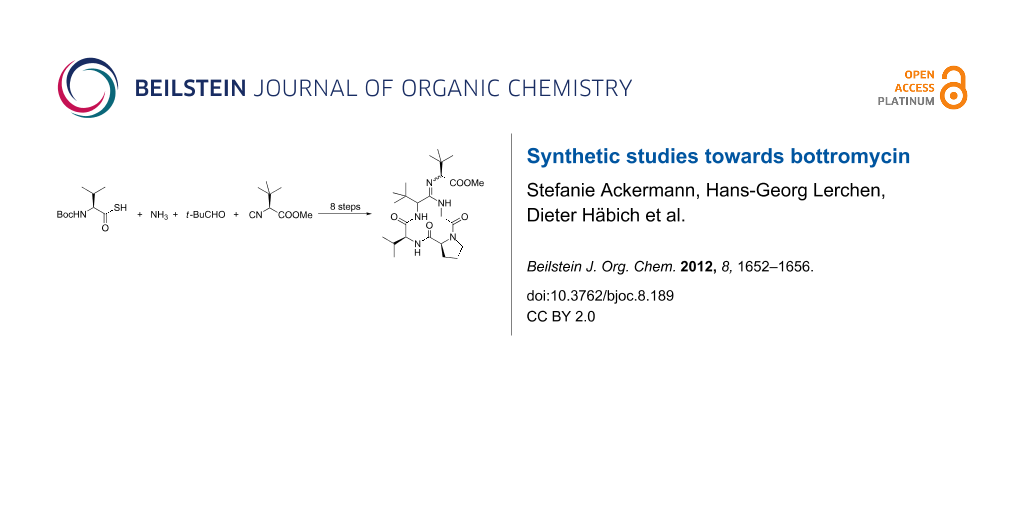
Abstract
Thio-Ugi reactions are described as an excellent synthetic tool for the synthesis of sterically highly hindered endothiopeptides. S-Methylation and subsequent amidine formation can be carried out in an inter- as well as in an intramolecular fashion. The intramolecular approach allows the synthesis of the bottromycin ring system in a straightforward manner.
Graphical Abstract
Introduction
Natural products are excellent sources as lead structures for the development of new antibiotics. Over millions of years microorganisms, such as bacteria and fungi, developed efficient defense strategies against their bacterial competitors [1-3]. Not surprising, a wide range of common antibiotics such as penicillin or vancomycin are natural products or derivatives thereof.
In 1957 Waiswisz et al. reported a new antibiotic peptide isolated from the fermentation broth of Streptomyces bottropensis, called bottromycin [4-6]. This antibiotic inhibits the growth of a wide range of microorganisms by interfering with their protein biosynthesis [7-12]. In 1965 Nakamura et al. isolated closely related antibiotics from the strain Streptomyces No. 3668-L2, named bottromycin A and B [13,14]. Acidic hydrolysis provided a mixture of all-(S)-configured amino acids containing 3-methylphenylalanine [15], tert-leucine, valine, β-(2-thiazolyl)-β-alanine [16] and glycine. While also proline was found in bottromycin B, cis-3-methylproline is a component in bottromycin A [17]. Originally Nakamura postulated a linear N-acylated iminohexapeptide structure, a proposal which was revised after synthetic studies [18] as well as NMR spectroscopic investigations by Takita et al., which proposed a cyclic iminopeptide structure [19]. This proposal was verified by Schipper [20] and Kaneda [21], based on detailed NMR studies. According to them, the bottromycins are cyclic tetrapeptides, connected to a tripeptidic side chain through an amidine structure. The different bottromycins differ only in the substitution pattern of the proline (Figure 1). The three-dimensional structure was reported recently by Gouda et al. [22]. This structure is quite unusual, not only because of the amidine moiety but also because most amino acids are found in β-methylated form (tert-leucine can be seen as β-methylvaline).
Although a series of synthetic studies towards linear bottromycin sequences have been published [23,24], no total synthesis was reported for a long time. The first and only synthesis so far was described by Sunazuka and Ōmura et al. in 2009 [25]. Their synthesis was based on the formation of the amidine structure by reaction of the tripeptide side chain with an endothiopeptide and ring closure between proline and glycine. The same group also undertook some modifications on the natural product [26].
Results and Discussion
Our group is also involved in the synthesis of peptide-based natural products [27-32], and of course the structure of bottromycin is highly fascinating from a synthetic point of view. In our previous investigations we observed that for the synthesis of sterically demanding peptides, especially those containing N-alkylated amide bonds, Ugi reactions are especially suited [33,34]. For N-unsubstituted peptides the Ugi reactions should be carried out with ammonia as the amine component, which is a protocol that is often accompanied by a range of side reactions. But in general the yields are good if sterically demanding aldehydes, such as pivaldehyde are used [35,36]. With thiocarboxylic acids as acid components, this approach allows also the synthesis of endothiopeptides [37-40], and therefore this protocol should be extremely suitable for the synthesis of bottromycins.
To prove this option, we reacted Boc-protected (S)-thiovaline [41] with pivaldehyde, ethyl isocyanoacetate, and a 2 M NH3 solution in CH3OH. In trifluoroethanol, which is the best solvent for ammonia Ugi reactions [35], the expected endothiopeptide 1 was formed in high yield as a 1:1 diastereomeric mixture (Scheme 1). With this building block in hand, we were interested to see whether we would be able to generate the required cyclic endothiopeptide and if we could even subsequently connect the side chain.
Scheme 1: Syntheses of endothiopeptides by thio-Ugi reaction.
Scheme 1: Syntheses of endothiopeptides by thio-Ugi reaction.
Therefore, we prolonged the peptide chain 2 under standard peptide coupling conditions and tried to activate the linear peptide chain. We chose the pentafluorophenylester protocol developed by Schmidt et al. for cyclization [42]. But unfortunately this approach failed (as did all other activations investigated) because of the formation of thiazolone side product, which could not be cyclized.
In parallel, we tried to figure out if the amidine formation is possible with sterically hindered endothiopeptides (Scheme 2). As a model substrate we chose thiopeptide 3 [37], easily obtained by thio-Ugi reaction in excellent yield. The reaction was very fast and was finished already after 15 min, and peptide 3 crystallized directly from the reaction mixture. Because our first attempts to couple 3 directly with amines to the corresponding amidine 5 failed [43], we decided to convert 3 into the corresponding thioimidate 4, which was reacted with (S)-valine methyl ester as an amine component in the presence of Hg salts [44]. Hg(OCOCF3)2 was found to be more suitable than Hg(OCOCH3)2 (Table 1, entries 1 and 2) and from the different solvents tested, THF was the solvent of choice. With a twofold excess of the amine component a yield of up to 72% of 5 could be obtained as a 1:1 diastereomeric mixture. The diastereomers could be separated by flash chromatography, but unfortunately these reaction conditions could not be transferred to the thioimidoester obtained from endothiopeptide 2. Here, the expected amidine was only formed in a trace amount, and a range of side products was obtained.
Scheme 2: Synthesis of amidine 5 by thio-Ugi reaction.
Scheme 2: Synthesis of amidine 5 by thio-Ugi reaction.
Therefore, we decided to change our strategy and to replace the intermolecular amidine formation by an intramolecular one. In this case the peptide ring should be formed in the amidination step. For this approach we first needed the isocyanide 7 derived from (S)-tert-leucine methyl ester. According to Ugi et al. this isocyanide was obtained in enantiomerically pure form from the corresponding formamide 6 by dehydration with POCl3/NEt3 (Scheme 3).
Scheme 3: Synthesis of the bottromycin ring system 12 by thio-Ugi reaction.
Scheme 3: Synthesis of the bottromycin ring system 12 by thio-Ugi reaction.
This isocyanide was subjected to a thio-Ugi reaction as described before, and the expected sterically highly demanding endothiopeptide 8 was obtained in high yield as a 1:1 diastereomeric mixture. In this case, the diastereomers could not be separated. Elongation of the peptide chain under standard peptide coupling conditions provided the linear precursor 10 for the ring-closing amidination. After removal of the Boc-protecting group the resulting salt showed a low solubility in THF, and therefore we had to run the amidination in CH3CN, although this was not the solvent of choice in our model system.
The amidination was carried out under high-dilution conditions. The peptide salt was dissolved in CH3CN and was added slowly through a syringe to a solution of Hg(OCOCF3)2 in CH3CN at 50 °C over a period of 10 h. Interestingly, the best result was obtained not with the free peptide amine but with the hydrochloride salt. Here the cyclic amidine was obtained in 51% yield.
Conclusion
In conclusion, we could show that thio-Ugi reactions are an excellent synthetic tool for the synthesis of highly sterically hindered endothiopeptides. S-Methylation and subsequent amidine formations can be carried out in an inter- as well as in an intramolecular fashion. The intramolecular approach allows the synthesis of the bottromycin ring system in a straightforward manner. The synthesis of the bottromycins and derivatives thereof is currently under investigation.
Supporting Information
| Supporting Information File 1: Detailed experimental procedures, NMR and analytical data of all compounds. | ||
| Format: PDF | Size: 322.9 KB | Download |
References
-
Gräfe, U. Biochemie der Antibiotika; Spektrum Akademischer Verlag: Heidelberg, Germany, 1992.
Return to citation in text: [1] -
Eberle, A. E. Chimia 1991, 45, 145–153.
Return to citation in text: [1] -
von Nussbaum, F.; Brands, M.; Hinzen, B.; Weigand, S.; Häbich, D. Angew. Chem. 2006, 118, 5194–5254. doi:10.1002/ange.200600350
Angew. Chem., Int. Ed. 2006, 45, 5072–5129. doi:10.1002/anie.200600350
Return to citation in text: [1] -
Waisvisz, J. M.; van der Hoeven, M. G.; van Peppen, J.; Zwennis, W. C. M. J. Am. Chem. Soc. 1957, 79, 4520–4521. doi:10.1021/ja01573a072
Return to citation in text: [1] -
Waisvisz, J. M.; van der Hoeven, M. G.; Hölscher, J. F.; te Nijenhuis, B. J. Am. Chem. Soc. 1957, 79, 4522–4524. doi:10.1021/ja01573a073
Return to citation in text: [1] -
Waisvisz, J. M.; van der Hoeven, M. G.; Te Nijenhuis, B. J. Am. Chem. Soc. 1957, 79, 4524–4527. doi:10.1021/ja01573a074
Return to citation in text: [1] -
Nakamura, S.; Chikaike, T.; Karasawa, K.; Tanaka, N.; Yonehara, H.; Umezawa, H. J. Antibiot. 1965, 18, 47–52.
Return to citation in text: [1] -
Tanaka, N.; Sashika, K.; Yamaguchi, H.; Umezawa, H. J. Biochem. 1966, 60, 405–410.
Return to citation in text: [1] -
Nakamura, S.; Yajima, T.; Lin, Y.-C.; Umezawa, H. J. Antibiot. 1967, 20, 1–5.
Return to citation in text: [1] -
Tanaka, N.; Lin, Y.-C. J. Biochem. 1968, 63, 1–7.
Return to citation in text: [1] -
Otaka, T.; Kaji, A. J. Biol. Chem. 1976, 251, 2299–2306.
Return to citation in text: [1] -
Otaka, T.; Kaji, A. FEBS Lett. 1983, 153, 53–59. doi:10.1016/0014-5793(83)80118-5
Return to citation in text: [1] -
Nakamura, S.; Chikaike, T.; Karasawa, K.; Tanaka, N.; Yonehara, H.; Umezawa, H. J. Antibiot. 1965, 9, 1860–1861.
Return to citation in text: [1] -
Nakamura, S.; Umezawa, H. Chem. Pharm. Bull. 1966, 14, 981–986. doi:10.1248/cpb.14.981
Return to citation in text: [1] -
Arold, H.; Eule, M.; Reissmann, S. Z. Chem. 1969, 9, 447–448.
Return to citation in text: [1] -
Seto, Y.; Torii, K.; Bori, K.; Inabata, K.; Kuwata, S.; Watanabe, H. Bull. Chem. Soc. Jpn. 1974, 47, 151–155. doi:10.1246/bcsj.47.151
Return to citation in text: [1] -
Nakamura, S.; Chikaike, T.; Yonehara, H.; Umezawa, H. Chem. Pharm. Bull. 1965, 13, 599–602. doi:10.1248/cpb.13.599
Return to citation in text: [1] -
Yamada, Y.; Takashima, K.; Miyazawa, T.; Kuwata, S.; Watanabe, H. Bull. Chem. Soc. Jpn. 1978, 51, 878–883. doi:10.1246/bcsj.51.878
Return to citation in text: [1] -
Takahashi, Y.; Naganawa, H.; Takita, T.; Umezawa, H.; Nakamura, S. J. Antibiot. 1976, 29, 1120–1123. doi:10.7164/antibiotics.29.1120
Return to citation in text: [1] -
Schipper, D. J. Antibiot. 1983, 36, 1076–1077. doi:10.7164/antibiotics.36.1076
Return to citation in text: [1] -
Kaneda, M. J. Antibiot. 1992, 45, 792–796. doi:10.7164/antibiotics.45.792
Return to citation in text: [1] -
Gouda, H.; Kobayashi, Y.; Yamada, T.; Ideguchi, T.; Sugawara, A.; Hirose, T.; Ōmura, S.; Sunazuka, T.; Hirono, S. Chem. Pharm. Bull. 2012, 60, 169–171. doi:10.1248/cpb.60.169
Return to citation in text: [1] -
Kataoka, Y.; Seto, Y.; Yamamoto, M.; Yamada, T.; Kuwata, S.; Watanabe, H. Bull. Chem. Soc. Jpn. 1976, 49, 1081–1084. doi:10.1246/bcsj.49.1081
Return to citation in text: [1] -
Yamada, T.; Miyazawa, T.; Kuwata, S.; Watanabe, H. Bull. Chem. Soc. Jpn. 1977, 50, 1827–1830. doi:10.1246/bcsj.50.1827
Return to citation in text: [1] -
Shimamura, H.; Gouda, H.; Nagai, K.; Hirose, T.; Ichioka, M.; Furuya, Y.; Kobayashi, Y.; Hirono, S.; Sunazuka, T.; Ōmura, S. Angew. Chem., Int. Ed. 2009, 48, 914–917. doi:10.1002/anie.200804138
Return to citation in text: [1] -
Kobayashi, Y.; Ichioka, M.; Hirose, T.; Nagai, K.; Matsumoto, A.; Matsui, H.; Hanaki, H.; Masuma, R.; Takahashi, Y.; Ōmura, S.; Sunazuka, T. Bioorg. Med. Chem. Lett. 2010, 20, 6116–6120. doi:10.1016/j.bmcl.2010.08.037
Return to citation in text: [1] -
Quirin, C.; Kazmaier, U. Eur. J. Org. Chem. 2009, 371–377. doi:10.1002/ejoc.200800890
Return to citation in text: [1] -
Ullrich, A.; Chai, Y.; Pistorius, D.; Elnakady, Y. A.; Herrmann, J. E.; Weissman, K. J.; Kazmaier, U.; Müller, R. Angew. Chem. 2009, 121, 4486–4489. doi:10.1002/ange.200900406
Angew. Chem., Int. Ed. 2009, 48, 4422–4425. doi:10.1002/anie.200900406
Return to citation in text: [1] -
Ullrich, A.; Herrmann, J.; Müller, R.; Kazmaier, U. Eur. J. Org. Chem. 2009, 6367–6378. doi:10.1002/ejoc.200900999
Return to citation in text: [1] -
Gawas, D.; Kazmaier, U. Org. Biomol. Chem. 2010, 8, 457–462. doi:10.1039/b917589j
Return to citation in text: [1] -
Burkhart, J. L.; Müller, R.; Kazmaier, U. Eur. J. Org. Chem. 2011, 3050–3059. doi:10.1002/ejoc.201100155
Return to citation in text: [1] -
Deska, J.; Hähn, S.; Kazmaier, U. Org. Lett. 2011, 13, 3210–3213. doi:10.1021/ol201120k
Return to citation in text: [1] -
Hebach, C.; Kazmaier, U. Chem. Commun. 2003, 596–597. doi:10.1039/b210952b
Return to citation in text: [1] -
Kazmaier, U.; Hebach, C.; Watzke, A.; Maier, S.; Mues, H.; Huch, V. Org. Biomol. Chem. 2005, 3, 136–145. doi:10.1039/b411228h
Return to citation in text: [1] -
Kazmaier, U.; Hebach, C. Synlett 2003, 1591–1594. doi:10.1055/s-2003-40987
Return to citation in text: [1] [2] -
Pick, R.; Bauer, M.; Kazmaier, U.; Hebach, C. Synlett 2005, 757–760. doi:10.1055/s-2005-863722
Return to citation in text: [1] -
Kazmaier, U.; Ackermann, S. Org. Biomol. Chem. 2005, 3, 3184–3187. doi:10.1039/b507028g
Return to citation in text: [1] [2] -
Bauer, M.; Maurer, F.; Hoffmann, S. M.; Kazmaier, U. Synlett 2008, 3203–3207. doi:10.1055/s-0028-1087366
Return to citation in text: [1] -
Kazmaier, U.; Persch, A. Org. Biomol. Chem. 2010, 8, 5442–5447. doi:10.1039/c0ob00453g
Return to citation in text: [1] -
Burkhart, J. L.; Kazmaier, U. Synthesis 2011, 4033–4036. doi:10.1055/s-0031-1289594
Return to citation in text: [1] -
Lehmann, J.; Linden, A.; Heimgartner, H. Tetrahedron 1998, 54, 8721–8736. doi:10.1016/S0040-4020(98)00506-7
Return to citation in text: [1] -
Schmidt, U.; Lieberknecht, A.; Griesser, H.; Talbiersky, J. J. Org. Chem. 1982, 47, 3261–3264. doi:10.1021/jo00138a012
Return to citation in text: [1] -
Micheel, F.; Flitsch, W. Justus Liebigs Ann. Chem. 1952, 577, 234–237. doi:10.1002/jlac.19525770306
Return to citation in text: [1] -
Bredereck, H.; Gompper, R.; Seiz, H. Chem. Ber. 1957, 90, 1837–1843. doi:10.1002/cber.19570900922
Return to citation in text: [1]
| 1. | Gräfe, U. Biochemie der Antibiotika; Spektrum Akademischer Verlag: Heidelberg, Germany, 1992. |
| 2. | Eberle, A. E. Chimia 1991, 45, 145–153. |
| 3. |
von Nussbaum, F.; Brands, M.; Hinzen, B.; Weigand, S.; Häbich, D. Angew. Chem. 2006, 118, 5194–5254. doi:10.1002/ange.200600350
Angew. Chem., Int. Ed. 2006, 45, 5072–5129. doi:10.1002/anie.200600350 |
| 26. | Kobayashi, Y.; Ichioka, M.; Hirose, T.; Nagai, K.; Matsumoto, A.; Matsui, H.; Hanaki, H.; Masuma, R.; Takahashi, Y.; Ōmura, S.; Sunazuka, T. Bioorg. Med. Chem. Lett. 2010, 20, 6116–6120. doi:10.1016/j.bmcl.2010.08.037 |
| 13. | Nakamura, S.; Chikaike, T.; Karasawa, K.; Tanaka, N.; Yonehara, H.; Umezawa, H. J. Antibiot. 1965, 9, 1860–1861. |
| 14. | Nakamura, S.; Umezawa, H. Chem. Pharm. Bull. 1966, 14, 981–986. doi:10.1248/cpb.14.981 |
| 27. | Quirin, C.; Kazmaier, U. Eur. J. Org. Chem. 2009, 371–377. doi:10.1002/ejoc.200800890 |
| 28. |
Ullrich, A.; Chai, Y.; Pistorius, D.; Elnakady, Y. A.; Herrmann, J. E.; Weissman, K. J.; Kazmaier, U.; Müller, R. Angew. Chem. 2009, 121, 4486–4489. doi:10.1002/ange.200900406
Angew. Chem., Int. Ed. 2009, 48, 4422–4425. doi:10.1002/anie.200900406 |
| 29. | Ullrich, A.; Herrmann, J.; Müller, R.; Kazmaier, U. Eur. J. Org. Chem. 2009, 6367–6378. doi:10.1002/ejoc.200900999 |
| 30. | Gawas, D.; Kazmaier, U. Org. Biomol. Chem. 2010, 8, 457–462. doi:10.1039/b917589j |
| 31. | Burkhart, J. L.; Müller, R.; Kazmaier, U. Eur. J. Org. Chem. 2011, 3050–3059. doi:10.1002/ejoc.201100155 |
| 32. | Deska, J.; Hähn, S.; Kazmaier, U. Org. Lett. 2011, 13, 3210–3213. doi:10.1021/ol201120k |
| 7. | Nakamura, S.; Chikaike, T.; Karasawa, K.; Tanaka, N.; Yonehara, H.; Umezawa, H. J. Antibiot. 1965, 18, 47–52. |
| 8. | Tanaka, N.; Sashika, K.; Yamaguchi, H.; Umezawa, H. J. Biochem. 1966, 60, 405–410. |
| 9. | Nakamura, S.; Yajima, T.; Lin, Y.-C.; Umezawa, H. J. Antibiot. 1967, 20, 1–5. |
| 10. | Tanaka, N.; Lin, Y.-C. J. Biochem. 1968, 63, 1–7. |
| 11. | Otaka, T.; Kaji, A. J. Biol. Chem. 1976, 251, 2299–2306. |
| 12. | Otaka, T.; Kaji, A. FEBS Lett. 1983, 153, 53–59. doi:10.1016/0014-5793(83)80118-5 |
| 23. | Kataoka, Y.; Seto, Y.; Yamamoto, M.; Yamada, T.; Kuwata, S.; Watanabe, H. Bull. Chem. Soc. Jpn. 1976, 49, 1081–1084. doi:10.1246/bcsj.49.1081 |
| 24. | Yamada, T.; Miyazawa, T.; Kuwata, S.; Watanabe, H. Bull. Chem. Soc. Jpn. 1977, 50, 1827–1830. doi:10.1246/bcsj.50.1827 |
| 4. | Waisvisz, J. M.; van der Hoeven, M. G.; van Peppen, J.; Zwennis, W. C. M. J. Am. Chem. Soc. 1957, 79, 4520–4521. doi:10.1021/ja01573a072 |
| 5. | Waisvisz, J. M.; van der Hoeven, M. G.; Hölscher, J. F.; te Nijenhuis, B. J. Am. Chem. Soc. 1957, 79, 4522–4524. doi:10.1021/ja01573a073 |
| 6. | Waisvisz, J. M.; van der Hoeven, M. G.; Te Nijenhuis, B. J. Am. Chem. Soc. 1957, 79, 4524–4527. doi:10.1021/ja01573a074 |
| 25. | Shimamura, H.; Gouda, H.; Nagai, K.; Hirose, T.; Ichioka, M.; Furuya, Y.; Kobayashi, Y.; Hirono, S.; Sunazuka, T.; Ōmura, S. Angew. Chem., Int. Ed. 2009, 48, 914–917. doi:10.1002/anie.200804138 |
| 19. | Takahashi, Y.; Naganawa, H.; Takita, T.; Umezawa, H.; Nakamura, S. J. Antibiot. 1976, 29, 1120–1123. doi:10.7164/antibiotics.29.1120 |
| 18. | Yamada, Y.; Takashima, K.; Miyazawa, T.; Kuwata, S.; Watanabe, H. Bull. Chem. Soc. Jpn. 1978, 51, 878–883. doi:10.1246/bcsj.51.878 |
| 22. | Gouda, H.; Kobayashi, Y.; Yamada, T.; Ideguchi, T.; Sugawara, A.; Hirose, T.; Ōmura, S.; Sunazuka, T.; Hirono, S. Chem. Pharm. Bull. 2012, 60, 169–171. doi:10.1248/cpb.60.169 |
| 17. | Nakamura, S.; Chikaike, T.; Yonehara, H.; Umezawa, H. Chem. Pharm. Bull. 1965, 13, 599–602. doi:10.1248/cpb.13.599 |
| 16. | Seto, Y.; Torii, K.; Bori, K.; Inabata, K.; Kuwata, S.; Watanabe, H. Bull. Chem. Soc. Jpn. 1974, 47, 151–155. doi:10.1246/bcsj.47.151 |
| 20. | Schipper, D. J. Antibiot. 1983, 36, 1076–1077. doi:10.7164/antibiotics.36.1076 |
| 37. | Kazmaier, U.; Ackermann, S. Org. Biomol. Chem. 2005, 3, 3184–3187. doi:10.1039/b507028g |
| 38. | Bauer, M.; Maurer, F.; Hoffmann, S. M.; Kazmaier, U. Synlett 2008, 3203–3207. doi:10.1055/s-0028-1087366 |
| 39. | Kazmaier, U.; Persch, A. Org. Biomol. Chem. 2010, 8, 5442–5447. doi:10.1039/c0ob00453g |
| 40. | Burkhart, J. L.; Kazmaier, U. Synthesis 2011, 4033–4036. doi:10.1055/s-0031-1289594 |
| 33. | Hebach, C.; Kazmaier, U. Chem. Commun. 2003, 596–597. doi:10.1039/b210952b |
| 34. | Kazmaier, U.; Hebach, C.; Watzke, A.; Maier, S.; Mues, H.; Huch, V. Org. Biomol. Chem. 2005, 3, 136–145. doi:10.1039/b411228h |
| 35. | Kazmaier, U.; Hebach, C. Synlett 2003, 1591–1594. doi:10.1055/s-2003-40987 |
| 36. | Pick, R.; Bauer, M.; Kazmaier, U.; Hebach, C. Synlett 2005, 757–760. doi:10.1055/s-2005-863722 |
| 43. | Micheel, F.; Flitsch, W. Justus Liebigs Ann. Chem. 1952, 577, 234–237. doi:10.1002/jlac.19525770306 |
| 44. | Bredereck, H.; Gompper, R.; Seiz, H. Chem. Ber. 1957, 90, 1837–1843. doi:10.1002/cber.19570900922 |
| 42. | Schmidt, U.; Lieberknecht, A.; Griesser, H.; Talbiersky, J. J. Org. Chem. 1982, 47, 3261–3264. doi:10.1021/jo00138a012 |
| 37. | Kazmaier, U.; Ackermann, S. Org. Biomol. Chem. 2005, 3, 3184–3187. doi:10.1039/b507028g |
| 41. | Lehmann, J.; Linden, A.; Heimgartner, H. Tetrahedron 1998, 54, 8721–8736. doi:10.1016/S0040-4020(98)00506-7 |
© 2012 Ackermann et al; licensee Beilstein-Institut.
This is an Open Access article under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
The license is subject to the Beilstein Journal of Organic Chemistry terms and conditions: (http://www.beilstein-journals.org/bjoc)