Abstract
A novel method of photoinduced synthesis of unsymmetrical diaryl selenides from triarylbismuthines and diaryl diselenides has been developed. Although the arylation reactions with triarylbismuthines are usually catalyzed by transition-metal complexes, the present arylation of diaryl diselenides with triarylbismuthines proceeds upon photoirradiation in the absence of transition-metal catalysts. A variety of unsymmetrical diaryl selenides can be conveniently prepared by using this arylation method.
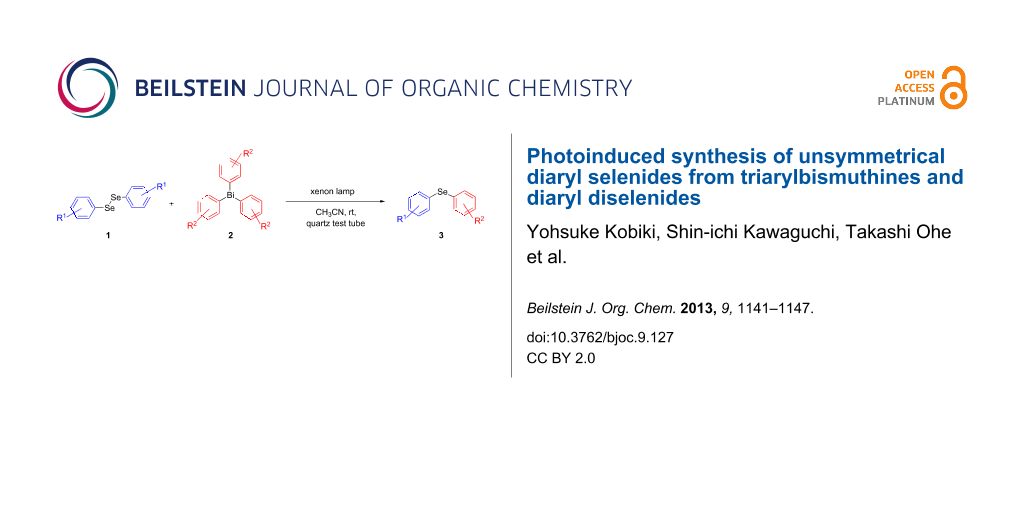
Graphical Abstract
Introduction
A number of organoselenium compounds are known to be biologically active [1-4]. In particular, diaryl selenides are known to have antioxidative effects [5]. Therefore, many studies on the synthetic methods for unsymmetrical diaryl selenides have recently been reported [6-32]. Most of these methods use coupling reactions catalyzed by transition-metal complexes. To avoid the contamination of product selenides with transition-metals, the development of synthetic methods for unsymmetrical diaryl selenides in the absence of transition-metal catalysts is desirable. On the other hand, triarylbismuthines are gaining interest as useful arylation reagents, because organobismuth compounds are nontoxic and have excellent reactivity, which has led to several applications in organic synthesis [33]. Therefore, numerous transition-metal-catalyzed coupling reactions with organobismuth compounds have been reported [34-53]. Although triphenylbismuthine can generate a phenyl radical [33,54] in the absence of a radical initiator simply by photoirradiation, few arylation reactions using this mechanism have been reported [55,56]. We presume that a phenyl radical generated from triphenylbismuthine can be captured by organic diselenides, which have a high carbon-radical-capturing ability [57-64] and as a result, diaryl selenide will be generated (Scheme 1). In 1999, Barton and co-workers reported that diaryl selenide was obtained by the reaction of triarylbismuthine with diphenyl diselenide under heating at high temperature (140 °C) [65], but the photoinduced reaction was not investigated. In this letter, we will report the radical reaction of diaryl diselenides with triarylbismuthines from the viewpoint of a photoinduced reaction in the synthesis of unsymmetrical diaryl selenides.
Scheme 1: Photoinduced radical reaction of diaryl diselenide with triphenylbismuthine.
Scheme 1: Photoinduced radical reaction of diaryl diselenide with triphenylbismuthine.
Results and Discussion
First, we investigated the photoinduced reaction of diphenyl diselenide with triphenylbismuthine. Diphenyl diselenide (1a, 0.1 mmol) and triphenylbismuthine (2a, 0.5 mmol) were placed in a Pyrex test tube ( = 9 mm) with CHCl3 (4 mL), and the mixture was irradiated by a xenon lamp for 5 h at room temperature. As a result, 0.042 mmol (21% yield based on the amount of selenium atoms) of diphenyl selenide (3aa) was obtained after the isolation by silica gel chromatography (the yield was determined by HPLC). Next, optimization of the reaction conditions was investigated as shown in Table 1. Irradiation by a tungsten lamp instead of a xenon lamp did not induce the desired arylation reaction (Table 1, entry 2), and in the dark, the reaction did not proceed at all (Table 1, entry 3). When 2,2′-azobis(isobutyronitrile) (AIBN) was used as a radical initiator, the desired reaction proceeded ineffectively (Table 1, entry 4). Among several solvents, such as benzene, DMSO and CH3CN, the use of CH3CN improved the yield of 3aa (Table 1, entries 5–7). Although the solubility of 2a is different depending on the solvent, the yield of 3aa is not correlated with the solubility of 2a. It may be more important to choose a solvent that does not react with the generated aryl radical. Moreover, a lower amount of solvent and the utilization of a quartz test tube (
= 9 mm) contributed to the increase in the yield of 3aa (Table 1, entries 8 and 9).
Table 1: Reaction of diphenyl diselenide with triphenylbismuthine under different conditions.
|
|
||
| entry | reaction conditions | yield of 3aaa |
|---|---|---|
| 1 | CHCl3 (4 mL), xenon lamp, Pyrex test tube, 5 h | 0.042 mmol, 21% |
| 2 | CHCl3 (4 mL), tungsten lamp, Pyrex test tube, 5 h | 0.004 mmol, 2% |
| 3 | CHCl3 (4 mL), dark, 24 h | 0% |
| 4 | C6H6 (5 mL), AIBN (1.5 mmol), 80 °C, two-necked flask, 8 h | 0.012 mmol, 6% |
| 5 | C6H6 (4 mL), xenon lamp, Pyrex test tube, 5 h | 0.102 mmol, 51% |
| 6 | DMSO (4 mL), xenon lamp, Pyrex test tube, 5 h | 0.034 mmol, 17% |
| 7 | CH3CN (4 mL), xenon lamp, Pyrex test tube, 5 h | 0.114 mmol, 57% |
| 8 | CH3CN (2 mL), xenon lamp, Pyrex test tube, 5 h | 0.126 mmol, 63% |
| 9 | CH3CN (2 mL), xenon lamp, quartz test tube, 5 h | 0.138 mmol, 69% |
aThe yields were determined by HPLC.
Next, we investigated the scope of the synthesis of unsymmetrical diaryl selenides by using different diaryl diselenides and triarylbismuthines (Table 2). The employed diaryl diselenides were diphenyl diselenide (1a), bis(4-fluorophenyl) diselenide (1b), bis(4-(trifluoromethyl)phenyl) diselenide (1c), bis(1-naphthyl) diselenide (1d), and bis(2-naphthyl) diselenide (1e). The used triarylbismuthines were triphenylbismuthine (2a), tris(4-methylphenyl)bismuthine (2b), tris(4-chlorophenyl)bismuthine (2c), and tris(4-fluorophenyl)bismuthine (2d). A number of combinations of 1 and 2 were examined and as a result, unsymmetrical diaryl selenides 3 were obtained in moderate to high yields (45–86%) in every case (Table 2, entries 1–10) after the isolation by preparative TLC on silica gel. The chemical shifts of 77Se NMR spectra of diaryl selenides 3 are also shown in Table 2, because 77Se NMR is a tool well suited to identify diorganyl monoselenides.
Table 2: Syntheses of unsymmetrical diaryl selenides.
|
|
|||||
| entry | (ArSe)2 1 | Ar’3Bi 2 | product 3 (ArSeAr’) |
77Se NMR,
δ ppm |
yielda |
|---|---|---|---|---|---|
| 1b |
1a |
2b |
3ab |
407 | 65% |
| 2b |
1a |
2c |
3ac |
416 | 45% |
| 3c |
1a |
2d |
3ad |
411 | 66% |
| 4b |
1b |
2a |
3ba |
411 | 57% |
| 5b |
1b |
2b |
3bb |
404 | 86% |
| 6b |
1c |
2a |
3ca |
427 | 67% |
| 7b |
1c |
2b |
3cb |
418 | 51% |
| 8c |
1d |
2a |
3da |
355 | 51% |
| 9c |
1e |
2a |
3ea |
418 | 71% |
| 10c |
1d |
2c |
3dc |
354 | 57% |
aThe yields were determined after isolation. b0.5 mmol of triarylbismuthine was used. c0.3 mmol of triarylbismuthine was used.
To get information about the reaction pathway of this arylation, we first investigated the arylation of diphenyl diselenide by varying the 1a/2a molar ratio (Table 3). When excess amounts of either starting substrate were employed, the yields of 3aa increased (Table 3, entries 1, 2 and 5).
Table 3: The yield of diphenyl selenide 3aa upon changing the ratio 1a/2a.
|
|
|||
| entry | amount of 1a | amount of 2a | yield of 3aaa |
|---|---|---|---|
| 1 | 0.1 mmol | 0.5 mmol |
69%
0.138 mmol |
| 2 | 0.1 mmol | 0.3 mmol |
69%
0.138 mmol |
| 3 | 0.1 mmol | 0.1 mmol |
59%
0.118 mmol |
| 4 | 0.1 mmol |
0.067 mmol
(2/3 equiv) |
57%
0.118 mmol |
| 5 | 0.2 mmol | 0.1 mmol |
88%b
0.263 mmol |
aThe yields were determined by HPLC based on the amount of 1a. bThe yield was calculated based on the amount of 2a.
In the case of the reaction of triphenylbismuthine with diphenyl disulfide (4) instead of diphenyl diselenide, diphenyl sulfide 5 was obtained in lower yield with unidentified byproducts, unlike in the case of diphenyl selenide 3aa (Scheme 2).
Scheme 2: Photoinduced reaction of diphenyl disulfide with triphenylbismuthine.
Scheme 2: Photoinduced reaction of diphenyl disulfide with triphenylbismuthine.
Additionally, air is entrained in the reaction system, since a test tube with a septum was used in which a needle was inserted. When the reaction of diaryl diselenide with triarylbismuthine was conducted with a strictly sealed tube in Ar atmosphere, a bismuth mirror was observed and the yield of 3aa decreased. We assume that the reaction proceeds with bismuth residue getting oxidized.
A plausible reaction pathway for the photoinduced reaction of diaryl diselenide with triarylbismuthine is shown in Scheme 3. First, an aryl radical is generated from triarylbismuthine by near-UV light irradiation [33,54,55]. The generated aryl radical is captured by diaryl diselenide to produce diaryl selenide and a seleno radical. The seleno radical may dimerize to re-form diselenide. Diphenyl diselenide has its absorption maximum (λmax) at 340 nm (ε = 103) [66] and accordingly, the seleno radical could be produced by the irradiation with a tungsten lamp. However, the irradiation by a tungsten lamp instead of a xenon lamp did not result in the desired reaction (Table 1, entry 3). This fact strongly suggests that the formation of a phenylseleno radical is not important for the formation of diphenyl selenide. Conceivably, when the reaction proceeds, a phenyl radical may be formed directly from triphenylbismuthine upon photoirradiation. Moreover, the use of an excess amount of 1, which has a relatively high carbon-radical-capturing ability, increased the yield of 3, and the use of diphenyl disulfide (4), which has a lower carbon-radical-capturing ability than diselenide, decreased the yield of 5. (The exact capturing abilities of diselenide and disulfide toward the phenyl radical are not known, but they have been reported toward vinyl radicals, where diselenide has a higher capturing ability than disulfide: kSe/kS = 160 [57-59].) These facts also support that the reaction starts from the generation of an aryl radical. On the other hand, a pale yellow solid, insoluble in organic solvents, was obtained as a byproduct after the reaction. We assume that this solid is a bismuth residue, which can consist of bismuth oxides and/or bismuth selenides. Moreover, it may form biaryls (Ar–Ar) as byproducts, but no biaryl was observed after the reaction.
Scheme 3: A plausible reaction pathway for the photoinduced reaction of diaryl diselenide with triarylbismuthine.
Scheme 3: A plausible reaction pathway for the photoinduced reaction of diaryl diselenide with triarylbismuth...
Conclusion
We have found that the photoinduced reaction of diaryl diselenides with triarylbismuthines affords unsymmetrical diaryl selenides in good yields. This method is efficient, because two arylseleno groups from diaryl selenides can be used as a selenium source, and its advantage is that the reaction proceeds in the absence of transition-metal catalysts.
Experimental
General comments
Compounds 1a, 2a, 3aa, 4, and 5 were obtained from commercially available materials. Diaryl diselenides 1b–e [67] and triarylbismuthines 2b–d [68] were synthesized according to the literature procedures.
General procedure for the photoinduced synthesis of unsymmetrical diaryl selenides from diaryl diselenide and triarylbismuthine
(Ar1Se)2 (0.1 mmol), and Ar23Bi (0.3 mmol) were dispersed in CH3CN (2 mL) with a stirring bar in a quartz test tube ( = 9 mm) with a septum in which a needle was inserted. The mixture was stirred and irradiated by a xenon lamp for 5 h at room temperature. The reaction mixture was filtered through a bed of celite (Celite 535). The crude product was purified by preparative TLC on silica gel (eluent: hexane/EtOAc). Details about compounds 3ab [30], 3ac [14], 3ad [17], 3ba [17], 3bb [31], 3ca [17], 3da [32], 3ea [28] and 3dc [30] were reported in the corresponding articles.
Supporting Information
| Supporting Information File 1: Spectral and analytical data of the new compound 3cb. | ||
| Format: PDF | Size: 1.4 MB | Download |
References
-
Klayman, D. L.; Günther, W. H. H. Organic Selenium Compounds: Their Chemistry and Biology; John Wiley & Sons: New York, 1973.
Return to citation in text: [1] -
Wirth, T., Ed. Organoselenium Chemistry; Topics in Current Chemistry, Vol. 208; Springer: Berlin, 2000. doi:10.1007/3-540-48171-0
Return to citation in text: [1] -
Ogawa, A. Selenium and Tellurium in Organic Synthesis. In Main Group Metals in Organic Synthesis; Yamamoto, H.; Oshima, K., Eds.; Wiley-VCH: Weinheim, Germany, 2004; Vol. 2, pp 813–866.
Return to citation in text: [1] -
Nogueira, C. W.; Rocha, J. B. Arch. Toxicol. 2011, 85, 1313–1359. doi:10.1007/s00204-011-0720-3
Return to citation in text: [1] -
Andersson, C.-M.; Hallberg, A.; Linden, M.; Brattsand, R.; Moldéus, P.; Cotgreave, I. Free Radical Biol. Med. 1994, 16, 17–28. doi:10.1016/0891-5849(94)90238-0
Return to citation in text: [1] -
Campbell, T. W.; Walker, H. G.; Coppinger, G. M. Chem. Rev. 1952, 50, 279–349. doi:10.1021/cr60156a003
Return to citation in text: [1] -
Greenberg, B.; Gould, E. S.; Burlant, W. J. Am. Chem. Soc. 1956, 78, 4028–4029. doi:10.1021/ja01597a043
Return to citation in text: [1] -
Cristau, H. J.; Chabaud, B.; Labaudiniere, R.; Christol, H. Organometallics 1985, 4, 657–661. doi:10.1021/om00123a007
Return to citation in text: [1] -
Beletskaya, I. P.; Sigeev, A. S.; Peregudov, A. S.; Petrovskii, P. V. J. Organomet. Chem. 2000, 605, 96–101. doi:10.1016/S0022-328X(00)00265-5
Return to citation in text: [1] -
Gujadhur, R. K.; Venkataraman, D. Tetrahedron Lett. 2003, 44, 81–84. doi:10.1016/S0040-4039(02)02480-2
Return to citation in text: [1] -
Taniguchi, N.; Onami, T. J. Org. Chem. 2004, 69, 915–920. doi:10.1021/jo030300+
Return to citation in text: [1] -
Kumar, S.; Engman, L. J. Org. Chem. 2006, 71, 5400–5403. doi:10.1021/jo060690a
Return to citation in text: [1] -
Varala, R.; Ramu, E.; Adapa, S. R. Bull. Chem. Soc. Jpn. 2006, 79, 140–141. doi:10.1246/bcsj.79.140
Return to citation in text: [1] -
Taniguchi, N. J. Org. Chem. 2007, 72, 1241–1245. doi:10.1021/jo062131+
Return to citation in text: [1] [2] -
Alves, D.; Santos, C. G.; Paixão, M. W.; Soares, L. C.; Souza, D. D.; Rodrigues, O. E. D.; Braga, A. L. Tetrahedron Lett. 2009, 50, 6635–6638. doi:10.1016/j.tetlet.2009.09.052
Return to citation in text: [1] -
Murthy, S. N.; Madhav, B.; Reddy, V. P.; Nageswar, Y. V. D. Eur. J. Org. Chem. 2009, 5902–5905. doi:10.1002/ejoc.200900989
Return to citation in text: [1] -
Reddy, V. P.; Kumar, A. V.; Swapna, K.; Rao, K. R. Org. Lett. 2009, 11, 951–953. doi:10.1021/ol802734f
Return to citation in text: [1] [2] [3] [4] -
Singh, D.; Alberto, E. E.; Rodrigues, O. E. D.; Braga, A. L. Green Chem. 2009, 11, 1521–1524. doi:10.1039/b916266f
Return to citation in text: [1] -
Bhadra, S.; Saha, A.; Ranu, B. C. J. Org. Chem. 2010, 75, 4864–4867. doi:10.1021/jo100755g
Return to citation in text: [1] -
Li, Y.; Wang, H.; Li, X.; Chen, T.; Zhao, D. Tetrahedron 2010, 66, 8583–8586. doi:10.1016/j.tet.2010.09.061
Return to citation in text: [1] -
Freitas, C. S.; Barcellos, A. M.; Ricordi, V. G.; Pena, J. M.; Perin, G.; Jacob, R. G.; Lenardão, E. J.; Alves, D. Green Chem. 2011, 13, 2931–2938. doi:10.1039/c1gc15725f
Return to citation in text: [1] -
Swapna, K.; Murthy, S. N.; Nageswar, Y. V. D. Eur. J. Org. Chem. 2011, 1940–1946. doi:10.1002/ejoc.201001639
Return to citation in text: [1] -
Zhao, H.; Hao, W.; Xi, Z.; Cai, M. New J. Chem. 2011, 35, 2661. doi:10.1039/c1nj20514e
Return to citation in text: [1] -
Ricordi, V. G.; Freitas, C. S.; Perin, G.; Lenardão, E. J.; Jacob, R. G.; Savegnago, L.; Alves, D. Green Chem. 2012, 14, 1030–1034. doi:10.1039/c2gc16427b
Return to citation in text: [1] -
Beletskaya, I. P.; Sigeev, A. S.; Peregudov, A. S.; Petrovskii, P. V.; Khrustalev, V. N. Chem. Lett. 2010, 39, 720–722. doi:10.1246/cl.2010.720
Return to citation in text: [1] -
Beletskaya, I. P.; Sigeev, A. S.; Peregudov, A. S.; Petrovskii, P. V. Russ. J. Org. Chem. 2001, 37, 1463–1475. doi:10.1023/A:1013460213633
Return to citation in text: [1] -
Ren, K.; Wang, M.; Wang, L. Org. Biomol. Chem. 2009, 7, 4858–4861. doi:10.1039/b914533h
Return to citation in text: [1] -
Reddy, K. H. V.; Satish, G.; Ramesh, K.; Karnakar, K.; Nageswar, Y. V. D. Chem. Lett. 2012, 41, 585–587. doi:10.1246/cl.2012.585
Return to citation in text: [1] [2] -
Wang, M.; Ren, K.; Wang, L. Adv. Synth. Catal. 2009, 351, 1586–1594. doi:10.1002/adsc.200900095
Return to citation in text: [1] -
Hayashi, S.; Yamane, K.; Nakanishi, W. J. Org. Chem. 2007, 72, 7587–7596. doi:10.1021/jo070988g
Return to citation in text: [1] [2] [3] -
Beletskaya, I. P.; Sigeev, A. S.; Peregudov, A. S.; Petrovskii, P. V. Tetrahedron Lett. 2003, 44, 7039–7041. doi:10.1016/S0040-4039(03)01756-8
Return to citation in text: [1] [2] -
Prasad, C. D.; Balkrishna, S. J.; Kumar, A.; Bhakuni, B. S.; Shrimali, K.; Biswas, S.; Kumar, S. J. Org. Chem. 2013, 78, 1434–1443. doi:10.1021/jo302480j
Return to citation in text: [1] [2] -
Suzuki, H.; Matano, Y. Organobismuth Chemistry; Elsevier: Amsterdam, 2001.
Return to citation in text: [1] [2] [3] -
Finet, J. P. Chem. Rev. 1989, 89, 1487–1501. doi:10.1021/cr00097a005
Return to citation in text: [1] -
Elliott, G. I.; Konopelski, J. P. Tetrahedron 2001, 57, 5683–5705. doi:10.1016/S0040-4020(01)00385-4
Return to citation in text: [1] -
Leonard, N. M.; Wieland, L. C.; Mohan, R. S. Tetrahedron 2002, 58, 8373–8397. doi:10.1016/S0040-4020(02)01000-1
Return to citation in text: [1] -
Cho, C. S.; Yoshimori, Y.; Uemura, S. Bull. Chem. Soc. Jpn. 1995, 68, 950–957. doi:10.1246/bcsj.68.950
Return to citation in text: [1] -
Arnauld, T.; Barton, D. H. R.; Doris, E. Tetrahedron 1997, 53, 4137–4144. doi:10.1016/S0040-4020(97)00133-6
Return to citation in text: [1] -
Arnauld, T.; Barton, D. H. R.; Normant, J.-F.; Doris, E. J. Org. Chem. 1999, 64, 6915–6917. doi:10.1021/jo9905928
Return to citation in text: [1] -
Ohe, T.; Tanaka, T.; Kuroda, M.; Cho, C. S.; Ohe, K.; Uemura, S. Bull. Chem. Soc. Jpn. 1999, 72, 1851–1855. doi:10.1246/bcsj.72.1851
Return to citation in text: [1] -
Ikegai, K.; Mukaiyama, T. Chem. Lett. 2005, 34, 1496–1497. doi:10.1246/cl.2005.1496
Return to citation in text: [1] -
Moiseev, D. V.; Malysheva, Y. B.; Shavyrin, A. S.; Kurskii, Y. A.; Gushchin, A. V. J. Organomet. Chem. 2005, 690, 3652–3663. doi:10.1016/j.jorganchem.2005.04.051
Return to citation in text: [1] -
Finet, J.-P.; Fedorov, A. Y. J. Organomet. Chem. 2006, 691, 2386–2393. doi:10.1016/j.jorganchem.2006.01.022
Return to citation in text: [1] -
Gagnon, A.; St-Onge, M.; Little, K.; Duplessis, M.; Barabé, F. J. Am. Chem. Soc. 2007, 129, 44–45. doi:10.1021/ja0676758
Return to citation in text: [1] -
Ikegai, K.; Fukumoto, K.; Mukaiyama, T. Chem. Lett. 2006, 35, 612–613. doi:10.1246/cl.2006.612
Return to citation in text: [1] -
Rao, M. L. N.; Venkatesh, V.; Banerjee, D. Tetrahedron 2007, 63, 12917–12926. doi:10.1016/j.tet.2007.10.047
Return to citation in text: [1] -
Gagnon, A.; Duplessis, M.; Alsabeh, P.; Barabé, F. J. Org. Chem. 2008, 73, 3604–3607. doi:10.1021/jo702377h
Return to citation in text: [1] -
Rao, M. L. N.; Jadhav, D. N.; Banerjee, D. Tetrahedron 2008, 64, 5762–5772. doi:10.1016/j.tet.2008.04.011
Return to citation in text: [1] -
Rahman, A. F. M. M.; Murafuji, T.; Ishibashi, M.; Miyoshi, Y.; Sugihara, Y. J. Organomet. Chem. 2004, 689, 3395–3401. doi:10.1016/j.jorganchem.2004.07.055
Return to citation in text: [1] -
Chaudhari, K. R.; Wadawale, A. P.; Jain, V. K. J. Organomet. Chem. 2012, 698, 15–21. doi:10.1016/j.jorganchem.2011.09.024
Return to citation in text: [1] -
Barton, D. H. R.; Ozbalik, N.; Ramesh, M. Tetrahedron 1988, 44, 5661–5668. doi:10.1016/S0040-4020(01)81427-7
Return to citation in text: [1] -
Rao, M. L. N.; Dasgupta, P. Tetrahedron Lett. 2012, 53, 162–165. doi:10.1016/j.tetlet.2011.10.156
Return to citation in text: [1] -
Rao, M. L. N.; Awasthi, D. K.; Talode, J. B. Tetrahedron Lett. 2012, 53, 2662–2666. doi:10.1016/j.tetlet.2012.03.059
Return to citation in text: [1] -
Kopinke, F. D.; Zimmermann, G.; Anders, K. J. Org. Chem. 1989, 54, 3571–3576. doi:10.1021/jo00276a014
Return to citation in text: [1] [2] -
Hey, D. H.; Shingleton, D. A.; Williams, G. H. J. Chem. Soc. 1963, 5612–5619. doi:10.1039/jr9630005612
Return to citation in text: [1] [2] -
Yamago, S.; Kayahara, E.; Kotani, M.; Ray, B.; Kwak, Y.; Goto, A.; Fukuda, T. Angew. Chem., Int. Ed. 2007, 46, 1304–1306. doi:10.1002/anie.200604473
Return to citation in text: [1] -
Perkins, M. J.; Turner, E. S. J. Chem. Soc., Chem. Commun. 1981, 139–140. doi:10.1039/C39810000139
Return to citation in text: [1] [2] -
Russell, G. A.; Tashtoush, H. J. Am. Chem. Soc. 1983, 105, 1398–1399. doi:10.1021/ja00343a069
Return to citation in text: [1] [2] -
Russell, G. A.; Ngoviwatchai, P.; Tashtoush, H. I.; Pla-Dalmau, A.; Khanna, R. K. J. Am. Chem. Soc. 1988, 110, 3530–3538. doi:10.1021/ja00219a030
Return to citation in text: [1] [2] -
Ogawa, A.; Tanaka, H.; Yokoyama, H.; Obayashi, R.; Yokoyama, K.; Sonoda, N. J. Org. Chem. 1992, 57, 111–115. doi:10.1021/jo00027a021
Return to citation in text: [1] -
Ogawa, A.; Obayashi, R.; Ine, H.; Tsuboi, Y.; Sonoda, N.; Hirao, T. J. Org. Chem. 1998, 63, 881–884. doi:10.1021/jo971652h
Return to citation in text: [1] -
Kawaguchi, S.-i.; Shirai, T.; Ohe, T.; Nomoto, A.; Sonoda, M.; Ogawa, A. J. Org. Chem. 2009, 74, 1751–1754. doi:10.1021/jo8020067
Return to citation in text: [1] -
Kawaguchi, S.-i.; Ogawa, A. J. Synth. Org. Chem., Jpn. 2010, 68, 705–717. doi:10.5059/yukigoseikyokaishi.68.705
Return to citation in text: [1] -
Kawaguchi, S.-i.; Ohe, T.; Shirai, T.; Nomoto, A.; Sonoda, M.; Ogawa, A. Organometallics 2010, 29, 312–316. doi:10.1021/om9008982
Return to citation in text: [1] -
Arnauld, T.; Barton, D. H. R.; Normant, J.-F. J. Org. Chem. 1999, 64, 3722–3725. doi:10.1021/jo982093x
Return to citation in text: [1] -
Ogawa, A.; Obayashi, R.; Doi, M.; Sonoda, N.; Hirao, T. J. Org. Chem. 1998, 63, 4277–4281. doi:10.1021/jo972253p
Return to citation in text: [1] -
Reich, H. J.; Renga, J. M.; Reich, I. L. J. Am. Chem. Soc. 1975, 97, 5434–5447. doi:10.1021/ja00852a019
Return to citation in text: [1] -
Barton, D. H. R.; Bhatnagar, N. Y.; Finet, J. P.; Motherwell, W. B. Tetrahedron 1986, 42, 3111–3122. doi:10.1016/S0040-4020(01)87378-6
Return to citation in text: [1]
| 31. | Beletskaya, I. P.; Sigeev, A. S.; Peregudov, A. S.; Petrovskii, P. V. Tetrahedron Lett. 2003, 44, 7039–7041. doi:10.1016/S0040-4039(03)01756-8 |
| 17. | Reddy, V. P.; Kumar, A. V.; Swapna, K.; Rao, K. R. Org. Lett. 2009, 11, 951–953. doi:10.1021/ol802734f |
| 17. | Reddy, V. P.; Kumar, A. V.; Swapna, K.; Rao, K. R. Org. Lett. 2009, 11, 951–953. doi:10.1021/ol802734f |
| 1. | Klayman, D. L.; Günther, W. H. H. Organic Selenium Compounds: Their Chemistry and Biology; John Wiley & Sons: New York, 1973. |
| 2. | Wirth, T., Ed. Organoselenium Chemistry; Topics in Current Chemistry, Vol. 208; Springer: Berlin, 2000. doi:10.1007/3-540-48171-0 |
| 3. | Ogawa, A. Selenium and Tellurium in Organic Synthesis. In Main Group Metals in Organic Synthesis; Yamamoto, H.; Oshima, K., Eds.; Wiley-VCH: Weinheim, Germany, 2004; Vol. 2, pp 813–866. |
| 4. | Nogueira, C. W.; Rocha, J. B. Arch. Toxicol. 2011, 85, 1313–1359. doi:10.1007/s00204-011-0720-3 |
| 34. | Finet, J. P. Chem. Rev. 1989, 89, 1487–1501. doi:10.1021/cr00097a005 |
| 35. | Elliott, G. I.; Konopelski, J. P. Tetrahedron 2001, 57, 5683–5705. doi:10.1016/S0040-4020(01)00385-4 |
| 36. | Leonard, N. M.; Wieland, L. C.; Mohan, R. S. Tetrahedron 2002, 58, 8373–8397. doi:10.1016/S0040-4020(02)01000-1 |
| 37. | Cho, C. S.; Yoshimori, Y.; Uemura, S. Bull. Chem. Soc. Jpn. 1995, 68, 950–957. doi:10.1246/bcsj.68.950 |
| 38. | Arnauld, T.; Barton, D. H. R.; Doris, E. Tetrahedron 1997, 53, 4137–4144. doi:10.1016/S0040-4020(97)00133-6 |
| 39. | Arnauld, T.; Barton, D. H. R.; Normant, J.-F.; Doris, E. J. Org. Chem. 1999, 64, 6915–6917. doi:10.1021/jo9905928 |
| 40. | Ohe, T.; Tanaka, T.; Kuroda, M.; Cho, C. S.; Ohe, K.; Uemura, S. Bull. Chem. Soc. Jpn. 1999, 72, 1851–1855. doi:10.1246/bcsj.72.1851 |
| 41. | Ikegai, K.; Mukaiyama, T. Chem. Lett. 2005, 34, 1496–1497. doi:10.1246/cl.2005.1496 |
| 42. | Moiseev, D. V.; Malysheva, Y. B.; Shavyrin, A. S.; Kurskii, Y. A.; Gushchin, A. V. J. Organomet. Chem. 2005, 690, 3652–3663. doi:10.1016/j.jorganchem.2005.04.051 |
| 43. | Finet, J.-P.; Fedorov, A. Y. J. Organomet. Chem. 2006, 691, 2386–2393. doi:10.1016/j.jorganchem.2006.01.022 |
| 44. | Gagnon, A.; St-Onge, M.; Little, K.; Duplessis, M.; Barabé, F. J. Am. Chem. Soc. 2007, 129, 44–45. doi:10.1021/ja0676758 |
| 45. | Ikegai, K.; Fukumoto, K.; Mukaiyama, T. Chem. Lett. 2006, 35, 612–613. doi:10.1246/cl.2006.612 |
| 46. | Rao, M. L. N.; Venkatesh, V.; Banerjee, D. Tetrahedron 2007, 63, 12917–12926. doi:10.1016/j.tet.2007.10.047 |
| 47. | Gagnon, A.; Duplessis, M.; Alsabeh, P.; Barabé, F. J. Org. Chem. 2008, 73, 3604–3607. doi:10.1021/jo702377h |
| 48. | Rao, M. L. N.; Jadhav, D. N.; Banerjee, D. Tetrahedron 2008, 64, 5762–5772. doi:10.1016/j.tet.2008.04.011 |
| 49. | Rahman, A. F. M. M.; Murafuji, T.; Ishibashi, M.; Miyoshi, Y.; Sugihara, Y. J. Organomet. Chem. 2004, 689, 3395–3401. doi:10.1016/j.jorganchem.2004.07.055 |
| 50. | Chaudhari, K. R.; Wadawale, A. P.; Jain, V. K. J. Organomet. Chem. 2012, 698, 15–21. doi:10.1016/j.jorganchem.2011.09.024 |
| 51. | Barton, D. H. R.; Ozbalik, N.; Ramesh, M. Tetrahedron 1988, 44, 5661–5668. doi:10.1016/S0040-4020(01)81427-7 |
| 52. | Rao, M. L. N.; Dasgupta, P. Tetrahedron Lett. 2012, 53, 162–165. doi:10.1016/j.tetlet.2011.10.156 |
| 53. | Rao, M. L. N.; Awasthi, D. K.; Talode, J. B. Tetrahedron Lett. 2012, 53, 2662–2666. doi:10.1016/j.tetlet.2012.03.059 |
| 30. | Hayashi, S.; Yamane, K.; Nakanishi, W. J. Org. Chem. 2007, 72, 7587–7596. doi:10.1021/jo070988g |
| 6. | Campbell, T. W.; Walker, H. G.; Coppinger, G. M. Chem. Rev. 1952, 50, 279–349. doi:10.1021/cr60156a003 |
| 7. | Greenberg, B.; Gould, E. S.; Burlant, W. J. Am. Chem. Soc. 1956, 78, 4028–4029. doi:10.1021/ja01597a043 |
| 8. | Cristau, H. J.; Chabaud, B.; Labaudiniere, R.; Christol, H. Organometallics 1985, 4, 657–661. doi:10.1021/om00123a007 |
| 9. | Beletskaya, I. P.; Sigeev, A. S.; Peregudov, A. S.; Petrovskii, P. V. J. Organomet. Chem. 2000, 605, 96–101. doi:10.1016/S0022-328X(00)00265-5 |
| 10. | Gujadhur, R. K.; Venkataraman, D. Tetrahedron Lett. 2003, 44, 81–84. doi:10.1016/S0040-4039(02)02480-2 |
| 11. | Taniguchi, N.; Onami, T. J. Org. Chem. 2004, 69, 915–920. doi:10.1021/jo030300+ |
| 12. | Kumar, S.; Engman, L. J. Org. Chem. 2006, 71, 5400–5403. doi:10.1021/jo060690a |
| 13. | Varala, R.; Ramu, E.; Adapa, S. R. Bull. Chem. Soc. Jpn. 2006, 79, 140–141. doi:10.1246/bcsj.79.140 |
| 14. | Taniguchi, N. J. Org. Chem. 2007, 72, 1241–1245. doi:10.1021/jo062131+ |
| 15. | Alves, D.; Santos, C. G.; Paixão, M. W.; Soares, L. C.; Souza, D. D.; Rodrigues, O. E. D.; Braga, A. L. Tetrahedron Lett. 2009, 50, 6635–6638. doi:10.1016/j.tetlet.2009.09.052 |
| 16. | Murthy, S. N.; Madhav, B.; Reddy, V. P.; Nageswar, Y. V. D. Eur. J. Org. Chem. 2009, 5902–5905. doi:10.1002/ejoc.200900989 |
| 17. | Reddy, V. P.; Kumar, A. V.; Swapna, K.; Rao, K. R. Org. Lett. 2009, 11, 951–953. doi:10.1021/ol802734f |
| 18. | Singh, D.; Alberto, E. E.; Rodrigues, O. E. D.; Braga, A. L. Green Chem. 2009, 11, 1521–1524. doi:10.1039/b916266f |
| 19. | Bhadra, S.; Saha, A.; Ranu, B. C. J. Org. Chem. 2010, 75, 4864–4867. doi:10.1021/jo100755g |
| 20. | Li, Y.; Wang, H.; Li, X.; Chen, T.; Zhao, D. Tetrahedron 2010, 66, 8583–8586. doi:10.1016/j.tet.2010.09.061 |
| 21. | Freitas, C. S.; Barcellos, A. M.; Ricordi, V. G.; Pena, J. M.; Perin, G.; Jacob, R. G.; Lenardão, E. J.; Alves, D. Green Chem. 2011, 13, 2931–2938. doi:10.1039/c1gc15725f |
| 22. | Swapna, K.; Murthy, S. N.; Nageswar, Y. V. D. Eur. J. Org. Chem. 2011, 1940–1946. doi:10.1002/ejoc.201001639 |
| 23. | Zhao, H.; Hao, W.; Xi, Z.; Cai, M. New J. Chem. 2011, 35, 2661. doi:10.1039/c1nj20514e |
| 24. | Ricordi, V. G.; Freitas, C. S.; Perin, G.; Lenardão, E. J.; Jacob, R. G.; Savegnago, L.; Alves, D. Green Chem. 2012, 14, 1030–1034. doi:10.1039/c2gc16427b |
| 25. | Beletskaya, I. P.; Sigeev, A. S.; Peregudov, A. S.; Petrovskii, P. V.; Khrustalev, V. N. Chem. Lett. 2010, 39, 720–722. doi:10.1246/cl.2010.720 |
| 26. | Beletskaya, I. P.; Sigeev, A. S.; Peregudov, A. S.; Petrovskii, P. V. Russ. J. Org. Chem. 2001, 37, 1463–1475. doi:10.1023/A:1013460213633 |
| 27. | Ren, K.; Wang, M.; Wang, L. Org. Biomol. Chem. 2009, 7, 4858–4861. doi:10.1039/b914533h |
| 28. | Reddy, K. H. V.; Satish, G.; Ramesh, K.; Karnakar, K.; Nageswar, Y. V. D. Chem. Lett. 2012, 41, 585–587. doi:10.1246/cl.2012.585 |
| 29. | Wang, M.; Ren, K.; Wang, L. Adv. Synth. Catal. 2009, 351, 1586–1594. doi:10.1002/adsc.200900095 |
| 30. | Hayashi, S.; Yamane, K.; Nakanishi, W. J. Org. Chem. 2007, 72, 7587–7596. doi:10.1021/jo070988g |
| 31. | Beletskaya, I. P.; Sigeev, A. S.; Peregudov, A. S.; Petrovskii, P. V. Tetrahedron Lett. 2003, 44, 7039–7041. doi:10.1016/S0040-4039(03)01756-8 |
| 32. | Prasad, C. D.; Balkrishna, S. J.; Kumar, A.; Bhakuni, B. S.; Shrimali, K.; Biswas, S.; Kumar, S. J. Org. Chem. 2013, 78, 1434–1443. doi:10.1021/jo302480j |
| 67. | Reich, H. J.; Renga, J. M.; Reich, I. L. J. Am. Chem. Soc. 1975, 97, 5434–5447. doi:10.1021/ja00852a019 |
| 5. | Andersson, C.-M.; Hallberg, A.; Linden, M.; Brattsand, R.; Moldéus, P.; Cotgreave, I. Free Radical Biol. Med. 1994, 16, 17–28. doi:10.1016/0891-5849(94)90238-0 |
| 68. | Barton, D. H. R.; Bhatnagar, N. Y.; Finet, J. P.; Motherwell, W. B. Tetrahedron 1986, 42, 3111–3122. doi:10.1016/S0040-4020(01)87378-6 |
| 65. | Arnauld, T.; Barton, D. H. R.; Normant, J.-F. J. Org. Chem. 1999, 64, 3722–3725. doi:10.1021/jo982093x |
| 66. | Ogawa, A.; Obayashi, R.; Doi, M.; Sonoda, N.; Hirao, T. J. Org. Chem. 1998, 63, 4277–4281. doi:10.1021/jo972253p |
| 28. | Reddy, K. H. V.; Satish, G.; Ramesh, K.; Karnakar, K.; Nageswar, Y. V. D. Chem. Lett. 2012, 41, 585–587. doi:10.1246/cl.2012.585 |
| 57. | Perkins, M. J.; Turner, E. S. J. Chem. Soc., Chem. Commun. 1981, 139–140. doi:10.1039/C39810000139 |
| 58. | Russell, G. A.; Tashtoush, H. J. Am. Chem. Soc. 1983, 105, 1398–1399. doi:10.1021/ja00343a069 |
| 59. | Russell, G. A.; Ngoviwatchai, P.; Tashtoush, H. I.; Pla-Dalmau, A.; Khanna, R. K. J. Am. Chem. Soc. 1988, 110, 3530–3538. doi:10.1021/ja00219a030 |
| 60. | Ogawa, A.; Tanaka, H.; Yokoyama, H.; Obayashi, R.; Yokoyama, K.; Sonoda, N. J. Org. Chem. 1992, 57, 111–115. doi:10.1021/jo00027a021 |
| 61. | Ogawa, A.; Obayashi, R.; Ine, H.; Tsuboi, Y.; Sonoda, N.; Hirao, T. J. Org. Chem. 1998, 63, 881–884. doi:10.1021/jo971652h |
| 62. | Kawaguchi, S.-i.; Shirai, T.; Ohe, T.; Nomoto, A.; Sonoda, M.; Ogawa, A. J. Org. Chem. 2009, 74, 1751–1754. doi:10.1021/jo8020067 |
| 63. | Kawaguchi, S.-i.; Ogawa, A. J. Synth. Org. Chem., Jpn. 2010, 68, 705–717. doi:10.5059/yukigoseikyokaishi.68.705 |
| 64. | Kawaguchi, S.-i.; Ohe, T.; Shirai, T.; Nomoto, A.; Sonoda, M.; Ogawa, A. Organometallics 2010, 29, 312–316. doi:10.1021/om9008982 |
| 57. | Perkins, M. J.; Turner, E. S. J. Chem. Soc., Chem. Commun. 1981, 139–140. doi:10.1039/C39810000139 |
| 58. | Russell, G. A.; Tashtoush, H. J. Am. Chem. Soc. 1983, 105, 1398–1399. doi:10.1021/ja00343a069 |
| 59. | Russell, G. A.; Ngoviwatchai, P.; Tashtoush, H. I.; Pla-Dalmau, A.; Khanna, R. K. J. Am. Chem. Soc. 1988, 110, 3530–3538. doi:10.1021/ja00219a030 |
| 30. | Hayashi, S.; Yamane, K.; Nakanishi, W. J. Org. Chem. 2007, 72, 7587–7596. doi:10.1021/jo070988g |
| 55. | Hey, D. H.; Shingleton, D. A.; Williams, G. H. J. Chem. Soc. 1963, 5612–5619. doi:10.1039/jr9630005612 |
| 56. | Yamago, S.; Kayahara, E.; Kotani, M.; Ray, B.; Kwak, Y.; Goto, A.; Fukuda, T. Angew. Chem., Int. Ed. 2007, 46, 1304–1306. doi:10.1002/anie.200604473 |
| 17. | Reddy, V. P.; Kumar, A. V.; Swapna, K.; Rao, K. R. Org. Lett. 2009, 11, 951–953. doi:10.1021/ol802734f |
| 33. | Suzuki, H.; Matano, Y. Organobismuth Chemistry; Elsevier: Amsterdam, 2001. |
| 54. | Kopinke, F. D.; Zimmermann, G.; Anders, K. J. Org. Chem. 1989, 54, 3571–3576. doi:10.1021/jo00276a014 |
| 33. | Suzuki, H.; Matano, Y. Organobismuth Chemistry; Elsevier: Amsterdam, 2001. |
| 54. | Kopinke, F. D.; Zimmermann, G.; Anders, K. J. Org. Chem. 1989, 54, 3571–3576. doi:10.1021/jo00276a014 |
| 55. | Hey, D. H.; Shingleton, D. A.; Williams, G. H. J. Chem. Soc. 1963, 5612–5619. doi:10.1039/jr9630005612 |
| 32. | Prasad, C. D.; Balkrishna, S. J.; Kumar, A.; Bhakuni, B. S.; Shrimali, K.; Biswas, S.; Kumar, S. J. Org. Chem. 2013, 78, 1434–1443. doi:10.1021/jo302480j |
© 2013 Kobiki et al; licensee Beilstein-Institut.
This is an Open Access article under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
The license is subject to the Beilstein Journal of Organic Chemistry terms and conditions: (http://www.beilstein-journals.org/bjoc)