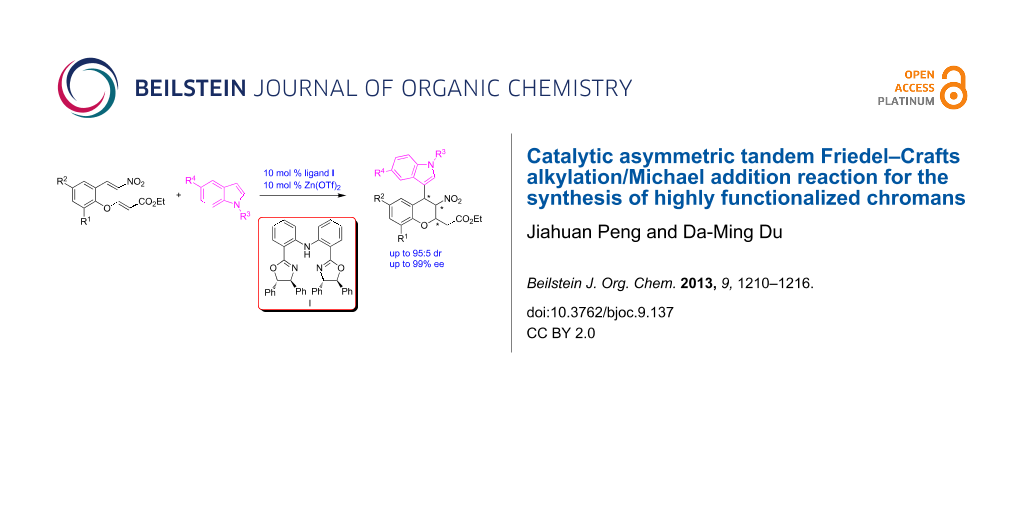
Abstract
The enantioselective tandem Friedel–Crafts alkylation/Michael addition reaction of indoles with nitroolefin enoates catalyzed by a diphenylamine-linked bis(oxazoline)-Zn(OTf)2 complex was investigated. This tandem reaction afforded functionalized chiral chromans in good yields with moderate to high stereoselectivities (up to 95:5 dr, up to 99% ee).
Graphical Abstract
Introduction
The development of efficient and convenient methods to access complex compounds with multiple stereogenic centers is one of the significant challenges in organic chemistry. Catalytic asymmetric tandem or cascade reactions are powerful tools to afford complex molecules with multiple stereogenic centers [1-13]. Newly developed tandem/domino reactions are increasingly applied in the synthesis of natural products and other biologically active compounds [14-16].
Dihydrocoumarins, chromans, and chromenes can be found in many natural products and synthetic molecules, and they also possess potentially useful biological properties [17-20]. The benzopyran framework has attracted considerable attention because of the importance of chromans and their biological properties. Numerous synthetic routes have been reported over the past few decades [21-32]. Chiral indolyl(nitro)chromans have been successfully synthesized in our previous study [33]. Good results were obtained in the diastereo- and enantioselective Friedel–Crafts alkylation of indoles with 3-nitro-2H-chromenes catalyzed by diphenylamine-linked bis(oxazoline)-Zn(II) complexes. On the other hand, indole and its derivatives are one of the most intensively investigated classical heterocycles owing to their prevalence in bioactive compounds. Indoles have been successfully utilized in asymmetric Friedel–Crafts reactions with nitroolefin and its derivatives in previous reports [34-49]. During the preparation of this manuscript, a similar report on the enantioselective synthesis of highly substituted chromans by a Zinc(II)-catalyzed tandem Friedel–Crafts alkylation/Michael addition reaction has appeared [50]. Herein, we wish to detail our independent research on the asymmetric tandem Friedel–Crafts alkylation/Michael addition reaction of indoles with nitroolefin enoates catalyzed by bis(oxazoline)-Zn(OTf)2 complexes, resulting in functionalized chiral chromans (dihydrobenzopyrans) in moderate to high diastereoselectivities (up to 95:5 dr) and enantioselectivities (up to 99% ee).
Results and Discussion
Our initial exploratory efforts began with the optimization of the model reaction between nitroolefin enoate 1a and indole 2a. First, a series of chiral bis(oxazoline) ligands (I–V) with Zn(OTf)2 as catalysts were investigated in this reaction (Figure 1). The results are summarized in Table 1. It was found that the reaction exhibited good yield and high stereoselectivity with catalysis by the I-Zn(OTf)2 complex (Table 1, entry 1). Interestingly, with the ligands III–V (Table 1, entries 3–5), the reaction gave the opposite stereoselectivities probably because of the lower steric hindrance compared with I and II. Although the opposite diastereomer was obtained in good yield and stereoselectivity with the use of ligand IV, ligand I was the preferred one according to the results.
Figure 1: Diphenylamine-linked bis(oxazoline).
Figure 1: Diphenylamine-linked bis(oxazoline).
Table 1: Effect of ligands on the asymmetric tandem Friedel–Crafts alkylation/Michael addition reaction.
|
|
||||
| entrya | ligand | yield (%)b | drc | ee (%)c,d |
|---|---|---|---|---|
| 1 | I | 48 | 96:4 | 83/– |
| 2 | II | 59 | 88:12 | 55/– |
| 3 | III | 43 | 33:67 | –/68 |
| 4 | IV | 56 | 17:83 | –/73 |
| 5 | V | 35 | 29:71 | –/53 |
aReaction conditions: nitroolefin enoate 1a (0.1 mmol) with indole 2a (0.1 mmol) in 1.5 mL of toluene catalyzed by 10 mol % ligand-Zn(OTf)2 complex for 24 h at room temperature. bIsolated yields by column chromatography. cDetermined by HPLC on Daicel Chiralpak IA column (n-hexane/2-propanol 85:15, 0.5 mL/min). dee for the major diastereomer.
In order to increase the yield and stereoselectivity of the desired product, further screening of reaction parameters such as the ratio of substrates and temperature were investigated. When 1.5 equiv of indole was used in the reaction, a significant improvement of the yield was realized (Table 2, entry 2). The enantioselectivity of the product 3a was improved slightly by lowering the reaction temperature (Table 2, entries 7 and 8). Raising the temperature to 50 °C led to a decrease of yield, diastereoselectivity and enantioselectivity. After a brief screening of the solvent, toluene was found to be the best choice.
Table 2: Optimization of reaction conditions.
|
|
||||
| entrya | solvent | yield (%)b | drc | ee (%)c |
|---|---|---|---|---|
| 1d | toluene | 48 | 96:4 | 83 |
| 2 | toluene | 58 | 96:4 | 84 |
| 3 | xylene | 49 | 94:6 | 82 |
| 4 | α,α,α-trifluorotoluene | 56 | 95:5 | 54 |
| 5 | CH2ClCH2Cl | 37 | 89:11 | 69 |
| 6 | THF | trace | – | – |
| 7e | toluene | 55 | 97:3 | 87 |
| 8f | toluene | 58 | 96:4 | 87 |
| 9g | toluene | 45 | 93:7 | 74 |
aReaction conditions: nitroolefin enoate 1a (0.1 mmol) with indole 2a (0.15 mmol) in 1.5 mL of toluene catalyzed by 10 mol % ligand I-Zn(OTf)2 complex for 24 h at room temperature. bIsolated yields by column chromatography. cDetermined by HPLC on Daicel Chiralpak IA column (n-hexane/2-propanol 85:15, 0.5 mL/min). d1 equiv of indole 2a (0.1 mmol) was used. eThe reaction was performed at 0 °C for 48 h. fThe reaction was performed at –10 °C for 48 h. gThe reaction was performed at 50 °C for 24 h.
Different additives (10 mol %) were then tested in the presence of 10 mol % of I-Zn(OTf)2 complex. Remarkably, a substantial improvement of the yield was realized when Et3N was added; however, the diastereo- and enantioselectivity dropped significantly (Table 3, entry 2). With both NH(C2H5)2 and TMEDA, no desired product was observed (Table 3, entries 3 and 4). DABCO led to no significant increase in yield and stereoselectivity (Table 3, entry 5). A substantial increase in enantioselectivity was observed with the use of LiOt-Bu, but the yield remained moderate (Table 3, entry 7). Among the additives probed, the best results (73% yield, 96:4 dr and 89% ee) were achieved when NaOt-Bu was used as an additive in the reaction (Table 3, entry 8).
Table 3: Effect of additives on asymmetric tandem Friedel–Crafts alkylation/Michael addition reaction.
|
|
||||
| entrya | additive | yield (%)b | drc | ee (%)c |
|---|---|---|---|---|
| 1 | – | 58 | 96:4 | 83 |
| 2 | Et3N | 76 | 79:21 | 63 |
| 3 | NH(C2H5)2 | 0 | – | – |
| 4 | TMEDA | 0 | – | – |
| 5 | DABCO | 41 | 93:7 | 82 |
| 6 | CsCO3 | 62 | 98:2 | 80 |
| 7 | LiOt-Bu | 47 | 95:5 | 92 |
| 8 | NaOt-Bu | 73 | 96:4 | 89 |
| 9 | KOt-Bu | 62 | 96:4 | 84 |
aReaction conditions: nitroolefin enoate 1a (0.1 mmol) with indole 2a (0.15 mmol) in 1.5 mL of toluene catalyzed by 10 mol % ligand I-Zn(OTf)2 complex with 10 mol % additive for 24 h at room temperature. bIsolated yields by column chromatography. cDetermined by HPLC on Daicel Chiralpak IA column (n-hexane/2-propanol 85:15, 0.5 mL/min).
After optimization of the reaction conditions, the substrate scope of the enantioselective Friedel–Crafts alkylation/Michael addition of nitroolefin enoates 1 with indoles 2 was explored. The results are summarized in Table 4 (see Supporting Information File 1 for full experimental data). Both electron withdrawing and electron-rich substituents in the 5-position of the indole caused moderate decrease in enantioselectivity and diastereoselectivity (Table 4, entries 2–4). Nitroolefin enoates 1b or 1c with chlorine or bromine on the aromatic ring reacted smoothly to afford products 3f or 3g with good yields and stereoselectivities (Table 4, entries 6 and 7). We found that incorporation of an electron-donating methoxy group on the aromatic ring of the nitroolefin enaote had a significant effect on both yield and stereoselectivity. In the case of methoxy-substituted nitroolefin enoates 1e (Table 4, entry 9), the yield of the product 3i decreased to 12% and the enantioselectivity decreased to 24% even though the reaction time was prolonged to 96 h. A similar result was observed in the case of product 3j (Table 4, entry 10). A steric effect was also observed in this reaction. When the substrate 1g bearing two sterically hindered bromine atoms on the phenyl ring was used, the yield and stereoselectivity of the desired product 3k decreased significantly (Table 4, entry 11). The configuration of the major diastereomer of 3g was determined to be C15(S), C16(R), C17(S) (Figure 2), and those of other products were assigned by analogy [51].
Table 4: Asymmetric tandem Friedel–Crafts alkylation/Michael addition reaction of nitroolefin enoates with indoles.
|
|
||||||||
| entrya | R1 | R2 | R3 | R4 | product | yield (%)b | drc | ee (%)c,d |
|---|---|---|---|---|---|---|---|---|
| 1e | H | H | H | H | 3a | 73 | 96:4 | 89 |
| 2 | H | H | H | CH3 | 3b | 69 | 90:10 | 80 |
| 3 | H | H | H | OCH3 | 3c | 76 | 93:7f | 72 |
| 4 | H | H | H | Cl | 3d | 49 | 82:18f | 62 |
| 5 | H | H | CH3 | H | 3e | 60 | 88:12f | 67 |
| 6 | H | Cl | H | H | 3f | 65 | 95:5 | 91 |
| 7 | H | Br | H | H | 3g | 66 | 93:7 | 87 |
| 8 | H | NO2 | H | H | 3h | 56 | 85:15f | 80 |
| 9g | OCH3 | H | H | H | 3i | 12 | –h | 24 |
| 10g | OC2H5 | H | H | H | 3j | 47 | 85:15f | 31 |
| 11 | Br | Br | H | H | 3k | 21 | 72:28 | 57 |
aReaction conditions: nitroolefin enoates 1 (0.2 mmol) with indoles 2 (0.3 mmol) in 3 mL of toluene catalyzed by 10 mol % ligand-Zn(OTf)2 complex with 10 mol % NaOt-Bu for 24 h at room temperature. bIsolated yields by column chromatography. cDetermined by HPLC. dee for the major diastereomer. eNitroolefin enoate 1a (0.1 mmol) with indole 2a (0.15 mmol). fDetermined by the weight ratio of isolated diastereomers. gThe reaction time was 96 h. hThe minor diastereomer was not detected.
![[1860-5397-9-137-2]](/bjoc/content/figures/1860-5397-9-137-2.png?scale=2.0&max-width=1024&background=FFFFFF)
Figure 2: X-ray crystal structure of the major diastereomer of 3g (one symmetric molecule and two solvent molecules are not labeled for clarity).
Figure 2: X-ray crystal structure of the major diastereomer of 3g (one symmetric molecule and two solvent mol...
The results of the substrate scope are unsatisfactory. The yields of the desired products 3 were affected by the side products 4, which were the Friedel–Crafts alkylation products of nitroolefin enoates and indoles. Fortunately, it was found that the model Friedel–Crafts alkylation product 4a, which was isolated, could be transformed to the desired cycloadduct 3a with high stereoselectivity in the presence of 5 equiv of Et3N at room temperature (Scheme 1). With the success of this model reaction, the substrate scope in Table 4 was reinvestigated. The corresponding reactions proceeded smoothly to afford desired products 3 and side products 4 at –10 °C. After the nitroolefin enoates were consumed, 5 equiv of Et3N was added to the reaction at room temperature. The new results are summarized in Table 5. All reactions proceeded smoothly affording desired products 3 with good to excellent yields. However, the diastereoselectivities of the products 3 decreased in all cases. Excellent enantioselectivities were observed with indoles bearing electron-rich substituents (Table 5, entries 2 and 3). Both the electron-withdrawing and electron-rich substituents on the aromatic ring of nitroolefin enoates afforded 3 in low diastereoselectivities (Table 5, entries 6–10). Good to excellent enantioselectivities were observed in these cases. 3k was obtained in moderate stereoselectivity (Table 5, entry 11).
Scheme 1: The transformation of Friedel–Crafts alkylation product 4a to cycloadduct 3a.
Scheme 1: The transformation of Friedel–Crafts alkylation product 4a to cycloadduct 3a.
Table 5: Asymmetric tandem Friedel–Crafts alkylation/Michael addition reaction of nitroolefin enoates with indoles.
|
|
||||||||
| entrya | R1 | R2 | R3 | R4 | product | Yield (%)b | drc | ee (%)c,d |
|---|---|---|---|---|---|---|---|---|
| 1 | H | H | H | H | 3a | 88 | 92:8 | 92/88 |
| 2 | H | H | H | CH3 | 3b | 88 | 44:56e | 62/98 |
| 3 | H | H | H | OCH3 | 3c | 96 | 28:72e | 73/99 |
| 4 | H | H | H | Cl | 3d | 58 | 76:24 | 82/53 |
| 5 | H | H | CH3 | H | 3e | 89 | 82:18e | 65/79 |
| 6 | H | Cl | H | H | 3f | 86 | 74:26 | 95/95 |
| 7 | H | Br | H | H | 3g | 100 | 60:40 | 91/93 |
| 8 | H | NO2 | H | H | 3h | 89 | 49:51 | 95/95 |
| 9 | OCH3 | H | H | H | 3i | 85 | 58:42e | 39/83 |
| 10 | OC2H5 | H | H | H | 3j | 94 | 54:46e | 30/97 |
| 11 | Br | Br | H | H | 3k | 75 | 38:62 | 53/90 |
aReaction conditions: nitroolefin enoate 1a (0.2 mmol) with indole 2a (0.3 mmol) in 3 mL of toluene catalyzed by 10 mol % ligand-Zn(OTf)2 complex for 72 h at –10 °C. Subsequently, 5 equiv of Et3N was added. bIsolated yields by column chromatography. cDetermined by HPLC. dee for both diastereomers. eDetermined by the weight ratio of isolated diastereomers.
Conclusion
In conclusion, we have developed a convenient catalytic asymmetric tandem Friedel–Crafts alkylation/Michael addition reaction of nitroolefin enoates 1 with indoles 2 catalyzed by a tridentate bis(oxazoline) I-Zn(OTf)2 complex. Moderate to high stereoselectivities (up to 95:5 dr, up to 99% ee) and good to excellent yields of the functionalized chiral chromans were obtained. Further applications of these catalysts in other reactions are underway in our laboratory.
Experimental
General procedure A for the catalytic asymmetric tandem Friedel–Crafts alkylation/Michael addition reaction of indoles with nitroolefin enoates: Into a dried Schlenk tube were added Zn(OTf)2 (7.3 mg, 0.02 mmol), ligand I (12.2 mg, 0.02 mmol) and NaOt-Bu (1.9 mg, 0.02 mmol) under argon followed by the addition of toluene (3 mL). The solution was stirred at room temperature for 0.5 h, and then nitroolefin enoate 1 (0.2 mmol) was added. The mixture was stirred for 10 min then the indole 2 (0.3 mmol) was added. After stirring for 48 h at room temperature, the solvent was removed under vacuum. Purification by column chromatography afforded the desired products 3.
General procedure B for the catalytic asymmetric tandem Friedel–Crafts alkylation/Michael addition reaction of indoles with nitroolefin enoates: Into a dried Schlenk tube were added Zn(OTf)2 (7.3 mg, 0.02 mmol) and ligand I (12.2 mg, 0.02 mmol) under argon followed by the addition of toluene (3 mL). The solution was stirred at room temperature for 0.5 h and then nitroolefin enoate 1 (0.2 mmol) was added. The mixture was stirred for 10 min then the indole 2 (0.3 mmol) was added. After stirring for 72 h at –10 °C, Et3N (100 mg, 1 mmol) was added, and the mixture was stirred for another 24 h at room temperature. The solvent was removed under vacuum. Purification by column chromatography afforded the desired products 3.
Acknowledgements
We are grateful for financial support from the National Natural Science Foundation of China (Grant Nos. 20772006, 21072020), the Science and Technology Innovation Program of Beijing Institute of Technology (Grant No. 2011CX01008) and the Development Program for Distinguished Young and Middle-aged Teachers of Beijing Institute of Technology.
References
-
Posner, G. H. Chem. Rev. 1986, 86, 831–844. doi:10.1021/cr00075a007
Return to citation in text: [1] -
Tietze, L. F.; Beifuss, U. Angew. Chem., Int. Ed. Engl. 1993, 32, 131–163. doi:10.1002/anie.199301313
Return to citation in text: [1] -
Tietze, L. F. Chem. Rev. 1996, 96, 115–136. doi:10.1021/cr950027e
Return to citation in text: [1] -
Wasilke, J.-C.; Obrey, S. J.; Baker, R. T.; Bazan, G. C. Chem. Rev. 2005, 105, 1001–1020. doi:10.1021/cr020018n
Return to citation in text: [1] -
Ramón, D. J.; Yus, M. Angew. Chem., Int. Ed. 2005, 44, 1602–1634. doi:10.1002/anie.200460548
Return to citation in text: [1] -
Pellissier, H. Tetrahedron 2006, 62, 1619–1665. doi:10.1016/j.tet.2005.10.040
Return to citation in text: [1] -
Pellissier, H. Tetrahedron 2006, 62, 2143–2173. doi:10.1016/j.tet.2005.10.041
Return to citation in text: [1] -
Guo, H.-C.; Ma, J.-A. Angew. Chem., Int. Ed. 2006, 45, 354–366. doi:10.1002/anie.200500195
Return to citation in text: [1] -
Enders, D.; Grondal, C.; Hüttl, M. R. M. Angew. Chem., Int. Ed. 2007, 46, 1570–1581. doi:10.1002/anie.200603129
Return to citation in text: [1] -
Grondal, C.; Jeanty, M.; Enders, D. Nat. Chem. 2010, 2, 167–178. doi:10.1038/nchem.539
Return to citation in text: [1] -
Moyano, A.; Rios, R. Chem. Rev. 2011, 111, 4703–4832. doi:10.1021/cr100348t
Return to citation in text: [1] -
Pellissier, H. Adv. Synth. Catal. 2012, 354, 237–294. doi:10.1002/adsc.201100714
Return to citation in text: [1] -
Schreiber, S. L. Science 2000, 287, 1964–1969. doi:10.1126/science.287.5460.1964
Return to citation in text: [1] -
Nicolaou, K. C.; Edmonds, D. J.; Bulger, P. G. Angew. Chem., Int. Ed. 2006, 45, 7134–7186. doi:10.1002/anie.200601872
Return to citation in text: [1] -
de Figueiredo, R. M.; Christmann, M. Eur. J. Org. Chem. 2007, 2575–2600. doi:10.1002/ejoc.200700032
Return to citation in text: [1] -
Rueping, M.; Dufour, J.; Schoepke, F. R. Green Chem. 2011, 13, 1084–1105. doi:10.1039/c1gc15027h
Return to citation in text: [1] -
Conti, C.; Monaco, L. P.; Desideri, N. Bioorg. Med. Chem. 2011, 19, 7357–7364. doi:10.1016/j.bmc.2011.10.060
Return to citation in text: [1] -
Schweizer, E. E.; Meeder-Nycz, O. In Chromenes, Chromanes, Chromones; Ellis, G. P., Ed.; Wiley-Interscience: New York, NY, 1977; pp 11–139.
Return to citation in text: [1] -
Engler, T. A.; LaTessa, K. O.; Iyengar, R.; Chai, W.; Agrios, K. Bioorg. Med. Chem. 1996, 4, 1755–1769. doi:10.1016/0968-0896(96)00192-7
Return to citation in text: [1] -
Broggini, G.; Folcio, F.; Sardone, N.; Sonzogni, M.; Zecchi, G. Tetrahedron: Asymmetry 1996, 7, 797–806. doi:10.1016/0957-4166(96)00076-6
Return to citation in text: [1] -
Sugimoto, H.; Nakamura, S.; Ohwada, T. Adv. Synth. Catal. 2007, 349, 669–679. doi:10.1002/adsc.200600508
Return to citation in text: [1] -
Fukamizu, K.; Miyake, Y.; Nishibayashi, Y. J. Am. Chem. Soc. 2008, 130, 10498–10499. doi:10.1021/ja8038745
Return to citation in text: [1] -
Xu, D.-Q.; Wang, Y.-F.; Luo, S.-P.; Zhang, S.; Zhong, A.-G.; Chen, H.; Xu, Z.-Y. Adv. Synth. Catal. 2008, 350, 2610–2616. doi:10.1002/adsc.200800535
Return to citation in text: [1] -
Ramachary, D. B.; Sakthidevi, R. Chem.–Eur. J. 2009, 15, 4516–4522. doi:10.1002/chem.200900066
Return to citation in text: [1] -
Xie, J.-W.; Huang, X.; Fan, L.-P.; Xu, D.-C.; Li, X.-S.; Su, H.; Wen, Y.-H. Adv. Synth. Catal. 2009, 351, 3077–3082. doi:10.1002/adsc.200900579
Return to citation in text: [1] -
Shen, H. C. Tetrahedron 2009, 65, 3931–3952. doi:10.1016/j.tet.2009.02.002
Return to citation in text: [1] -
Ferreira, S. B.; da Silva, F. C.; Pinto, A. C.; Gonzaga, D. T. G.; Ferreira, V. F. J. Heterocycl. Chem. 2009, 46, 1080–1097. doi:10.1002/jhet.232
Return to citation in text: [1] -
Lim, H. J.; RajanBabu, T. V. Org. Lett. 2009, 11, 2924–2927. doi:10.1021/ol900961m
Return to citation in text: [1] -
Pearson, E. L.; Kanizaj, N.; Willis, A. C.; Paddon-Row, M. N.; Sherburn, M. S. Chem.–Eur. J. 2010, 16, 8280–8284. doi:10.1002/chem.201001176
Return to citation in text: [1] -
Rueping, M.; Lin, M.-Y. Chem.–Eur. J. 2010, 16, 4169–4172. doi:10.1002/chem.201000203
Return to citation in text: [1] -
Zhang, X.; Zhang, S.; Wang, W. Angew. Chem., Int. Ed. 2010, 49, 1481–1484. doi:10.1002/anie.200906050
Return to citation in text: [1] -
Lu, L.-Q.; Chen, J.-R.; Xiao, W.-J. Acc. Chem. Res. 2012, 45, 1278–1293. doi:10.1021/ar200338s
Return to citation in text: [1] -
Jia, Y.; Yang, W.; Du, D.-M. Org. Biomol. Chem. 2012, 10, 4739–4746. doi:10.1039/c2ob25360g
Return to citation in text: [1] -
Poulsen, T. B.; Jørgensen, K. A. Chem. Rev. 2008, 108, 2903–2915. doi:10.1021/cr078372e
Return to citation in text: [1] -
Bandini, M.; Eichholzer, A. Angew. Chem., Int. Ed. 2009, 48, 9608–9644. doi:10.1002/anie.200901843
Return to citation in text: [1] -
You, S.-L.; Cai, Q.; Zeng, M. Chem. Soc. Rev. 2009, 38, 2190–2201. doi:10.1039/b817310a
Return to citation in text: [1] -
Herrera, R. P.; Sgarzani, V.; Bernardi, L.; Ricci, A. Angew. Chem., Int. Ed. 2005, 44, 6576–6579. doi:10.1002/anie.200500227
Return to citation in text: [1] -
Jia, Y.-X.; Zhu, S.-F.; Yang, Y.; Zhou, Q.-L. J. Org. Chem. 2006, 71, 75–80. doi:10.1021/jo0516537
Return to citation in text: [1] -
Lu, S.-F.; Du, D.-M.; Xu, J. Org. Lett. 2006, 8, 2115–2118. doi:10.1021/ol060586f
Return to citation in text: [1] -
Singh, P. K.; Bisai, A.; Singh, V. K. Tetrahedron Lett. 2007, 48, 1127–1129. doi:10.1016/j.tetlet.2006.12.081
Return to citation in text: [1] -
Liu, H.; Lu, S.-F.; Xu, J.; Du, D.-M. Chem.–Asian J. 2008, 3, 1111–1121. doi:10.1002/asia.200800071
Return to citation in text: [1] -
Ganesh, M.; Seidel, D. J. Am. Chem. Soc. 2008, 130, 16464–16465. doi:10.1021/ja8063292
Return to citation in text: [1] -
Itoh, J.; Fuchibe, K.; Akiyama, T. Angew. Chem., Int. Ed. 2008, 47, 4016–4018. doi:10.1002/anie.200800770
Return to citation in text: [1] -
McKeon, S. C.; Müller-Bunz, H.; Guiry, P. J. Eur. J. Org. Chem. 2009, 4833–4841. doi:10.1002/ejoc.200900683
Return to citation in text: [1] -
Yokoyama, N.; Arai, T. Chem. Commun. 2009, 3285–3287. doi:10.1039/b904275j
Return to citation in text: [1] -
Liu, H.; Du, D.-M. Adv. Synth. Catal. 2010, 352, 1113–1118. doi:10.1002/adsc.201000111
Return to citation in text: [1] -
Liu, H.; Du, D.-M. Eur. J. Org. Chem. 2010, 2121–2131. doi:10.1002/ejoc.200901434
Return to citation in text: [1] -
Guo, F.; Lai, G.; Xiong, S.; Wang, S.; Wang, Z. Chem.–Eur. J. 2010, 16, 6438–6441. doi:10.1002/chem.201000540
Return to citation in text: [1] -
Peng, J.; Du, D.-M. Eur. J. Org. Chem. 2012, 4042–4051. doi:10.1002/ejoc.201200382
Return to citation in text: [1] -
Li, C.; Liu, F.-L.; Zou, Y.-Q.; Lu, L.-Q.; Chen, J.-R.; Xiao, W.-J. Synthesis 2013, 45, 601–608. doi:10.1055/s-0032-1318200
Return to citation in text: [1] -
CCDC-936409 contains the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic DataCentre via http://www.ccdc.cam.ac.uk/ data_request/cif.
Return to citation in text: [1]
| 1. | Posner, G. H. Chem. Rev. 1986, 86, 831–844. doi:10.1021/cr00075a007 |
| 2. | Tietze, L. F.; Beifuss, U. Angew. Chem., Int. Ed. Engl. 1993, 32, 131–163. doi:10.1002/anie.199301313 |
| 3. | Tietze, L. F. Chem. Rev. 1996, 96, 115–136. doi:10.1021/cr950027e |
| 4. | Wasilke, J.-C.; Obrey, S. J.; Baker, R. T.; Bazan, G. C. Chem. Rev. 2005, 105, 1001–1020. doi:10.1021/cr020018n |
| 5. | Ramón, D. J.; Yus, M. Angew. Chem., Int. Ed. 2005, 44, 1602–1634. doi:10.1002/anie.200460548 |
| 6. | Pellissier, H. Tetrahedron 2006, 62, 1619–1665. doi:10.1016/j.tet.2005.10.040 |
| 7. | Pellissier, H. Tetrahedron 2006, 62, 2143–2173. doi:10.1016/j.tet.2005.10.041 |
| 8. | Guo, H.-C.; Ma, J.-A. Angew. Chem., Int. Ed. 2006, 45, 354–366. doi:10.1002/anie.200500195 |
| 9. | Enders, D.; Grondal, C.; Hüttl, M. R. M. Angew. Chem., Int. Ed. 2007, 46, 1570–1581. doi:10.1002/anie.200603129 |
| 10. | Grondal, C.; Jeanty, M.; Enders, D. Nat. Chem. 2010, 2, 167–178. doi:10.1038/nchem.539 |
| 11. | Moyano, A.; Rios, R. Chem. Rev. 2011, 111, 4703–4832. doi:10.1021/cr100348t |
| 12. | Pellissier, H. Adv. Synth. Catal. 2012, 354, 237–294. doi:10.1002/adsc.201100714 |
| 13. | Schreiber, S. L. Science 2000, 287, 1964–1969. doi:10.1126/science.287.5460.1964 |
| 33. | Jia, Y.; Yang, W.; Du, D.-M. Org. Biomol. Chem. 2012, 10, 4739–4746. doi:10.1039/c2ob25360g |
| 21. | Sugimoto, H.; Nakamura, S.; Ohwada, T. Adv. Synth. Catal. 2007, 349, 669–679. doi:10.1002/adsc.200600508 |
| 22. | Fukamizu, K.; Miyake, Y.; Nishibayashi, Y. J. Am. Chem. Soc. 2008, 130, 10498–10499. doi:10.1021/ja8038745 |
| 23. | Xu, D.-Q.; Wang, Y.-F.; Luo, S.-P.; Zhang, S.; Zhong, A.-G.; Chen, H.; Xu, Z.-Y. Adv. Synth. Catal. 2008, 350, 2610–2616. doi:10.1002/adsc.200800535 |
| 24. | Ramachary, D. B.; Sakthidevi, R. Chem.–Eur. J. 2009, 15, 4516–4522. doi:10.1002/chem.200900066 |
| 25. | Xie, J.-W.; Huang, X.; Fan, L.-P.; Xu, D.-C.; Li, X.-S.; Su, H.; Wen, Y.-H. Adv. Synth. Catal. 2009, 351, 3077–3082. doi:10.1002/adsc.200900579 |
| 26. | Shen, H. C. Tetrahedron 2009, 65, 3931–3952. doi:10.1016/j.tet.2009.02.002 |
| 27. | Ferreira, S. B.; da Silva, F. C.; Pinto, A. C.; Gonzaga, D. T. G.; Ferreira, V. F. J. Heterocycl. Chem. 2009, 46, 1080–1097. doi:10.1002/jhet.232 |
| 28. | Lim, H. J.; RajanBabu, T. V. Org. Lett. 2009, 11, 2924–2927. doi:10.1021/ol900961m |
| 29. | Pearson, E. L.; Kanizaj, N.; Willis, A. C.; Paddon-Row, M. N.; Sherburn, M. S. Chem.–Eur. J. 2010, 16, 8280–8284. doi:10.1002/chem.201001176 |
| 30. | Rueping, M.; Lin, M.-Y. Chem.–Eur. J. 2010, 16, 4169–4172. doi:10.1002/chem.201000203 |
| 31. | Zhang, X.; Zhang, S.; Wang, W. Angew. Chem., Int. Ed. 2010, 49, 1481–1484. doi:10.1002/anie.200906050 |
| 32. | Lu, L.-Q.; Chen, J.-R.; Xiao, W.-J. Acc. Chem. Res. 2012, 45, 1278–1293. doi:10.1021/ar200338s |
| 17. | Conti, C.; Monaco, L. P.; Desideri, N. Bioorg. Med. Chem. 2011, 19, 7357–7364. doi:10.1016/j.bmc.2011.10.060 |
| 18. | Schweizer, E. E.; Meeder-Nycz, O. In Chromenes, Chromanes, Chromones; Ellis, G. P., Ed.; Wiley-Interscience: New York, NY, 1977; pp 11–139. |
| 19. | Engler, T. A.; LaTessa, K. O.; Iyengar, R.; Chai, W.; Agrios, K. Bioorg. Med. Chem. 1996, 4, 1755–1769. doi:10.1016/0968-0896(96)00192-7 |
| 20. | Broggini, G.; Folcio, F.; Sardone, N.; Sonzogni, M.; Zecchi, G. Tetrahedron: Asymmetry 1996, 7, 797–806. doi:10.1016/0957-4166(96)00076-6 |
| 14. | Nicolaou, K. C.; Edmonds, D. J.; Bulger, P. G. Angew. Chem., Int. Ed. 2006, 45, 7134–7186. doi:10.1002/anie.200601872 |
| 15. | de Figueiredo, R. M.; Christmann, M. Eur. J. Org. Chem. 2007, 2575–2600. doi:10.1002/ejoc.200700032 |
| 16. | Rueping, M.; Dufour, J.; Schoepke, F. R. Green Chem. 2011, 13, 1084–1105. doi:10.1039/c1gc15027h |
| 51. | CCDC-936409 contains the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic DataCentre via http://www.ccdc.cam.ac.uk/ data_request/cif. |
| 50. | Li, C.; Liu, F.-L.; Zou, Y.-Q.; Lu, L.-Q.; Chen, J.-R.; Xiao, W.-J. Synthesis 2013, 45, 601–608. doi:10.1055/s-0032-1318200 |
| 34. | Poulsen, T. B.; Jørgensen, K. A. Chem. Rev. 2008, 108, 2903–2915. doi:10.1021/cr078372e |
| 35. | Bandini, M.; Eichholzer, A. Angew. Chem., Int. Ed. 2009, 48, 9608–9644. doi:10.1002/anie.200901843 |
| 36. | You, S.-L.; Cai, Q.; Zeng, M. Chem. Soc. Rev. 2009, 38, 2190–2201. doi:10.1039/b817310a |
| 37. | Herrera, R. P.; Sgarzani, V.; Bernardi, L.; Ricci, A. Angew. Chem., Int. Ed. 2005, 44, 6576–6579. doi:10.1002/anie.200500227 |
| 38. | Jia, Y.-X.; Zhu, S.-F.; Yang, Y.; Zhou, Q.-L. J. Org. Chem. 2006, 71, 75–80. doi:10.1021/jo0516537 |
| 39. | Lu, S.-F.; Du, D.-M.; Xu, J. Org. Lett. 2006, 8, 2115–2118. doi:10.1021/ol060586f |
| 40. | Singh, P. K.; Bisai, A.; Singh, V. K. Tetrahedron Lett. 2007, 48, 1127–1129. doi:10.1016/j.tetlet.2006.12.081 |
| 41. | Liu, H.; Lu, S.-F.; Xu, J.; Du, D.-M. Chem.–Asian J. 2008, 3, 1111–1121. doi:10.1002/asia.200800071 |
| 42. | Ganesh, M.; Seidel, D. J. Am. Chem. Soc. 2008, 130, 16464–16465. doi:10.1021/ja8063292 |
| 43. | Itoh, J.; Fuchibe, K.; Akiyama, T. Angew. Chem., Int. Ed. 2008, 47, 4016–4018. doi:10.1002/anie.200800770 |
| 44. | McKeon, S. C.; Müller-Bunz, H.; Guiry, P. J. Eur. J. Org. Chem. 2009, 4833–4841. doi:10.1002/ejoc.200900683 |
| 45. | Yokoyama, N.; Arai, T. Chem. Commun. 2009, 3285–3287. doi:10.1039/b904275j |
| 46. | Liu, H.; Du, D.-M. Adv. Synth. Catal. 2010, 352, 1113–1118. doi:10.1002/adsc.201000111 |
| 47. | Liu, H.; Du, D.-M. Eur. J. Org. Chem. 2010, 2121–2131. doi:10.1002/ejoc.200901434 |
| 48. | Guo, F.; Lai, G.; Xiong, S.; Wang, S.; Wang, Z. Chem.–Eur. J. 2010, 16, 6438–6441. doi:10.1002/chem.201000540 |
| 49. | Peng, J.; Du, D.-M. Eur. J. Org. Chem. 2012, 4042–4051. doi:10.1002/ejoc.201200382 |
© 2013 Peng and Du; licensee Beilstein-Institut.
This is an Open Access article under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
The license is subject to the Beilstein Journal of Organic Chemistry terms and conditions: (http://www.beilstein-journals.org/bjoc)