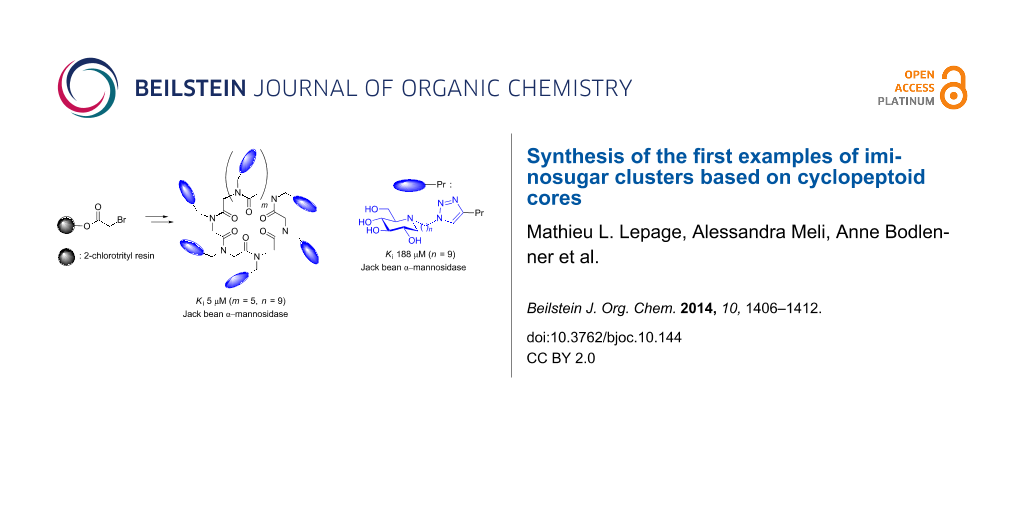
Abstract
Cyclic N-propargyl α-peptoids of various sizes were prepared by way of macrocyclizations of linear N-substituted oligoglycines. These compounds were used as molecular platforms to synthesize a series of iminosugar clusters with different valency and alkyl spacer lengths by means of Cu(I)-catalysed azide–alkyne cycloadditions. Evaluation of these compounds as α-mannosidase inhibitors led to significant multivalent effects and further demonstrated the decisive influence of scaffold rigidity on binding affinity enhancements.
Graphical Abstract
Introduction
Within a few years, the field of multivalent glycosidase inhibitors has witnessed tremendous advancement. Since the report in 2009 of the first quantifiable multivalent effect in glycosidase inhibition [1,2], the pace of progress has been breath-taking with the discovery of iminosugar clusters showing outstanding affinity enhancements of up to four orders of magnitude over the parent monovalent analogues [3-7]. The best results were obtained with multivalent systems based on C60 [3], β-cyclodextrin [4,5] and porphyrin [7] cores, and with nanoparticles prepared by self-assembly of iminosugar-based glycopolypetides [6]. So far, the largest multivalent effect (up to 610-fold relative inhibition potency increase on a valency-corrected basis) has been achieved on jack bean α-mannosidase with β-cyclodextrin-based analogues displaying 14 copies of 1-deoxynojirimycin (DNJ) [4]. Applications of the inhibitory multivalent effect to glycosidases of therapeutic interest were promptly performed and promising results were obtained in the field of Gaucher disease, the most common lysosomal storage disorder [8,9]. In 2013, the first description of a multivalent effect for correcting protein folding defects in cells was reported with trivalent DNJ clusters [10]. These compounds were found to overcome the processing defect of the mutant CFTR protein involved in cystic fibrosis, and to be up to 2 orders of magnitude more efficient as CFTR correctors than the clinical candidate miglustat (N-Bu-DNJ, 1, Figure 1).
Figure 1: N-Bu-DNJ (1), azide-armed DNJ derivatives 5 and cyclopeptoid scaffolds 2–4.
Figure 1: N-Bu-DNJ (1), azide-armed DNJ derivatives 5 and cyclopeptoid scaffolds 2–4.
The mechanisms underlying the inhibitory multivalent effect were studied with different methods such as isothermal titration calorimetry, competitive lectin-enzyme assays, X-ray crystallography or atomic force spectroscopy [7,11-13]. At this stage of research, one of the main challenges in the field is to design optimal systems that not only display large multivalent effects but also possess the desired properties for particular applications. In this context, the choice of the scaffold is crucial as it defines the valency, the size and the shape of the multivalent architectures. Due to their broad chemical diversity, rapid and convenient synthetic access, improved proteolytic stability and cell permeability over peptides, N-substituted glycine oligomers, called peptoids [14-17], appear as promising scaffolds for the synthesis of glycoconjugates of biological interest [14-18]. Combination of these advantages has led to many examples of biologically active peptoids [19-21]. So far, some syntheses of N-, O-, C- and S-linked glycopeptoids have been reported [22-31] and few of them are related to cyclopeptoids [32,33]. One of the most intriguing features of peptoids is their capacity to generate cyclic structures, which can expand the utility of this platform to multivalent chemical achitectures [34]. Conformation, size, charge and branching of these cyclic scaffolds influence the pharmacological profile of the products [35-39]. Moreover, macrocyclization enforces the rigidity of the more flexible linear peptoid skeleton and generally produces enhancement in biological activities [21,37]. In this context cyclopeptoids 2–4 appear as ideal building blocks because of their simplicity of synthesis and easy functionalization by click reaction (Figure 1). In the present paper, we report the synthesis of the first examples of cyclopeptoid-based iminosugar clusters. The influence of valency, size, linker and scaffold structure on jack bean α-mannosidase inhibition was evaluated with a series of 6- to 10-valent DNJ derivatives with two different alkyl spacer lengths (C6 or C9).
Results and Discussion
Our synthetic strategy was based on a convergent approach involving the attachment of azide-armed iminosugars 5 onto polyalkyne “clickable” scaffolds 2–4 by Cu(I)-catalyzed azide–alkyne cycloaddition (CuAAC) reactions [40,41] (Figure 1). N-alkyl derivatives of DNJ were logically chosen as the peripheral ligands because of the therapeutic relevance of these compounds [42]. In addition, most of the glycosidase inhibitor clusters published in the literature are based on these binding motifs [1-7,11,43] providing thus the opportunity to assess the relevance of cyclopeptoid cores by comparison with the other platforms already described.
Scaffold synthesis
The linear precursors of cyclic scaffolds (2–4, Figure 1) were prepared using the sub-monomer approach developed by Zuckerman et al. [44] through a two-step sequence, repeated iteratively, to obtain the desired oligomers (Scheme 1). Each monomer is constructed on the 2-chlorotrityl resin from C- to N-terminus using N,N’-diisopropylcarbodiimide (DIC)-mediated acylation with bromoacetic acid, followed by amination with the propargyl amine. After the completion of synthesis, the oligomers were cleaved from the resin using a 4:1 solution of CH2Cl2/hexafluoroisopropanol (HFIP). Macrocyclizations of the linear N-substituted oligoglycines 6–8 proceeded smoothly giving, under high dilution conditions (3.0 × 10−3 M) and in the presence of the efficient coupling agent HATU, the desired cyclic peptoids 2–4 (Scheme 1). After purification, compounds 2–4 were recovered in 31%, 32% and 12% overall yield respectively.
Scheme 1: Sub-monomer approach for the synthesis of cyclopeptoids 2–4: DIPEA = N,N-diisopropylethylamine; DIC = N,N’-diisopropylcarbodiimide; HATU O-(7-azabenzotriazole-1-yl)-N,N,N′,N′-tetramethyluronium hexafluorophosphate. (a) bromoacetic acid, DIPEA, CH2Cl2; (b) propargylamine (10 equiv), DMF; (c) bromoacetic acid, DIC, DMF; (d) CH2Cl2/HFIP (4:1); (e) HATU, DIPEA, DMF.
Scheme 1: Sub-monomer approach for the synthesis of cyclopeptoids 2–4: DIPEA = N,N-diisopropylethylamine; DIC...
DNJ cluster synthesis
The last stages of the DNJ cluster synthesis were based on a robust two-step process, recently developed in our group for the preparation of iminosugar click clusters [4,5,9-11]. The first step of the process involved the attachment of peracetylated azido iminosugars 5 [4] onto polyalkyne scaffolds 2–4 by microwave-assisted CuAAC reaction (Scheme 2).
Scheme 2: Synthesis of DNJ clusters 10: (a) CuSO4·5H2O cat., sodium ascorbate, DMF/H2O (5:1), MW, 80 °C; (b) Amberlite IRA 400 (OH–), MeOH/H2O (1:1), 40 °C. Overall yields from compounds 2, 3 or 4: 10a (n = 6, m = 1) 95%; 10b (n = 9, m = 1) 83%; 10c (n = 6, m = 3) 69%; 10d (n = 9, m = 3) 80%; 10e (n = 6, m = 5) 70%; 10f (n = 9, m = 5) 80%.
Scheme 2: Synthesis of DNJ clusters 10: (a) CuSO4·5H2O cat., sodium ascorbate, DMF/H2O (5:1), MW, 80 °C; (b) ...
The multiconjugation reaction proceeded smoothly to afford the six desired DNJ clusters 9 in 69–95% yields. With the exception of octavalent iminosugars 9c (n = 6) and 9d (n = 9), these compounds showed complex 1H NMR spectra at room temperature as exemplified by compound 9a (Figure 2i). This phenomenon, already observed for N-substituted cyclic α-peptoid derivatives [35-39], indicated the presence of more than one conformer in slow exchange on the NMR time scale. It is well known that the conformational heterogeneity is due to tertiary amide bonds, which can isomerize more readily than secondary amides, and to the absence of amide protons, which stabilize secondary structure by backbone hydrogen bonding [15,16]. As we have previousy demonstrated, this heterogeneity can be reduced by metal chelation [35,38]. Addition of an excess of sodium picrate to 9a indeed dramatically simplified the 1H NMR spectrum by inducing the formation of a sodium complex with a 6-fold symmetry (Figure 2ii).
![[1860-5397-10-144-2]](/bjoc/content/figures/1860-5397-10-144-2.png?scale=2.0&max-width=1024&background=FFFFFF)
Figure 2: i) Partial 1H NMR spectrum (400 MHz, CD3CN/CDCl3 9:1) of compound 9a; ii) Partial 1H NMR spectrum (400 MHz, CD3CN/CDCl3 9:1) of compound 9a with sodium picrate (11 equiv). * Residual solvent peak for CDCl3. • and X are assigned to protons a or b.
Figure 2: i) Partial 1H NMR spectrum (400 MHz, CD3CN/CDCl3 9:1) of compound 9a; ii) Partial 1H NMR spectrum (...
Subsequent O-deacetylation of compounds 9 using anion exchange Amberlite IRA-400 (OH−) resin provided the final deprotected iminosugar clusters 10 in high yields without affecting the potentially labile amide bond.
As indicated in the introduction, the best multivalent effects in glycosidase inhibition observed so far were obtained with jack bean α-mannosidase [1-7,11,12]. Accordingly, in order to complete these compelling investigations, evaluation of the inhibition potency of multivalent iminosugars 10 was performed on this peculiar enzyme (Table 1). Related monovalent controls 11 [3,4] as well as 7-valent β-cyclodextrin-based DNJ clusters 12 [4] have been included for comparative purposes (Figure 3). Our results clearly point out that all cyclopeptoid-based clusters 10 display a significant multivalent effect (rp/n > 1), with 6-valent iminosugar 10a as a single exception.
Figure 3: Monovalent models 11 and 7-valent DNJ derivatives 12.
Figure 3: Monovalent models 11 and 7-valent DNJ derivatives 12.
Table 1: Relative inhibition potency of cyclopeptoid-based clusters 10 and inhibitory activity (Ki, μM) against jack bean α-mannosidase.
aRelative inhibition potency = Ki (monovalent reference)/Ki (glycocluster). brp/n = Relative inhibition potency/number of iminosugar units. cSingle determination of Ki without duplicate.
Increasing the valency (from 6 to 10) or the linker length (from C6 to C9) resulted in increased inhibition potencies when compared to the corresponding monovalent models 11, the best result being obtained with 10-valent DNJ cluster 10f with a C9 linker (rp/n ~ 4). However, the binding enhancements were found to be 2- to 31-fold lower than the ones observed with the related 7-valent DNJ clusters 12 with identical alkyl spacer length but a different core (β-cyclodextrin). These results may indicate that the ligand spatial presentation in cyclopeptoid-based iminosugars 10 is not optimal to achieve a substantial multivalent effect. It has been shown recently that the use of rigid scaffolds such as porphyrin or C60 could lead to large multivalent effects (up to 200-fold on a valency-corrected basis) [3,7]. The modest inhibition enhancements observed with DNJ-cyclopeptoid conjugates 10 could thus be due to the high flexibility of their amide backbone [14-17].
Conclusion
In conclusion, we have reported the efficient synthesis of the first examples of cyclopeptoid-based iminosugar clusters and their evaluation as α-mannosidase inhibitors. Modest but significant inhibitory multivalent effects were observed for most of the compounds evaluated. This study further highlights the decisive impact of the scaffold rigidity on binding affinity enhancements. In connection with our recent work in the field of rare genetic diseases [8-10], further evaluation of neoglycopeptoids in cell systems are currently underway in our laboratory. The intrinsic advantages of cylopeptoid scaffolds including improved cell permeability and proteolytic stability are indeed expected to be most beneficial for this exploratory work.
Experimental
General information
NMR spectra were recorded on Bruker 300, 400 and 500 MHz spectrometers with solvent peaks as reference. Carbon multiplicities were assigned by distortionless enhancement by polarization transfer (DEPT) experiments. The 1H signals were assigned by 2D experiments (COSY). ESI–HRMS mass spectra were carried out on a Bruker MicroTOF spectrometer. Purifications were performed with silica gel 60 (230–400 mesh, 0.040–0.063 mm).
General procedure for the synthesis of cyclopeptoid-based iminosugar click clusters 9a–f
To a solution of the cyclopeptoid 2, 3 or 4 (typically 5 to 15 mg) and ligand 5a or 5b (1.1 equiv/alkyne moiety) in DMF (typically 0.5 to 1 mL) in a microwave vial was added a bright yellow suspension of CuSO4·5H2O (10 mol %/alkyne moiety) and sodium ascorbate (20 mol %/alkyne moiety) in water (typically 0.1 to 0.2 mL). The mixture was stirred and heated under microwave irradiation for 3 h at 80 °C. The mixture was concentrated, diluted in a 9:1:1 (v/v/v) mixture of MeCN/water/30 wt %-NH4OH and filtrated with the same eluent (25 mL) on a small pad of SiO2 (typically 1 cm thick), whose top surface became blue after copper complexation with NH3. The filtrate was concentrated and then filtrated on another pad of SiO2 (typically 1 cm wide and 2 cm thick), eluting it with AcOEt/PE 4:6 (25 mL) to recover clean unclicked ligand 5a or 5b, and then with MeCN/water 8:2 (25 mL) to afford iminosugar click clusters 9a–f as pale brown translucent wax after concentration.
General procedure for the synthesis of deprotected cyclopeptoid-based iminosugar click clusters 10a–f
To a solution of acetate-protected iminosugar click clusters 9a–f in a 1:1 mixture of water/MeOH (typically 600 µL/µmol) was added Amberlite IRA400 (OH–) (5.5n g/mmol of substrate; n = number of acetate groups). The suspension was softly stirred overnight at 40 °C. Then the mixture was filtrated and the filtrate was concentrated to afford deprotected iminosugar click clusters 10a–f in quantitative yields.
Compound 9a
+6.2 (c 1, CHCl3); 1H NMR (CD3CN/CDCl3 9:1 + 11 equiv sodium picrate, 400 MHz) δ 7.76 (s, 6H, H-1'), 4.97 (m, J = 10.3 Hz, 12H, H-3, H-4), 4.85 (d, J = 16.3 Hz, 6H, H-3' or H-5'), 4.83 (td, J = 9.8, 5.3 Hz, 6H, H-2), 4.71 (d, J = 16.3 Hz, 6H; H-3' or H-5'), 4.45 (d, J = 16.3 Hz, 6H, H-3' or H-5'), 4.32 (t, J = 7.0 Hz, 12H, H-12), 4.10 (dd, J = 19.4, 13.0 Hz, 12H, H-6), 3.97 (d, J = 16.3 Hz, 6H, H-3' or H-5'), 3.11 (dd, J = 11.1, 5.3 Hz, 6H, H-1a), 2.70 (m, 6H, H-7a), 2.68 (d, J = 8.8 Hz, 6H, H-5), 2.51 (m, 6H, H-7b), 2.35 (dd, J = 12.7, 11.1 Hz, 6H, H-1b), 1.95 (s, 72H, AcO), 1.85 (m, 12H, H-11), 1.40 (m, 12H, H-8), 1.28 (m, 24H, H-9, H-10) ppm; 13C NMR (CD3CN/CDCl3 9:1, 100 MHz) δ 171.4, 170.94, 170.88, 170.6, 170.6–168.9, 144.4–142.8, 124.7–123.8, 75.3, 70.5, 70.2, 62.1, 60.5, 53.4, 52.3, 50.8, 50.3–48.5, 44.9–42.6, 30.9, 27.3, 27.0, 25.2, 21.1 ppm; HRMS–ESI (m/z): [M + 2H]2+ calcd for C150H224N30O54 1654.7847; found: 1654.7827.
Compound 10a
−28.0 (c 0.1, H2O/DMSO 1:1 + 0.1% TFA); 1H NMR (D2O + 0.1% TFA, 500 MHz) δ 8.21–7.60 (m, 6H, H-1’), 5.20–3.72 (br m, 12H, H-3’ and H-5’), 4.35 (s, 12H, H-12), 3.85 (s, 12H, H-6), 3.59 (s, 6H, H-2), 3.43 (s, 6H, H-4), 3.31 (s, 6H, H-3), 3.16 (s, 6H, H-1a), 2.91 (s, 6H, H-7a), 2.78 (s, 6H, H-7b), 2.56 (s, 12H, H-1b and H-5), 1.84 (s, 12H, H-11), 1.51 (s, 12H, H-8), 1.25 (s, 24H, H-9 and H-10) ppm; 13C NMR (D2O + 0.1% TFA, 125 MHz) δ 173.4–169.3, 145.6–142.8, 127.4–124.6, 78.9, 70.4, 69.3, 66.8, 57.5, 55.9, 53.9, 51.9, 51.5–49.3, 46.3–43.3, 30.9, 27.3, 26.8, 24.0 ppm; HRMS–ESI (m/z): [M + 2H]2+ calcd for C102H174N30O30 1150.6579; found: 1150.6626.
Supporting Information
| Supporting Information File 1: Mannosidase inhibition assay procedures, synthesis and NMR spectra of all new compounds. | ||
| Format: PDF | Size: 5.5 MB | Download |
Acknowledgements
This work was supported by the Institut Universitaire de France (IUF), the CNRS (UMR 7509), the University of Strasbourg, the Agence Nationale de la Recherche (ANR, grant number 11-BS07-003-02), and a doctoral fellowship from the French Department of Research to Mathieu L. Lepage. We also thank Michel Schmitt and Lionel Allouche for NMR measurements and Hélène Nierengarten for MS measurements. We thank the University of Salerno (FARB) and the Italian MIUR (PRIN 20109Z2XRJ_006) for financial support. We thank Dr. Patrizia Iannece for ESI–MS and Miss. Dagmara Lubowiecka for experimental work (University of Salerno).
References
-
Diot, J.; Garcia-Moreno, M. I.; Gouin, S. G.; Ortiz Mellet, C.; Haupt, K.; Kovensky, J. Org. Biomol. Chem. 2009, 7, 357–363. doi:10.1039/b815408b
Return to citation in text: [1] [2] [3] -
Compain, P.; Bodlenner, A. ChemBioChem 2014, in press. doi:10.1002/cbic.201402026
See for a review on the multivalent effect in glycosidase inhibition.
Return to citation in text: [1] [2] [3] -
Compain, P.; Decroocq, C.; Iehl, J.; Holler, M.; Hazelard, D.; Mena Barragán, T.; Ortiz Mellet, C.; Nierengarten, J.-F. Angew. Chem., Int. Ed. 2010, 49, 5753–5756. doi:10.1002/anie.201002802
Return to citation in text: [1] [2] [3] [4] [5] [6] [7] [8] -
Decroocq, C.; Rodríguez-Lucena, D.; Russo, V.; Mena Barragán, T.; Ortiz Mellet, C.; Compain, P. Chem.–Eur. J. 2011, 17, 13825–13831. doi:10.1002/chem.201102266
Return to citation in text: [1] [2] [3] [4] [5] [6] [7] [8] [9] [10] [11] [12] [13] -
Joosten, A.; Schneider, J. P.; Lepage, M. L.; Tarnus, C.; Bodlenner, A.; Compain, P. Eur. J. Org. Chem. 2014, 1866–1872. doi:10.1002/ejoc.201301583
Return to citation in text: [1] [2] [3] [4] [5] -
Bonduelle, C.; Huang, J.; Mena-Barragán, T.; Ortiz Mellet, C.; Decroocq, C.; Etamé, E.; Heise, A.; Compain, P.; Lecommandoux, S. Chem. Commun. 2014, 50, 3350–3352. doi:10.1039/c3cc48190e
Return to citation in text: [1] [2] [3] [4] -
Brissonet, Y.; Ortiz Mellet, C.; Morandat, S.; Garcia-Moreno, M. I.; Deniaud, D.; Matthews, S. E.; Vidal, S.; Sestak, S.; El Kirat, K.; Gouin, S. G. J. Am. Chem. Soc. 2013, 135, 18427–18435. doi:10.1021/ja406931w
Return to citation in text: [1] [2] [3] [4] [5] [6] -
Decroocq, C.; Rodríguez-Lucena, D.; Ikeda, K.; Asano, N.; Compain, P. ChemBioChem 2012, 13, 661–664. doi:10.1002/cbic.201200005
Return to citation in text: [1] [2] -
Joosten, A.; Decroocq, C.; de Sousa, J.; Schneider, J.; Etamé, E.; Bodlenner, A.; Butters, T. D.; Compain, P. ChemBioChem 2014, 15, 309–319. doi:10.1002/cbic.201300442
Return to citation in text: [1] [2] [3] -
Compain, P.; Decroocq, C.; Joosten, A.; de Sousa, J.; Rodríguez-Lucena, D.; Butters, T. D.; Bertrand, J.; Clément, R.; Boinot, C.; Becq, F.; Norez, C. ChemBioChem 2013, 14, 2050–2058. doi:10.1002/cbic.201300312
Return to citation in text: [1] [2] [3] -
Decroocq, C.; Joosten, A.; Sergent, R.; Mena Barragán, T.; Ortiz Mellet, C.; Compain, P. ChemBioChem 2013, 14, 2038–2049. doi:10.1002/cbic.201300283
Return to citation in text: [1] [2] [3] [4] -
Rísquez-Cuadro, R.; García Fernández, J. M.; Nierengarten, J.-F.; Ortiz Mellet, C. Chem.–Eur. J. 2013, 19, 16791–16803. doi:10.1002/chem.201303158
Return to citation in text: [1] [2] -
Moreno-Clavijo, E.; Carmona, A. T.; Moreno-Vargas, A. J.; Molina, L.; Wright, D. W.; Davies, G. J.; Robina, I. Eur. J. Org. Chem. 2013, 7328–7336. doi:10.1002/ejoc.201300878
Return to citation in text: [1] -
Nielsen, P. E., Ed. Pseudo-Peptides in Drug Discovery; Wiley-VCH: Weinheim, 2004.
Return to citation in text: [1] [2] [3] -
Fowler, S. A.; Blackwell, H. E. Org. Biomol. Chem. 2009, 7, 1508–1524. doi:10.1039/B817980H
Return to citation in text: [1] [2] [3] [4] -
Culf, A. S.; Ouellette, R. J. Molecules 2010, 15, 5282–5335. doi:10.3390/molecules15085282
Return to citation in text: [1] [2] [3] [4] -
Seo, J.; Lee, B.-C.; Zuckermann, R. N. Compr. Biomater. 2011, 2, 53–76. doi:10.1016/B978-0-08-055294-1.00256-7
Return to citation in text: [1] [2] [3] -
Marcaurelle, L. A.; Bertozzi, C. R. Chem.–Eur. J. 1999, 5, 1384–1390. doi:10.1002/(SICI)1521-3765(19990503)5:5<1384::AID-CHEM1384>3.0.CO;2-X
Return to citation in text: [1] -
Yoo, B.; Kirshenbaum, K. Curr. Opin. Chem. Biol. 2008, 12, 714–721. doi:10.1016/j.cbpa.2008.08.015
Return to citation in text: [1] -
Zuckermann, R. N.; Kodadek, T. Curr. Opin. Mol. Ther. 2009, 11, 299–307.
Return to citation in text: [1] -
Huang, M. L.; Shin, S. B. Y.; Benson, M. A.; Torres, V. J.; Kirshenbaum, K. ChemMedChem 2012, 7, 114–122. doi:10.1002/cmdc.201100358
Return to citation in text: [1] [2] -
Saha, U. K.; Roy, R. Tetrahedron Lett. 1995, 36, 3635–3638. doi:10.1016/0040-4039(95)00620-R
Return to citation in text: [1] -
Yuasa, H.; Kamata, Y.; Kurono, S.; Hashimoto, H. Bioorg. Med. Chem. Lett. 1998, 8, 2139–2144. doi:10.1016/S0960-894X(98)00364-3
Return to citation in text: [1] -
Dechantsreiter, M. A.; Burkhart, F.; Kessler, H. Tetrahedron Lett. 1998, 39, 253–254. doi:10.1016/S0040-4039(97)10566-4
Return to citation in text: [1] -
Burger, K.; Böttcher, C.; Radics, G.; Hennig, L. Tetrahedron Lett. 2001, 42, 3061–3063. doi:10.1016/S0040-4039(01)00351-3
Return to citation in text: [1] -
Norgren, A. S.; Budke, C.; Majer, Z.; Heggemann, C.; Koop, T.; Sewald, N. Synthesis 2009, 3, 488–494. doi:10.1055/s-0028-1083302
Return to citation in text: [1] -
Ahn, M.; Murugan, R. N.; Nan, Y. H.; Cheong, C.; Sohn, H.; Kim, E.-H.; Hwang, E.; Ryu, E. K.; Kang, S. W.; Shin, S. Y.; Bang, J. K. Bioorg. Med. Chem. Lett. 2011, 21, 6148–6153. doi:10.1016/j.bmcl.2011.08.012
Return to citation in text: [1] -
Khan, S. N.; Kim, A.; Grubbs, R. H.; Kwon, Y.-U. Org. Lett. 2012, 14, 2952–2955. doi:10.1021/ol300808c
Return to citation in text: [1] -
Ham, H. O.; Park, S. H.; Kurutz, J. W.; Szleifer, I. G.; Messersmith, P. B. J. Am. Chem. Soc. 2013, 135, 13015–13022. doi:10.1021/ja404681x
Return to citation in text: [1] -
Singhamahapatra, A.; Sahoo, L.; Loganathan, D. J. Org. Chem. 2013, 78, 10329–10336. doi:10.1021/jo401720s
Return to citation in text: [1] -
Fürniss, D.; Mack, T.; Hahn, F.; Vollrath, S. B. L.; Koroniak, K.; Schepers, S.; Bräse, S. Beilstein J. Org. Chem. 2013, 9, 56–63. doi:10.3762/bjoc.9.7
Return to citation in text: [1] -
Comegna, D.; De Riccardis, F. Org. Lett. 2009, 11, 3898–3901. doi:10.1021/ol901524k
Return to citation in text: [1] -
Cecioni, S.; Faure, S.; Darbost, U.; Bonnamour, I.; Parrot-Lopez, H.; Roy, O.; Taillefumier, C.; Wimmerova, M.; Praly, J.-P.; Imberty, A.; Vidal, S. Chem.–Eur. J. 2011, 17, 2146–2159. doi:10.1002/chem.201002635
Return to citation in text: [1] -
Levine, P. M.; Carberry, T. P.; Holub, J. M.; Kirshenbaum, K. Med. Chem. Commun. 2013, 4, 493–509. doi:10.1039/c2md20338c
Return to citation in text: [1] -
Maulucci, N.; Izzo, I.; Bifulco, G.; Aliberti, A.; De Cola, C.; Comegna, D.; Gaeta, C.; Napolitano, A.; Pizza, C.; Tedesco, C.; Flot, D.; De Riccardis, F. Chem. Commun. 2008, 3927–3929. doi:10.1039/b806508j
Return to citation in text: [1] [2] [3] -
De Cola, C.; Licen, S.; Comegna, D.; Cafaro, E.; Bifulco, G.; Izzo, I.; Tecilla, P.; De Riccardis, F. Org. Biomol. Chem. 2009, 7, 2851–2854. doi:10.1039/b905665c
Return to citation in text: [1] [2] -
Comegna, D.; Benincasa, M.; Gennaro, R.; Izzo, I.; De Riccardis, F. Bioorg. Med. Chem. 2010, 18, 2010–2018. doi:10.1016/j.bmc.2010.01.026
Return to citation in text: [1] [2] [3] -
Izzo, I.; Ianniello, G.; De Cola, C.; Nardone, B.; Erra, L.; Vaughan, G.; Tedesco, C.; De Riccardis, F. Org. Lett. 2013, 15, 598–601. doi:10.1021/ol3034143
Return to citation in text: [1] [2] [3] -
Della Sala, G.; Nardone, B.; De Riccardis, F.; Izzo, I. Org. Biomol. Chem. 2013, 11, 726–731. doi:10.1039/c2ob26764k
Return to citation in text: [1] [2] -
Kolb, H. C.; Finn, M. G.; Sharpless, K. B. Angew. Chem., Int. Ed. 2001, 40, 2004–2021. doi:10.1002/1521-3773(20010601)40:11<2004::AID-ANIE2004>3.0.CO;2-5
Angew. Chem. 2001, 113, 2056–2075. doi:10.1002/1521-3757(20010601)113:11<2056::AID-ANGE2056>3.0.CO;2-W
Return to citation in text: [1] -
Tornøe, C. W.; Christensen, C.; Meldal, M. J. Org. Chem. 2002, 67, 3057–3064. doi:10.1021/jo011148j
Return to citation in text: [1] -
Compain, P.; Martin, O. R., Eds. Iminosugars: from Synthesis to Therapeutic Applications; Wiley & Sons: Chichester, 2007. doi:10.1002/9780470517437
Return to citation in text: [1] -
Cardona, F.; Isoldi, G.; Sansone, F.; Casnati, A.; Goti, A. J. Org. Chem. 2012, 77, 6980–6988. doi:10.1021/jo301155p
See for a recent example of multivalent pyrrolidinols based oncalix[4]arene scaffolds.
Return to citation in text: [1] -
Zuckermann, R. N.; Kerr, J. M.; Kent, S. B. H.; Moos, W. H. J. Am. Chem. Soc. 1992, 114, 10646–10647. doi:10.1021/ja00052a076
Return to citation in text: [1]
| 1. | Diot, J.; Garcia-Moreno, M. I.; Gouin, S. G.; Ortiz Mellet, C.; Haupt, K.; Kovensky, J. Org. Biomol. Chem. 2009, 7, 357–363. doi:10.1039/b815408b |
| 2. |
Compain, P.; Bodlenner, A. ChemBioChem 2014, in press. doi:10.1002/cbic.201402026
See for a review on the multivalent effect in glycosidase inhibition. |
| 3. | Compain, P.; Decroocq, C.; Iehl, J.; Holler, M.; Hazelard, D.; Mena Barragán, T.; Ortiz Mellet, C.; Nierengarten, J.-F. Angew. Chem., Int. Ed. 2010, 49, 5753–5756. doi:10.1002/anie.201002802 |
| 4. | Decroocq, C.; Rodríguez-Lucena, D.; Russo, V.; Mena Barragán, T.; Ortiz Mellet, C.; Compain, P. Chem.–Eur. J. 2011, 17, 13825–13831. doi:10.1002/chem.201102266 |
| 5. | Joosten, A.; Schneider, J. P.; Lepage, M. L.; Tarnus, C.; Bodlenner, A.; Compain, P. Eur. J. Org. Chem. 2014, 1866–1872. doi:10.1002/ejoc.201301583 |
| 6. | Bonduelle, C.; Huang, J.; Mena-Barragán, T.; Ortiz Mellet, C.; Decroocq, C.; Etamé, E.; Heise, A.; Compain, P.; Lecommandoux, S. Chem. Commun. 2014, 50, 3350–3352. doi:10.1039/c3cc48190e |
| 7. | Brissonet, Y.; Ortiz Mellet, C.; Morandat, S.; Garcia-Moreno, M. I.; Deniaud, D.; Matthews, S. E.; Vidal, S.; Sestak, S.; El Kirat, K.; Gouin, S. G. J. Am. Chem. Soc. 2013, 135, 18427–18435. doi:10.1021/ja406931w |
| 11. | Decroocq, C.; Joosten, A.; Sergent, R.; Mena Barragán, T.; Ortiz Mellet, C.; Compain, P. ChemBioChem 2013, 14, 2038–2049. doi:10.1002/cbic.201300283 |
| 12. | Rísquez-Cuadro, R.; García Fernández, J. M.; Nierengarten, J.-F.; Ortiz Mellet, C. Chem.–Eur. J. 2013, 19, 16791–16803. doi:10.1002/chem.201303158 |
| 3. | Compain, P.; Decroocq, C.; Iehl, J.; Holler, M.; Hazelard, D.; Mena Barragán, T.; Ortiz Mellet, C.; Nierengarten, J.-F. Angew. Chem., Int. Ed. 2010, 49, 5753–5756. doi:10.1002/anie.201002802 |
| 4. | Decroocq, C.; Rodríguez-Lucena, D.; Russo, V.; Mena Barragán, T.; Ortiz Mellet, C.; Compain, P. Chem.–Eur. J. 2011, 17, 13825–13831. doi:10.1002/chem.201102266 |
| 4. | Decroocq, C.; Rodríguez-Lucena, D.; Russo, V.; Mena Barragán, T.; Ortiz Mellet, C.; Compain, P. Chem.–Eur. J. 2011, 17, 13825–13831. doi:10.1002/chem.201102266 |
| 1. | Diot, J.; Garcia-Moreno, M. I.; Gouin, S. G.; Ortiz Mellet, C.; Haupt, K.; Kovensky, J. Org. Biomol. Chem. 2009, 7, 357–363. doi:10.1039/b815408b |
| 2. |
Compain, P.; Bodlenner, A. ChemBioChem 2014, in press. doi:10.1002/cbic.201402026
See for a review on the multivalent effect in glycosidase inhibition. |
| 7. | Brissonet, Y.; Ortiz Mellet, C.; Morandat, S.; Garcia-Moreno, M. I.; Deniaud, D.; Matthews, S. E.; Vidal, S.; Sestak, S.; El Kirat, K.; Gouin, S. G. J. Am. Chem. Soc. 2013, 135, 18427–18435. doi:10.1021/ja406931w |
| 32. | Comegna, D.; De Riccardis, F. Org. Lett. 2009, 11, 3898–3901. doi:10.1021/ol901524k |
| 33. | Cecioni, S.; Faure, S.; Darbost, U.; Bonnamour, I.; Parrot-Lopez, H.; Roy, O.; Taillefumier, C.; Wimmerova, M.; Praly, J.-P.; Imberty, A.; Vidal, S. Chem.–Eur. J. 2011, 17, 2146–2159. doi:10.1002/chem.201002635 |
| 8. | Decroocq, C.; Rodríguez-Lucena, D.; Ikeda, K.; Asano, N.; Compain, P. ChemBioChem 2012, 13, 661–664. doi:10.1002/cbic.201200005 |
| 9. | Joosten, A.; Decroocq, C.; de Sousa, J.; Schneider, J.; Etamé, E.; Bodlenner, A.; Butters, T. D.; Compain, P. ChemBioChem 2014, 15, 309–319. doi:10.1002/cbic.201300442 |
| 10. | Compain, P.; Decroocq, C.; Joosten, A.; de Sousa, J.; Rodríguez-Lucena, D.; Butters, T. D.; Bertrand, J.; Clément, R.; Boinot, C.; Becq, F.; Norez, C. ChemBioChem 2013, 14, 2050–2058. doi:10.1002/cbic.201300312 |
| 4. | Decroocq, C.; Rodríguez-Lucena, D.; Russo, V.; Mena Barragán, T.; Ortiz Mellet, C.; Compain, P. Chem.–Eur. J. 2011, 17, 13825–13831. doi:10.1002/chem.201102266 |
| 5. | Joosten, A.; Schneider, J. P.; Lepage, M. L.; Tarnus, C.; Bodlenner, A.; Compain, P. Eur. J. Org. Chem. 2014, 1866–1872. doi:10.1002/ejoc.201301583 |
| 34. | Levine, P. M.; Carberry, T. P.; Holub, J. M.; Kirshenbaum, K. Med. Chem. Commun. 2013, 4, 493–509. doi:10.1039/c2md20338c |
| 3. | Compain, P.; Decroocq, C.; Iehl, J.; Holler, M.; Hazelard, D.; Mena Barragán, T.; Ortiz Mellet, C.; Nierengarten, J.-F. Angew. Chem., Int. Ed. 2010, 49, 5753–5756. doi:10.1002/anie.201002802 |
| 19. | Yoo, B.; Kirshenbaum, K. Curr. Opin. Chem. Biol. 2008, 12, 714–721. doi:10.1016/j.cbpa.2008.08.015 |
| 20. | Zuckermann, R. N.; Kodadek, T. Curr. Opin. Mol. Ther. 2009, 11, 299–307. |
| 21. | Huang, M. L.; Shin, S. B. Y.; Benson, M. A.; Torres, V. J.; Kirshenbaum, K. ChemMedChem 2012, 7, 114–122. doi:10.1002/cmdc.201100358 |
| 3. | Compain, P.; Decroocq, C.; Iehl, J.; Holler, M.; Hazelard, D.; Mena Barragán, T.; Ortiz Mellet, C.; Nierengarten, J.-F. Angew. Chem., Int. Ed. 2010, 49, 5753–5756. doi:10.1002/anie.201002802 |
| 7. | Brissonet, Y.; Ortiz Mellet, C.; Morandat, S.; Garcia-Moreno, M. I.; Deniaud, D.; Matthews, S. E.; Vidal, S.; Sestak, S.; El Kirat, K.; Gouin, S. G. J. Am. Chem. Soc. 2013, 135, 18427–18435. doi:10.1021/ja406931w |
| 3. | Compain, P.; Decroocq, C.; Iehl, J.; Holler, M.; Hazelard, D.; Mena Barragán, T.; Ortiz Mellet, C.; Nierengarten, J.-F. Angew. Chem., Int. Ed. 2010, 49, 5753–5756. doi:10.1002/anie.201002802 |
| 4. | Decroocq, C.; Rodríguez-Lucena, D.; Russo, V.; Mena Barragán, T.; Ortiz Mellet, C.; Compain, P. Chem.–Eur. J. 2011, 17, 13825–13831. doi:10.1002/chem.201102266 |
| 5. | Joosten, A.; Schneider, J. P.; Lepage, M. L.; Tarnus, C.; Bodlenner, A.; Compain, P. Eur. J. Org. Chem. 2014, 1866–1872. doi:10.1002/ejoc.201301583 |
| 6. | Bonduelle, C.; Huang, J.; Mena-Barragán, T.; Ortiz Mellet, C.; Decroocq, C.; Etamé, E.; Heise, A.; Compain, P.; Lecommandoux, S. Chem. Commun. 2014, 50, 3350–3352. doi:10.1039/c3cc48190e |
| 7. | Brissonet, Y.; Ortiz Mellet, C.; Morandat, S.; Garcia-Moreno, M. I.; Deniaud, D.; Matthews, S. E.; Vidal, S.; Sestak, S.; El Kirat, K.; Gouin, S. G. J. Am. Chem. Soc. 2013, 135, 18427–18435. doi:10.1021/ja406931w |
| 22. | Saha, U. K.; Roy, R. Tetrahedron Lett. 1995, 36, 3635–3638. doi:10.1016/0040-4039(95)00620-R |
| 23. | Yuasa, H.; Kamata, Y.; Kurono, S.; Hashimoto, H. Bioorg. Med. Chem. Lett. 1998, 8, 2139–2144. doi:10.1016/S0960-894X(98)00364-3 |
| 24. | Dechantsreiter, M. A.; Burkhart, F.; Kessler, H. Tetrahedron Lett. 1998, 39, 253–254. doi:10.1016/S0040-4039(97)10566-4 |
| 25. | Burger, K.; Böttcher, C.; Radics, G.; Hennig, L. Tetrahedron Lett. 2001, 42, 3061–3063. doi:10.1016/S0040-4039(01)00351-3 |
| 26. | Norgren, A. S.; Budke, C.; Majer, Z.; Heggemann, C.; Koop, T.; Sewald, N. Synthesis 2009, 3, 488–494. doi:10.1055/s-0028-1083302 |
| 27. | Ahn, M.; Murugan, R. N.; Nan, Y. H.; Cheong, C.; Sohn, H.; Kim, E.-H.; Hwang, E.; Ryu, E. K.; Kang, S. W.; Shin, S. Y.; Bang, J. K. Bioorg. Med. Chem. Lett. 2011, 21, 6148–6153. doi:10.1016/j.bmcl.2011.08.012 |
| 28. | Khan, S. N.; Kim, A.; Grubbs, R. H.; Kwon, Y.-U. Org. Lett. 2012, 14, 2952–2955. doi:10.1021/ol300808c |
| 29. | Ham, H. O.; Park, S. H.; Kurutz, J. W.; Szleifer, I. G.; Messersmith, P. B. J. Am. Chem. Soc. 2013, 135, 13015–13022. doi:10.1021/ja404681x |
| 30. | Singhamahapatra, A.; Sahoo, L.; Loganathan, D. J. Org. Chem. 2013, 78, 10329–10336. doi:10.1021/jo401720s |
| 31. | Fürniss, D.; Mack, T.; Hahn, F.; Vollrath, S. B. L.; Koroniak, K.; Schepers, S.; Bräse, S. Beilstein J. Org. Chem. 2013, 9, 56–63. doi:10.3762/bjoc.9.7 |
| 14. | Nielsen, P. E., Ed. Pseudo-Peptides in Drug Discovery; Wiley-VCH: Weinheim, 2004. |
| 15. | Fowler, S. A.; Blackwell, H. E. Org. Biomol. Chem. 2009, 7, 1508–1524. doi:10.1039/B817980H |
| 16. | Culf, A. S.; Ouellette, R. J. Molecules 2010, 15, 5282–5335. doi:10.3390/molecules15085282 |
| 17. | Seo, J.; Lee, B.-C.; Zuckermann, R. N. Compr. Biomater. 2011, 2, 53–76. doi:10.1016/B978-0-08-055294-1.00256-7 |
| 10. | Compain, P.; Decroocq, C.; Joosten, A.; de Sousa, J.; Rodríguez-Lucena, D.; Butters, T. D.; Bertrand, J.; Clément, R.; Boinot, C.; Becq, F.; Norez, C. ChemBioChem 2013, 14, 2050–2058. doi:10.1002/cbic.201300312 |
| 14. | Nielsen, P. E., Ed. Pseudo-Peptides in Drug Discovery; Wiley-VCH: Weinheim, 2004. |
| 15. | Fowler, S. A.; Blackwell, H. E. Org. Biomol. Chem. 2009, 7, 1508–1524. doi:10.1039/B817980H |
| 16. | Culf, A. S.; Ouellette, R. J. Molecules 2010, 15, 5282–5335. doi:10.3390/molecules15085282 |
| 17. | Seo, J.; Lee, B.-C.; Zuckermann, R. N. Compr. Biomater. 2011, 2, 53–76. doi:10.1016/B978-0-08-055294-1.00256-7 |
| 3. | Compain, P.; Decroocq, C.; Iehl, J.; Holler, M.; Hazelard, D.; Mena Barragán, T.; Ortiz Mellet, C.; Nierengarten, J.-F. Angew. Chem., Int. Ed. 2010, 49, 5753–5756. doi:10.1002/anie.201002802 |
| 4. | Decroocq, C.; Rodríguez-Lucena, D.; Russo, V.; Mena Barragán, T.; Ortiz Mellet, C.; Compain, P. Chem.–Eur. J. 2011, 17, 13825–13831. doi:10.1002/chem.201102266 |
| 8. | Decroocq, C.; Rodríguez-Lucena, D.; Ikeda, K.; Asano, N.; Compain, P. ChemBioChem 2012, 13, 661–664. doi:10.1002/cbic.201200005 |
| 9. | Joosten, A.; Decroocq, C.; de Sousa, J.; Schneider, J.; Etamé, E.; Bodlenner, A.; Butters, T. D.; Compain, P. ChemBioChem 2014, 15, 309–319. doi:10.1002/cbic.201300442 |
| 14. | Nielsen, P. E., Ed. Pseudo-Peptides in Drug Discovery; Wiley-VCH: Weinheim, 2004. |
| 15. | Fowler, S. A.; Blackwell, H. E. Org. Biomol. Chem. 2009, 7, 1508–1524. doi:10.1039/B817980H |
| 16. | Culf, A. S.; Ouellette, R. J. Molecules 2010, 15, 5282–5335. doi:10.3390/molecules15085282 |
| 17. | Seo, J.; Lee, B.-C.; Zuckermann, R. N. Compr. Biomater. 2011, 2, 53–76. doi:10.1016/B978-0-08-055294-1.00256-7 |
| 18. | Marcaurelle, L. A.; Bertozzi, C. R. Chem.–Eur. J. 1999, 5, 1384–1390. doi:10.1002/(SICI)1521-3765(19990503)5:5<1384::AID-CHEM1384>3.0.CO;2-X |
| 4. | Decroocq, C.; Rodríguez-Lucena, D.; Russo, V.; Mena Barragán, T.; Ortiz Mellet, C.; Compain, P. Chem.–Eur. J. 2011, 17, 13825–13831. doi:10.1002/chem.201102266 |
| 4. | Decroocq, C.; Rodríguez-Lucena, D.; Russo, V.; Mena Barragán, T.; Ortiz Mellet, C.; Compain, P. Chem.–Eur. J. 2011, 17, 13825–13831. doi:10.1002/chem.201102266 |
| 3. | Compain, P.; Decroocq, C.; Iehl, J.; Holler, M.; Hazelard, D.; Mena Barragán, T.; Ortiz Mellet, C.; Nierengarten, J.-F. Angew. Chem., Int. Ed. 2010, 49, 5753–5756. doi:10.1002/anie.201002802 |
| 4. | Decroocq, C.; Rodríguez-Lucena, D.; Russo, V.; Mena Barragán, T.; Ortiz Mellet, C.; Compain, P. Chem.–Eur. J. 2011, 17, 13825–13831. doi:10.1002/chem.201102266 |
| 6. | Bonduelle, C.; Huang, J.; Mena-Barragán, T.; Ortiz Mellet, C.; Decroocq, C.; Etamé, E.; Heise, A.; Compain, P.; Lecommandoux, S. Chem. Commun. 2014, 50, 3350–3352. doi:10.1039/c3cc48190e |
| 7. | Brissonet, Y.; Ortiz Mellet, C.; Morandat, S.; Garcia-Moreno, M. I.; Deniaud, D.; Matthews, S. E.; Vidal, S.; Sestak, S.; El Kirat, K.; Gouin, S. G. J. Am. Chem. Soc. 2013, 135, 18427–18435. doi:10.1021/ja406931w |
| 11. | Decroocq, C.; Joosten, A.; Sergent, R.; Mena Barragán, T.; Ortiz Mellet, C.; Compain, P. ChemBioChem 2013, 14, 2038–2049. doi:10.1002/cbic.201300283 |
| 12. | Rísquez-Cuadro, R.; García Fernández, J. M.; Nierengarten, J.-F.; Ortiz Mellet, C. Chem.–Eur. J. 2013, 19, 16791–16803. doi:10.1002/chem.201303158 |
| 13. | Moreno-Clavijo, E.; Carmona, A. T.; Moreno-Vargas, A. J.; Molina, L.; Wright, D. W.; Davies, G. J.; Robina, I. Eur. J. Org. Chem. 2013, 7328–7336. doi:10.1002/ejoc.201300878 |
| 4. | Decroocq, C.; Rodríguez-Lucena, D.; Russo, V.; Mena Barragán, T.; Ortiz Mellet, C.; Compain, P. Chem.–Eur. J. 2011, 17, 13825–13831. doi:10.1002/chem.201102266 |
| 40. |
Kolb, H. C.; Finn, M. G.; Sharpless, K. B. Angew. Chem., Int. Ed. 2001, 40, 2004–2021. doi:10.1002/1521-3773(20010601)40:11<2004::AID-ANIE2004>3.0.CO;2-5
Angew. Chem. 2001, 113, 2056–2075. doi:10.1002/1521-3757(20010601)113:11<2056::AID-ANGE2056>3.0.CO;2-W |
| 41. | Tornøe, C. W.; Christensen, C.; Meldal, M. J. Org. Chem. 2002, 67, 3057–3064. doi:10.1021/jo011148j |
| 35. | Maulucci, N.; Izzo, I.; Bifulco, G.; Aliberti, A.; De Cola, C.; Comegna, D.; Gaeta, C.; Napolitano, A.; Pizza, C.; Tedesco, C.; Flot, D.; De Riccardis, F. Chem. Commun. 2008, 3927–3929. doi:10.1039/b806508j |
| 36. | De Cola, C.; Licen, S.; Comegna, D.; Cafaro, E.; Bifulco, G.; Izzo, I.; Tecilla, P.; De Riccardis, F. Org. Biomol. Chem. 2009, 7, 2851–2854. doi:10.1039/b905665c |
| 37. | Comegna, D.; Benincasa, M.; Gennaro, R.; Izzo, I.; De Riccardis, F. Bioorg. Med. Chem. 2010, 18, 2010–2018. doi:10.1016/j.bmc.2010.01.026 |
| 38. | Izzo, I.; Ianniello, G.; De Cola, C.; Nardone, B.; Erra, L.; Vaughan, G.; Tedesco, C.; De Riccardis, F. Org. Lett. 2013, 15, 598–601. doi:10.1021/ol3034143 |
| 39. | Della Sala, G.; Nardone, B.; De Riccardis, F.; Izzo, I. Org. Biomol. Chem. 2013, 11, 726–731. doi:10.1039/c2ob26764k |
| 21. | Huang, M. L.; Shin, S. B. Y.; Benson, M. A.; Torres, V. J.; Kirshenbaum, K. ChemMedChem 2012, 7, 114–122. doi:10.1002/cmdc.201100358 |
| 37. | Comegna, D.; Benincasa, M.; Gennaro, R.; Izzo, I.; De Riccardis, F. Bioorg. Med. Chem. 2010, 18, 2010–2018. doi:10.1016/j.bmc.2010.01.026 |
| 15. | Fowler, S. A.; Blackwell, H. E. Org. Biomol. Chem. 2009, 7, 1508–1524. doi:10.1039/B817980H |
| 16. | Culf, A. S.; Ouellette, R. J. Molecules 2010, 15, 5282–5335. doi:10.3390/molecules15085282 |
| 35. | Maulucci, N.; Izzo, I.; Bifulco, G.; Aliberti, A.; De Cola, C.; Comegna, D.; Gaeta, C.; Napolitano, A.; Pizza, C.; Tedesco, C.; Flot, D.; De Riccardis, F. Chem. Commun. 2008, 3927–3929. doi:10.1039/b806508j |
| 38. | Izzo, I.; Ianniello, G.; De Cola, C.; Nardone, B.; Erra, L.; Vaughan, G.; Tedesco, C.; De Riccardis, F. Org. Lett. 2013, 15, 598–601. doi:10.1021/ol3034143 |
| 4. | Decroocq, C.; Rodríguez-Lucena, D.; Russo, V.; Mena Barragán, T.; Ortiz Mellet, C.; Compain, P. Chem.–Eur. J. 2011, 17, 13825–13831. doi:10.1002/chem.201102266 |
| 35. | Maulucci, N.; Izzo, I.; Bifulco, G.; Aliberti, A.; De Cola, C.; Comegna, D.; Gaeta, C.; Napolitano, A.; Pizza, C.; Tedesco, C.; Flot, D.; De Riccardis, F. Chem. Commun. 2008, 3927–3929. doi:10.1039/b806508j |
| 36. | De Cola, C.; Licen, S.; Comegna, D.; Cafaro, E.; Bifulco, G.; Izzo, I.; Tecilla, P.; De Riccardis, F. Org. Biomol. Chem. 2009, 7, 2851–2854. doi:10.1039/b905665c |
| 37. | Comegna, D.; Benincasa, M.; Gennaro, R.; Izzo, I.; De Riccardis, F. Bioorg. Med. Chem. 2010, 18, 2010–2018. doi:10.1016/j.bmc.2010.01.026 |
| 38. | Izzo, I.; Ianniello, G.; De Cola, C.; Nardone, B.; Erra, L.; Vaughan, G.; Tedesco, C.; De Riccardis, F. Org. Lett. 2013, 15, 598–601. doi:10.1021/ol3034143 |
| 39. | Della Sala, G.; Nardone, B.; De Riccardis, F.; Izzo, I. Org. Biomol. Chem. 2013, 11, 726–731. doi:10.1039/c2ob26764k |
| 44. | Zuckermann, R. N.; Kerr, J. M.; Kent, S. B. H.; Moos, W. H. J. Am. Chem. Soc. 1992, 114, 10646–10647. doi:10.1021/ja00052a076 |
| 4. | Decroocq, C.; Rodríguez-Lucena, D.; Russo, V.; Mena Barragán, T.; Ortiz Mellet, C.; Compain, P. Chem.–Eur. J. 2011, 17, 13825–13831. doi:10.1002/chem.201102266 |
| 5. | Joosten, A.; Schneider, J. P.; Lepage, M. L.; Tarnus, C.; Bodlenner, A.; Compain, P. Eur. J. Org. Chem. 2014, 1866–1872. doi:10.1002/ejoc.201301583 |
| 9. | Joosten, A.; Decroocq, C.; de Sousa, J.; Schneider, J.; Etamé, E.; Bodlenner, A.; Butters, T. D.; Compain, P. ChemBioChem 2014, 15, 309–319. doi:10.1002/cbic.201300442 |
| 10. | Compain, P.; Decroocq, C.; Joosten, A.; de Sousa, J.; Rodríguez-Lucena, D.; Butters, T. D.; Bertrand, J.; Clément, R.; Boinot, C.; Becq, F.; Norez, C. ChemBioChem 2013, 14, 2050–2058. doi:10.1002/cbic.201300312 |
| 11. | Decroocq, C.; Joosten, A.; Sergent, R.; Mena Barragán, T.; Ortiz Mellet, C.; Compain, P. ChemBioChem 2013, 14, 2038–2049. doi:10.1002/cbic.201300283 |
| 42. | Compain, P.; Martin, O. R., Eds. Iminosugars: from Synthesis to Therapeutic Applications; Wiley & Sons: Chichester, 2007. doi:10.1002/9780470517437 |
| 1. | Diot, J.; Garcia-Moreno, M. I.; Gouin, S. G.; Ortiz Mellet, C.; Haupt, K.; Kovensky, J. Org. Biomol. Chem. 2009, 7, 357–363. doi:10.1039/b815408b |
| 2. |
Compain, P.; Bodlenner, A. ChemBioChem 2014, in press. doi:10.1002/cbic.201402026
See for a review on the multivalent effect in glycosidase inhibition. |
| 3. | Compain, P.; Decroocq, C.; Iehl, J.; Holler, M.; Hazelard, D.; Mena Barragán, T.; Ortiz Mellet, C.; Nierengarten, J.-F. Angew. Chem., Int. Ed. 2010, 49, 5753–5756. doi:10.1002/anie.201002802 |
| 4. | Decroocq, C.; Rodríguez-Lucena, D.; Russo, V.; Mena Barragán, T.; Ortiz Mellet, C.; Compain, P. Chem.–Eur. J. 2011, 17, 13825–13831. doi:10.1002/chem.201102266 |
| 5. | Joosten, A.; Schneider, J. P.; Lepage, M. L.; Tarnus, C.; Bodlenner, A.; Compain, P. Eur. J. Org. Chem. 2014, 1866–1872. doi:10.1002/ejoc.201301583 |
| 6. | Bonduelle, C.; Huang, J.; Mena-Barragán, T.; Ortiz Mellet, C.; Decroocq, C.; Etamé, E.; Heise, A.; Compain, P.; Lecommandoux, S. Chem. Commun. 2014, 50, 3350–3352. doi:10.1039/c3cc48190e |
| 7. | Brissonet, Y.; Ortiz Mellet, C.; Morandat, S.; Garcia-Moreno, M. I.; Deniaud, D.; Matthews, S. E.; Vidal, S.; Sestak, S.; El Kirat, K.; Gouin, S. G. J. Am. Chem. Soc. 2013, 135, 18427–18435. doi:10.1021/ja406931w |
| 11. | Decroocq, C.; Joosten, A.; Sergent, R.; Mena Barragán, T.; Ortiz Mellet, C.; Compain, P. ChemBioChem 2013, 14, 2038–2049. doi:10.1002/cbic.201300283 |
| 43. |
Cardona, F.; Isoldi, G.; Sansone, F.; Casnati, A.; Goti, A. J. Org. Chem. 2012, 77, 6980–6988. doi:10.1021/jo301155p
See for a recent example of multivalent pyrrolidinols based oncalix[4]arene scaffolds. |
© 2014 Lepage et al; licensee Beilstein-Institut.
This is an Open Access article under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
The license is subject to the Beilstein Journal of Organic Chemistry terms and conditions: (http://www.beilstein-journals.org/bjoc)