifluoromethyl-propargylamine 3 bearing the removable (R)-phenylglycinol chiral auxiliary (Scheme 2) [30-32].
Scheme 2: Cycloaddition of enantiopure propargylamine.
Scheme 2: Cycloaddition of enantiopure propargylamine.
The reaction was carried out under the same condition with azidoacetic acid methyl ester and afforded the cycloadduct 4 in good yield (79%) and as a single isomer without any racemization. This compound can easily afford the free amino ester which is a promising trifluoromethyl building block for the synthesis of new triazole-based trifluoromethyl oligomers.
Conclusion
In summary, this paper describes the synthesis of new trifluoromethyl triazole scaffolds from readily accessible propargylamines and azides through a copper (I) catalyzed 1,3-dipolar cycloaddition. The triazole derivatives were obtained in good yields and will be useful intermediates for further synthesis of new fluorinated foldamers and their conformational feature studies.
Supporting Information
| Supporting Information File 1: General methods, synthetic procedure and spectroscopic data of 2a-i and 4. | ||
| Format: DOC | Size: 298.5 KB | Download |
Acknowledgements
Claire Troufflard is gratefully acknowledged for NMR experiments. Central Glass is thanked for kind gift of fluoral hydrate and DSM company for donation of (R)-phenylglycine. We thank the European Community for the financial support (Marie Curie Early Stage training Fellowship of the European Community's Sixth Framework Programme: contract MEST-CT-2004-515968). We thank Julien Legros for helpful discussion.
References
-
Giguère, D.; Patnam, R.; Bellefleur, M. A.; St-Pierre, C.; Sato, S.; Roy, R. Chem. Commun. 2006, 2379–2381. doi:10.1039/b517529a
For recent references on the biological activity of 1,2,3-triazoles.
Return to citation in text: [1] -
Whitting, M.; Muldoon, J.; Lin, Y.-C.; Silverman, S. M.; Lindstrom, W.; Olson, A. J.; Kolb, H. C.; Finn, M. G.; Sharpless, K. B.; Elder, J. H.; Fokin, V. V. Angew. Chem., Int. Ed. 2006, 45, 1435–1439. doi:10.1002/anie.200502161
Return to citation in text: [1] -
Holla, B. S.; Mahalinga, M.; Karthikeyan, M. S.; Poojary, B.; Akberali, P. M.; Kumari, N. S. Eur. J. Med. Chem. 2005, 40, 1173–1178. doi:10.1016/j.ejmech.2005.02.013
Return to citation in text: [1] -
Pande, V.; Ramos, M. J. Bioorg. Med. Chem. Lett. 2005, 15, 5129–5135. doi:10.1016/j.bmcl.2005.08.077
Return to citation in text: [1] -
Boume, Y.; Kolb, H. C.; Radić, Z.; Sharpless, K. B.; Taylor, P.; Marchot, P. Proc. Natl. Acad. Sci. U. S. A. 2004, 101, 1449–1454. doi:10.1073/pnas.0308206100
And references therein.
Return to citation in text: [1] -
Borg, S.; Estenne-Bouhtou, G.; Luthman, K.; Csöregh, I.; Hesselink, W.; Hacksell, U. J. Org. Chem. 1995, 60, 3112–3120. doi:10.1021/jo00115a029
And references therein.
Return to citation in text: [1] -
Tomøe, C. W.; Christensen, C.; Meldal, M. J. Org. Chem. 2002, 67, 3057–3064. doi:10.1021/jo011148j
Return to citation in text: [1] -
Angelo, N. G.; Arora, P. S. J. Am. Chem. Soc. 2005, 127, 17134–17135. doi:10.1021/ja056406z
Return to citation in text: [1] [2] -
Angell, Y.; Burgess, K. J. Org. Chem. 2005, 70, 9595–9598. doi:10.1021/jo0516180
Return to citation in text: [1] -
Oh, K.; Guan, Z. Chem. Commun. 2006, 3069–3071. doi:10.1039/b606185k
Return to citation in text: [1] -
Pokorski, J. K.; Miller Jelnkins, L. M.; Feng, H.; Durell, S. R.; Bai, Y.; Apella, D. H. Org. Lett. 2007, 9, 2381–2383. doi:10.1021/ol070817y
Return to citation in text: [1] -
Angelo, N. G.; Arora, P. S. J. Org. Chem. 2007, 72, 7963–7967. doi:10.1021/jo701292h
Return to citation in text: [1] [2] -
Bock, V. D.; Speijer, D.; Hiemstra, H.; van Maarseveen, J. H. Org. Biomol. Chem. 2007, 5, 971–975. doi:10.1039/b616751a
And references therein.
Return to citation in text: [1] -
Bégué, J.-P.; Bonnet-Delpon, D. Chimie bioorganique et médicinale du fluor; CNRS Editions-EDP Sciences: Paris, 2005.
Return to citation in text: [1] -
Filler, R.; Kobayashi, Y.; Yagupolskii, L. M., Eds. Organofluorine Compounds in Medicinal Chemistry and Biomedical Applications; Elsevier: Amsterdam, 1993.
Return to citation in text: [1] -
Ojima, I.; McCarthy, J. R.; Welch, J. T., Eds. Biomedical Frontiers of Fluorine Chemistry; ACS Symposium Series 639; American Chemical Society: Washington, DC, 1996.
Return to citation in text: [1] -
Edwards, P. N. Uses of Fluorine in Chemotherapy. In Organofluorine Chemistry: Principles and Commercial Applications; Banks, R. E.; Smart, B. E.; Tatlow, J. C., Eds.; Plenum Press: New York, 1994; pp 501 ff.
Return to citation in text: [1] -
Molteni, M.; Volonterio, A.; Fossati, G.; Lazzari, P.; Zanda, M. Tetrahedron Lett. 2007, 48, 589–593. doi:10.1016/j.tetlet.2006.11.124
Return to citation in text: [1] -
Moreno, M.; Sani, M.; Raos, G.; Meille, S. V.; Belotti, D.; Giavazzi, R.; Bellosta, S.; Volonterio, A.; Zanda, M. Tetrahedron 2006, 62, 10171–10181. doi:10.1016/j.tet.2006.08.036
Return to citation in text: [1] -
Volonterio, A.; Bellosta, S.; Bravin, F.; Bellucci, M. C.; Bruche, L.; Colombo, G.; Malpezzi, L.; Mazzini, S.; Meille, S. V.; Meli, M.; Ramirez de Arellano, C.; Zanda, M. Chem.–Eur. J. 2003, 9, 4510–4522. doi:10.1002/chem.200304881
Return to citation in text: [1] -
Zanda, M. New J. Chem. 2004, 28, 1401–1411. doi:10.1039/b405955g
For a review on trifluoromethyl peptides.
Return to citation in text: [1] -
Bonnamour, J.; Legros, J.; Crousse, B.; Bonnet-Delpon, D. Tetrahedron Lett. 2007, 48, 8360–8362. doi:10.1016/j.tetlet.2007.09.118
Return to citation in text: [1] -
Rinaudo, G.; Narizuka, S.; Askari, N.; Crousse, B.; Bonnet-Delpon, D. Tetrahedron Lett. 2006, 47, 2065–2068. doi:10.1016/j.tetlet.2006.01.142
Return to citation in text: [1] -
Magueur, G.; Crousse, B.; Bonnet-Delpon, D. Tetrahedron Lett. 2005, 46, 2219–2221. doi:10.1016/j.tetlet.2005.02.030
Return to citation in text: [1] -
Ngoc Tam, N. T.; Magueur, G.; Ourévitch, M.; Crousse, B.; Bégué, J.-P.; Bonnet-Delpon, D. J. Org. Chem. 2005, 70, 699–702. doi:10.1021/jo0485233
Return to citation in text: [1] -
Magueur, G.; Legros, J.; Meyer, F.; Ourévitch, M.; Crousse, B.; Bonnet-Delpon, D. Eur. J. Org. Chem. 2005, 1258–1265. doi:10.1002/ejoc.200400719
Return to citation in text: [1] -
Huisgen, R. Proc. Chem. Soc., London 1961, 357–369.
Return to citation in text: [1] -
Huisgen, R. Angew. Chem., Int. Ed. Engl. 1963, 2, 565–578. doi:10.1002/anie.196305651
Return to citation in text: [1] -
Huisgen, R. In 1,3-Dipolar Cycloaddition Chemistry; Padwa, A., Ed.; Wiley: New York, 1984; pp 1–176.
Return to citation in text: [1] -
Magueur, G.; Crousse, B.; Bonnet-Delpon, D. Tetrahedron Lett. 2005, 46, 2219–2221. doi:10.1016/j.tetlet.2005.02.030
Return to citation in text: [1] [2] -
Magueur, G.; Crousse, B.; Bonnet-Delpon, D. Eur. J. Org. Chem. 2008, 9, 1527–1534. doi:10.1002/ejoc.200701090
Return to citation in text: [1] [2] -
Bégué, J.-P.; Bonnet-Delpon, D.; Crousse, B.; Legros, J. Chem. Soc. Rev. 2005, 34, 562–572. doi:10.1039/b401707m
Return to citation in text: [1] [2] -
Rostovtsev, V. V.; Green, L. G.; Fokin, V. V.; Sharpless, K. B. Angew. Chem., Int. Ed. 2002, 41, 2596–2599. doi:10.1002/1521-3773(20020715)41:14<2596::AID-ANIE2596>3.0.CO;2-4
Return to citation in text: [1] [2] [3] -
Wu, P.; Fokin, V. V. Aldrichimica Acta 2007, 40, 7–17.
For reviews on click chemistry.
Return to citation in text: [1] [2] [3] -
Gil, M. V.; Arévalo, M. J.; López, Ó. Synthesis 2007, 1589–1620. doi:10.1055/s-2007-966071
Return to citation in text: [1] [2] -
Bock, V. D.; Hiemstra, H.; van Maarseveen, J. H. Eur. J. Org. Chem. 2006, 51–68. doi:10.1002/ejoc.200500483
Return to citation in text: [1] [2] -
Kolb, H. C.; Finn, M. G.; Sharpless, K. B. Angew. Chem., Int. Ed. 2001, 40, 2004–2021. doi:10.1002/1521-3773(20010601)40:11<2004::AID-ANIE2004>3.0.CO;2-5
Return to citation in text: [1] [2] -
Himo, F.; Lovell, T.; Hilgraf, R.; Rostovtsev, V. V.; Noodleman, L.; Sharpless, K. B.; Fokin, V. V. J. Am. Chem. Soc. 2005, 127, 210–216. doi:10.1021/ja0471525
For mechanistic studies.
Return to citation in text: [1] [2] -
Kolb, H. C.; Sharpless, K. B. Drug Discovery Today 2003, 8, 1128–1137. doi:10.1016/S1359-6446(03)02933-7
For reviews on the application of click chemistry.
Return to citation in text: [1]
| 1. |
Giguère, D.; Patnam, R.; Bellefleur, M. A.; St-Pierre, C.; Sato, S.; Roy, R. Chem. Commun. 2006, 2379–2381. doi:10.1039/b517529a
For recent references on the biological activity of 1,2,3-triazoles. |
| 2. | Whitting, M.; Muldoon, J.; Lin, Y.-C.; Silverman, S. M.; Lindstrom, W.; Olson, A. J.; Kolb, H. C.; Finn, M. G.; Sharpless, K. B.; Elder, J. H.; Fokin, V. V. Angew. Chem., Int. Ed. 2006, 45, 1435–1439. doi:10.1002/anie.200502161 |
| 3. | Holla, B. S.; Mahalinga, M.; Karthikeyan, M. S.; Poojary, B.; Akberali, P. M.; Kumari, N. S. Eur. J. Med. Chem. 2005, 40, 1173–1178. doi:10.1016/j.ejmech.2005.02.013 |
| 4. | Pande, V.; Ramos, M. J. Bioorg. Med. Chem. Lett. 2005, 15, 5129–5135. doi:10.1016/j.bmcl.2005.08.077 |
| 5. |
Boume, Y.; Kolb, H. C.; Radić, Z.; Sharpless, K. B.; Taylor, P.; Marchot, P. Proc. Natl. Acad. Sci. U. S. A. 2004, 101, 1449–1454. doi:10.1073/pnas.0308206100
And references therein. |
| 14. | Bégué, J.-P.; Bonnet-Delpon, D. Chimie bioorganique et médicinale du fluor; CNRS Editions-EDP Sciences: Paris, 2005. |
| 15. | Filler, R.; Kobayashi, Y.; Yagupolskii, L. M., Eds. Organofluorine Compounds in Medicinal Chemistry and Biomedical Applications; Elsevier: Amsterdam, 1993. |
| 16. | Ojima, I.; McCarthy, J. R.; Welch, J. T., Eds. Biomedical Frontiers of Fluorine Chemistry; ACS Symposium Series 639; American Chemical Society: Washington, DC, 1996. |
| 17. | Edwards, P. N. Uses of Fluorine in Chemotherapy. In Organofluorine Chemistry: Principles and Commercial Applications; Banks, R. E.; Smart, B. E.; Tatlow, J. C., Eds.; Plenum Press: New York, 1994; pp 501 ff. |
| 8. | Angelo, N. G.; Arora, P. S. J. Am. Chem. Soc. 2005, 127, 17134–17135. doi:10.1021/ja056406z |
| 12. | Angelo, N. G.; Arora, P. S. J. Org. Chem. 2007, 72, 7963–7967. doi:10.1021/jo701292h |
| 7. | Tomøe, C. W.; Christensen, C.; Meldal, M. J. Org. Chem. 2002, 67, 3057–3064. doi:10.1021/jo011148j |
| 8. | Angelo, N. G.; Arora, P. S. J. Am. Chem. Soc. 2005, 127, 17134–17135. doi:10.1021/ja056406z |
| 9. | Angell, Y.; Burgess, K. J. Org. Chem. 2005, 70, 9595–9598. doi:10.1021/jo0516180 |
| 10. | Oh, K.; Guan, Z. Chem. Commun. 2006, 3069–3071. doi:10.1039/b606185k |
| 11. | Pokorski, J. K.; Miller Jelnkins, L. M.; Feng, H.; Durell, S. R.; Bai, Y.; Apella, D. H. Org. Lett. 2007, 9, 2381–2383. doi:10.1021/ol070817y |
| 12. | Angelo, N. G.; Arora, P. S. J. Org. Chem. 2007, 72, 7963–7967. doi:10.1021/jo701292h |
| 13. |
Bock, V. D.; Speijer, D.; Hiemstra, H.; van Maarseveen, J. H. Org. Biomol. Chem. 2007, 5, 971–975. doi:10.1039/b616751a
And references therein. |
| 33. | Rostovtsev, V. V.; Green, L. G.; Fokin, V. V.; Sharpless, K. B. Angew. Chem., Int. Ed. 2002, 41, 2596–2599. doi:10.1002/1521-3773(20020715)41:14<2596::AID-ANIE2596>3.0.CO;2-4 |
| 34. |
Wu, P.; Fokin, V. V. Aldrichimica Acta 2007, 40, 7–17.
For reviews on click chemistry. |
| 6. |
Borg, S.; Estenne-Bouhtou, G.; Luthman, K.; Csöregh, I.; Hesselink, W.; Hacksell, U. J. Org. Chem. 1995, 60, 3112–3120. doi:10.1021/jo00115a029
And references therein. |
| 30. | Magueur, G.; Crousse, B.; Bonnet-Delpon, D. Tetrahedron Lett. 2005, 46, 2219–2221. doi:10.1016/j.tetlet.2005.02.030 |
| 31. | Magueur, G.; Crousse, B.; Bonnet-Delpon, D. Eur. J. Org. Chem. 2008, 9, 1527–1534. doi:10.1002/ejoc.200701090 |
| 32. | Bégué, J.-P.; Bonnet-Delpon, D.; Crousse, B.; Legros, J. Chem. Soc. Rev. 2005, 34, 562–572. doi:10.1039/b401707m |
| 30. | Magueur, G.; Crousse, B.; Bonnet-Delpon, D. Tetrahedron Lett. 2005, 46, 2219–2221. doi:10.1016/j.tetlet.2005.02.030 |
| 31. | Magueur, G.; Crousse, B.; Bonnet-Delpon, D. Eur. J. Org. Chem. 2008, 9, 1527–1534. doi:10.1002/ejoc.200701090 |
| 32. | Bégué, J.-P.; Bonnet-Delpon, D.; Crousse, B.; Legros, J. Chem. Soc. Rev. 2005, 34, 562–572. doi:10.1039/b401707m |
| 39. |
Kolb, H. C.; Sharpless, K. B. Drug Discovery Today 2003, 8, 1128–1137. doi:10.1016/S1359-6446(03)02933-7
For reviews on the application of click chemistry. |
| 27. | Huisgen, R. Proc. Chem. Soc., London 1961, 357–369. |
| 28. | Huisgen, R. Angew. Chem., Int. Ed. Engl. 1963, 2, 565–578. doi:10.1002/anie.196305651 |
| 29. | Huisgen, R. In 1,3-Dipolar Cycloaddition Chemistry; Padwa, A., Ed.; Wiley: New York, 1984; pp 1–176. |
| 33. | Rostovtsev, V. V.; Green, L. G.; Fokin, V. V.; Sharpless, K. B. Angew. Chem., Int. Ed. 2002, 41, 2596–2599. doi:10.1002/1521-3773(20020715)41:14<2596::AID-ANIE2596>3.0.CO;2-4 |
| 34. |
Wu, P.; Fokin, V. V. Aldrichimica Acta 2007, 40, 7–17.
For reviews on click chemistry. |
| 35. | Gil, M. V.; Arévalo, M. J.; López, Ó. Synthesis 2007, 1589–1620. doi:10.1055/s-2007-966071 |
| 36. | Bock, V. D.; Hiemstra, H.; van Maarseveen, J. H. Eur. J. Org. Chem. 2006, 51–68. doi:10.1002/ejoc.200500483 |
| 37. | Kolb, H. C.; Finn, M. G.; Sharpless, K. B. Angew. Chem., Int. Ed. 2001, 40, 2004–2021. doi:10.1002/1521-3773(20010601)40:11<2004::AID-ANIE2004>3.0.CO;2-5 |
| 38. |
Himo, F.; Lovell, T.; Hilgraf, R.; Rostovtsev, V. V.; Noodleman, L.; Sharpless, K. B.; Fokin, V. V. J. Am. Chem. Soc. 2005, 127, 210–216. doi:10.1021/ja0471525
For mechanistic studies. |
| 22. | Bonnamour, J.; Legros, J.; Crousse, B.; Bonnet-Delpon, D. Tetrahedron Lett. 2007, 48, 8360–8362. doi:10.1016/j.tetlet.2007.09.118 |
| 23. | Rinaudo, G.; Narizuka, S.; Askari, N.; Crousse, B.; Bonnet-Delpon, D. Tetrahedron Lett. 2006, 47, 2065–2068. doi:10.1016/j.tetlet.2006.01.142 |
| 24. | Magueur, G.; Crousse, B.; Bonnet-Delpon, D. Tetrahedron Lett. 2005, 46, 2219–2221. doi:10.1016/j.tetlet.2005.02.030 |
| 25. | Ngoc Tam, N. T.; Magueur, G.; Ourévitch, M.; Crousse, B.; Bégué, J.-P.; Bonnet-Delpon, D. J. Org. Chem. 2005, 70, 699–702. doi:10.1021/jo0485233 |
| 26. | Magueur, G.; Legros, J.; Meyer, F.; Ourévitch, M.; Crousse, B.; Bonnet-Delpon, D. Eur. J. Org. Chem. 2005, 1258–1265. doi:10.1002/ejoc.200400719 |
| 18. | Molteni, M.; Volonterio, A.; Fossati, G.; Lazzari, P.; Zanda, M. Tetrahedron Lett. 2007, 48, 589–593. doi:10.1016/j.tetlet.2006.11.124 |
| 19. | Moreno, M.; Sani, M.; Raos, G.; Meille, S. V.; Belotti, D.; Giavazzi, R.; Bellosta, S.; Volonterio, A.; Zanda, M. Tetrahedron 2006, 62, 10171–10181. doi:10.1016/j.tet.2006.08.036 |
| 20. | Volonterio, A.; Bellosta, S.; Bravin, F.; Bellucci, M. C.; Bruche, L.; Colombo, G.; Malpezzi, L.; Mazzini, S.; Meille, S. V.; Meli, M.; Ramirez de Arellano, C.; Zanda, M. Chem.–Eur. J. 2003, 9, 4510–4522. doi:10.1002/chem.200304881 |
| 21. |
Zanda, M. New J. Chem. 2004, 28, 1401–1411. doi:10.1039/b405955g
For a review on trifluoromethyl peptides. |
| 33. | Rostovtsev, V. V.; Green, L. G.; Fokin, V. V.; Sharpless, K. B. Angew. Chem., Int. Ed. 2002, 41, 2596–2599. doi:10.1002/1521-3773(20020715)41:14<2596::AID-ANIE2596>3.0.CO;2-4 |
| 34. |
Wu, P.; Fokin, V. V. Aldrichimica Acta 2007, 40, 7–17.
For reviews on click chemistry. |
| 35. | Gil, M. V.; Arévalo, M. J.; López, Ó. Synthesis 2007, 1589–1620. doi:10.1055/s-2007-966071 |
| 36. | Bock, V. D.; Hiemstra, H.; van Maarseveen, J. H. Eur. J. Org. Chem. 2006, 51–68. doi:10.1002/ejoc.200500483 |
| 37. | Kolb, H. C.; Finn, M. G.; Sharpless, K. B. Angew. Chem., Int. Ed. 2001, 40, 2004–2021. doi:10.1002/1521-3773(20010601)40:11<2004::AID-ANIE2004>3.0.CO;2-5 |
| 38. |
Himo, F.; Lovell, T.; Hilgraf, R.; Rostovtsev, V. V.; Noodleman, L.; Sharpless, K. B.; Fokin, V. V. J. Am. Chem. Soc. 2005, 127, 210–216. doi:10.1021/ja0471525
For mechanistic studies. |
© 2008 Martinelli et al; licensee Beilstein-Institut.
This is an Open Access article under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
The license is subject to the Beilstein Journal of Organic Chemistry terms and conditions: (http://www.beilstein-journals.org/bjoc)