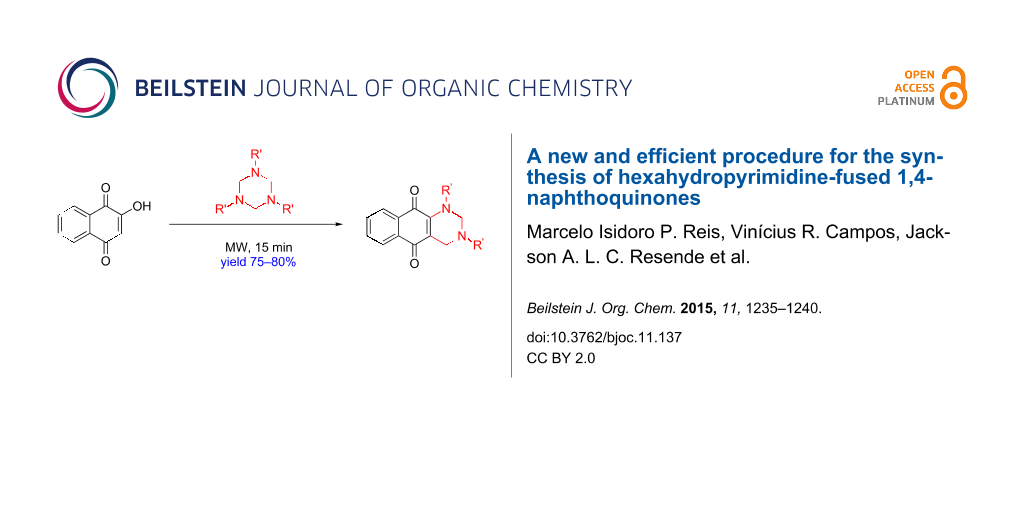
Abstract
A new and efficient method for the synthesis of hexahydropyrimidine-fused 1,4-naphthoquinones in one step with high yields from the reaction of lawsone with 1,3,5-triazinanes was developed.
Graphical Abstract
Introduction
Quinones represent a diverse family of naturally occurring secondary metabolites [1-3]. Interest in these substances has intensified in recent years due to their pharmacological importance [4]. Heterocycle-fused naphthoquinones (Figure 1) such as naphtho[2,3-b]furan [5-14], naphtho-pyran [15-18], benzo[f]indole [19-24], benzo[g]quinolone [25], benzo[b]carbazole [26], naphtho[2,3-b]thiophene [27-33] and naphtho[2,3-b]]oxazole [34] have been demonstrated to display various biological activities. There are several specific and general methods reported in the literature that describe the preparation and biological activity of heterocycle-fused naphthoquinone compounds [35]. Recently, Iida et al. described a general method for the construction of pyrrole-, furan- and thiophene-fused naphthoquinones [36].
Figure 1: General template for heterocycle-fused 1,4-naphthoquinones.
Figure 1: General template for heterocycle-fused 1,4-naphthoquinones.
For several years, our group has been interested in developing new synthetic methods for the preparation of heterocycle-fused 1,4-naphthoquinones or heterocycle-tethered 1,4-naphthoquinones. 1,3-Quinazolines are nitrogenated heterocycles that are present in several products. However, there are only three procedures for the preparation of hexahydropyrimidine-fused 1,4-naphthoquinones available. Some of them are restrictive, troublesome and produce the hexahydropyrimidine-1,4-naphthoquinones in low yields. Möhrle and Herbruggen synthesized unsymmetrical hexahydropyrimidine-fused 1,2- and 1,4-naphthoquinones by the reaction of 4-amino-l,2-naphthoquinone and 2-amino-1,4-naphthoquinone with amines and formaldehyde in moderate yields [37]. Ohta et al. studied the nucleophilic addition reaction of methylamine to 2-bromo-3-hydroxymethyl-1,4-naphthoquinone (1) and obtained hexahydropyrimidine-fused 1,4-naphthoquinone (5) as side product (Scheme 1) [38,39]. This reaction was non-selective and formed a mixture of four products (2–5) that are derived from the addition of methylamine to both of the electrophilic sites of the starting material 1. In the same publication, the authors reported another method that was more selective, which involved the addition of two equivalents of a monoalkyl amine (R-NH2) to menadione (6) to give hexahydropyrimidine-fused 1,4-naphthoquinones 5, 11–13 in low yields.
Scheme 1: Methods for the preparation of hexahydropyrimidine-fused 1,4-naphthoquinones.
Scheme 1: Methods for the preparation of hexahydropyrimidine-fused 1,4-naphthoquinones.
Results and Discussion
Herein we describe a new method for the synthesis of hexahydropyrimidine-fused 1,4-naphthoquinones (13 and 21–25) in high yields, from the sequential reaction of readily available 1,3,5-triazinanes 14–19 with 2-hydroxy-1,4-naphthoquinone (20, or lawsone) under microwave irradiation (Scheme 2).
Scheme 2: Synthesis of hexahydropyrimidine-fused 1,4-naphthoquinones 13 and 21–25.
Scheme 2: Synthesis of hexahydropyrimidine-fused 1,4-naphthoquinones 13 and 21–25.
The 1,3,5-triazinanes have several synthetic and biological applications [40]. These substances are easily prepared from commercially available amines and formaldehyde in toluene in yields ranging from 75–90%. Barluenga and coworkers [41] have previously shown that 1,3,5-triazinanes undergo fragmentation at elevated temperatures to form 3 equivalents of alkyl- or aryl-formimines in situ. The latter compounds may serve as electrophilic agents for aminoalkylation reactions. Our research group also investigated the aminoalkylation of 2-amino-1,4-naphthoquinone with formaldehyde under microwave irradiation to produce two series of N,O-acetals and N,S-acetals. These compounds were obtained in good yields, and several of them showed promising antibacterial activity [42].
The structures of the synthesized 1,3,5-triazinanes were confirmed by spectroscopic techniques such as NMR, 1H and 13C-APT, infrared spectroscopy (FTIR) and high resolution mass spectrometry. The synthesis of compounds 14 [43] and 17 [44] has been previously reported in the literature. The structure of compound 18 was confirmed by X-ray diffraction analysis and Figure 2 shows the ORTEP diagram of this compound. The details of the crystal data and refinements are collected in Supporting Information File 1, Table S1.
![[1860-5397-11-137-2]](/bjoc/content/figures/1860-5397-11-137-2.png?scale=2.0&max-width=1024&background=FFFFFF)
Figure 2: ORTEP diagram of compound 18 depicted with ellipsoids drawn at the 50% probability level and the atom-numbering scheme.
Figure 2: ORTEP diagram of compound 18 depicted with ellipsoids drawn at the 50% probability level and the at...
The crystal structure of compound 18 contains two molecules per asymmetric unit. Two factors explain the differences between molecules A and B (Figure 2): (1) different intermolecular interactions and (2) small differences in the torsion angles of the p-substituted benzyl groups. The 1,3,5-triazinane rings of 18 (molecules A and B) adopt a chair conformation, with Cremer–Pople puckering parameters q2 and Φ2 of 0.023(9) Å and 323.0(20)º, respectively, for molecule A, and q2 and Φ2 of 0.016(3) Å and 2.0(25)º, respectively, for molecule B.
Next the reaction between 1,3,5-triazinanes 14–19 and 2-hydroxy-1,4-naphthoquinone (20) was developed to prepare fused hexahydropyrimidine-1,4-naphthoquinones 13 and 21–25 using in situ generated alkyl formimines. Performing the reaction without heating proceeds very slow and after 24 hours extensive degradation products were observed. By elevating the temperature and or changing the solvent some product is formed but the yields were very low. On the other hand, when the reactions were conducted in an equimolar ratio under microwave irradiation (300 Monowave model brand Aanton Paar) in chloroform for 15 minutes at a temperature of 150 °C, the desired products were obtained in good yields (75–80%, Scheme 2). All structures of the benzo-fused tetrahydroquinazolines were characterized by 1H NMR and 13C-APT, infrared spectroscopy (FTIR) and high resolution mass spectrometry. Using compound 23 as an example, it can be observed that its 1H NMR spectrum contains a doublet of doublets (J = 0.98 and 7.8 Hz) at 7.98 ppm attributed to the hydrogen H-5 of the naphthoquinone moiety, a multiplet at 7.16–7.25 ppm related to the aromatic protons on the phenyl ring (H-3”’–H, 7”’), and four singulets corresponding to the H1’ (C–CH2–N), H1” (N–CH2–N), H-1”’ (N–CH2–Ph) and H-1”” (N–CH2–Ph) methylene protons at 3.60 ppm, 3.77 ppm, 3.84 ppm and 4.75 ppm, respectively. The high resolution mass spectrum of compound 23 showed a molecular ion [M + H]+ at m/z 395.2062, which corresponds with the calculated mass for C26H22N2O2H [M + H]+ of 395.1715. The structure of compound 23 was further elucidated by X-ray diffraction analysis (Figure 3). It confirmed the insertion of two imines to form a hexahydropyrimidine ring which is coupled to a 1,4-naphthoquinone moiety. Figure 3 shows the ORTEP diagram of compound 23 and the details of the crystal data and refinements are given in Supporting Information File 1, Table S2.
![[1860-5397-11-137-3]](/bjoc/content/figures/1860-5397-11-137-3.png?scale=2.0&max-width=1024&background=FFFFFF)
Figure 3: ORTEP diagram of compound 23 depicted with ellipsoids drawn at the 50% probability level and the atom-numbering scheme.
Figure 3: ORTEP diagram of compound 23 depicted with ellipsoids drawn at the 50% probability level and the at...
In the crystal structure, the six-membered nitrogen-containing ring of 23 adopts a half boat conformation (Cremer–Pople puckering parameters [45] q2 and Φ2 of 0.374(9) Å and 119.0(1)) in which the N(3) atom is located at 0.641(2) Å out of the plane of the other five atoms C(2), N(1), C(4a), C(10) and C(4).
A possible mechanism that could explain the formation of the tetrahydrobenzo[g]quinazolines 13 and 21–25 is shown in Scheme 3. It initially involves the in situ formation of three thermally generated iminium ions or the equivalent methylformimines from the corresponding triazinane which then react with lawsone (20) at its two nucleophilic sites, thus forming products (Scheme 3).
Scheme 3: Proposed mechanism for the formation of 13 and 21–25.
Scheme 3: Proposed mechanism for the formation of 13 and 21–25.
Conclusion
A new method for the synthesis of new heterocyclic hexahydropyrimidines fused to a 1,4-naphthoquinone system (13 and 21–25) was developed. The products were obtained in one step, under microwave irradiation and with excellent yields. This method is a more efficient alternative for the preparation of benzo-fused tetrahydroquinazolindiones than the method described in the literature. The structures of all starting 1,3,5-triazinanes and products were confirmed by spectroscopical methods and X-ray diffraction analysis.
Supporting Information
| Supporting Information File 1: Experimental procedures and spectral data. | ||
| Format: PDF | Size: 3.4 MB | Download |
References
-
Patai, S. The chemistry of the quinoid compounds; John Wiley & Sons: London, England, 1974.
Return to citation in text: [1] -
Patai, S.; Rappoport, Z. The Chemistry of the Functional Groups. The Chemistry of the Quinonoid Compounds; John Wiley & Sons: New York, USA, 1988.
Return to citation in text: [1] -
Thomson, R. H. Naturally Occurring Quinones IV; Blackie Academic & Professional: London, England, 1997.
Return to citation in text: [1] -
Ferreira, V. F.; da Rocha, D. R.; da Silva, F. C.; Ferreira, P. G.; Boechat, N. A.; Magalhães, J. L. Expert Opin. Ther. Pat. 2013, 23, 319–331. doi:10.1517/13543776.2013.749862
Return to citation in text: [1] -
Rao, M. M.; Kingston, D. G. I. J. Nat. Prod. 1982, 45, 600–604. doi:10.1021/np50023a014
Return to citation in text: [1] -
Cardoso, M. F. C.; Rodrigues, P. C.; Oliveira, M. E. I. M.; Gama, I. L.; da Silva, I. M. C. B.; Santos, I. O.; Rocha, D. R.; Pinho, R. T.; Ferreira, V. F.; de Souza, M. C. B. V.; da Silva, F. d. C.; Silva, F. P., Jr. Eur. J. Med. Chem. 2014, 84, 708–717. doi:10.1016/j.ejmech.2014.07.079
Return to citation in text: [1] -
Eyong, K. O.; Kumar, P. S.; Kuete, V.; Folefoc, G. N.; Nkengfack, E. A.; Baskaran, S. Bioorg. Med. Chem. Lett. 2008, 18, 5387–5390. doi:10.1016/j.bmcl.2008.09.053
Return to citation in text: [1] -
Ferreira, M. d. P. S. B. C.; Cardoso, M. F. d. C.; da Silva, F. d. C.; Ferreira, V. F.; Lima, E. S.; Souza, J. V. B. Ann. Clin. Microbiol. Antimicrob. 2014, 13, No. 26. doi:10.1186/1476-0711-13-26
Return to citation in text: [1] -
Teimouri, M. B.; Khavasi, H. R. Tetrahedron 2007, 63, 10269–10275. doi:10.1016/j.tet.2007.07.082
Return to citation in text: [1] -
da Silva, A. J. M.; Netto, C. D.; Pacienza-Lima, W.; Torres-Santos, E. C.; Rossi-Bergmann, B.; Maurel, S.; Valentin, A.; Costa, P. R. R. J. Braz. Chem. Soc. 2009, 20, 176–182. doi:10.1590/S0103-50532009000100026
Return to citation in text: [1] -
Ueda, S.; Umemura, T.; Dohguchi, K.; Matsuzaki, T.; Tokuda, H.; Nishino, H.; Iwashima, A. Phytochemistry 1994, 36, 323–325. doi:10.1016/S0031-9422(00)97069-9
Return to citation in text: [1] -
Wu, C.; Johnson, R. K.; Mattern, M. R.; Wong, J. C.; Kingston, D. G. I. J. Nat. Prod. 1999, 62, 963–968. doi:10.1021/np9900019
Return to citation in text: [1] -
Jiménez-Alonso, S.; Guasch, J.; Estévez-Braun, A.; Ratera, I.; Veciana, J.; Ravelo, A. G. J. Org. Chem. 2011, 76, 1634–1643. doi:10.1021/jo102233j
Return to citation in text: [1] -
Reichstein, A.; Vortherms, S.; Bannwitz, S.; Tentrop, J.; Prinz, H.; Müller, K. J. Med. Chem. 2012, 55, 7273–7284. doi:10.1021/jm3009597
Return to citation in text: [1] -
Ferreira, S. B.; da Silva, F. d. C.; Pinto, A. C.; Gonzaga, D. T. G.; Ferreira, V. F. J. Heterocycl. Chem. 2009, 46, 1080–1097. doi:10.1002/jhet.232
Return to citation in text: [1] -
da Costa, E. C. B.; Amorim, R.; da Silva, F. C.; Rocha, D. R.; Papa, M. P.; de Arruda, L. B.; Mohana-Borges, R.; Ferreira, V. F.; Tanuri, A.; da Costa, L. J.; Ferreira, S. B. PLoS One 2013, 8, e82504. doi:10.1371/journal.pone.0082504
Return to citation in text: [1] -
da Rocha, D. R.; de Souza, A. C. G.; Resende, J. A. L. C.; Santos, W. C.; dos Santos, E. A.; Pessoa, C.; de Moraes, M. O.; Costa-Lotufo, L. V.; Montenegro, R. C.; Ferreira, V. F. Org. Biomol. Chem. 2011, 9, 4315–4322. doi:10.1039/c1ob05209h
Return to citation in text: [1] -
Ferreira, V. F.; Ferreira, S. B.; da Silva, F. d. C. Org. Biomol. Chem. 2010, 8, 4793–4802. doi:10.1039/c0ob00277a
Return to citation in text: [1] -
Tseng, C.-M.; Wu, Y.-L.; Chuang, C.-P. Tetrahedron 2004, 60, 12249–12260. doi:10.1016/j.tet.2004.10.029
Return to citation in text: [1] -
Shvartsberg, M. S.; Kolodina, E. A.; Lebedeva, N. I.; Fedenok, L. G. Tetrahedron Lett. 2009, 50, 6769–6771. doi:10.1016/j.tetlet.2009.09.110
Return to citation in text: [1] -
Inman, M.; Moody, C. J. J. Org. Chem. 2010, 75, 6023–6026. doi:10.1021/jo101071c
Return to citation in text: [1] -
Acosta, A.; de la Cruz, P.; De Miguel, P.; Diez-Barra, E.; de la Hoz, A.; Langa, F.; Loupy, A.; Majdoub, M.; Martin, N.; Sanchez, C.; Seoane, C. Tetrahedron Lett. 1995, 36, 2165–2168. doi:10.1016/0040-4039(95)00202-N
Return to citation in text: [1] -
Oostveen, E. A.; Speckamp, W. N. Tetrahedron 1987, 43, 255–262. doi:10.1016/S0040-4020(01)89952-X
Return to citation in text: [1] -
Comer, E.; Murphy, W. S. ARKIVOC 2003, 7, 286–296.
Return to citation in text: [1] -
Bala, B. D.; Muthusaravanan, S.; Perumal, S. Tetrahedron Lett. 2013, 54, 3735–3739. doi:10.1016/j.tetlet.2013.04.125
Return to citation in text: [1] -
Inoue, A.; Nomura, Y.; Kuroki, N.; Konishi, K. J. Synth. Org. Chem., Jpn. 1958, 16, 536–540. doi:10.5059/yukigoseikyokaishi.16.536
Return to citation in text: [1] -
Kobayashi, K.; Yoneda, K.; Uchida, M.; Matsuoka, H.; Morikawa, O.; Konishi, H. Heterocycles 2001, 55, 2423–2430. doi:10.3987/COM-01-9358
Return to citation in text: [1] -
Valderrama, J. A.; Astudillo, C.; Tapia, R. A.; Prina, E.; Estrabaud, E.; Mahieux, R.; Fournet, A. Chem. Pharm. Bull. 2002, 50, 1215–1218. doi:10.1248/cpb.50.1215
Return to citation in text: [1] -
Tapia, R. A.; Alegria, L.; Pessoa, C. D.; Salas, C.; Cortés, M. J.; Valderrama, J. A.; Sarciron, M.-E.; Pautet, F.; Walchshofer, N.; Fillion, H. Bioorg. Med. Chem. 2003, 11, 2175–2182. doi:10.1016/S0968-0896(03)00122-6
Return to citation in text: [1] -
Ryu, C.-K.; Choi, I. H.; Lee, J. Y.; Jung, S. H. Heterocycles 2005, 65, 1205–1214. doi:10.3987/COM-05-10341
Return to citation in text: [1] -
Valderrama, J. A.; Espinoza, O.; Rodriguez, J.; Theoduloz, C. Lett. Org. Chem. 2009, 6, 278–281. doi:10.2174/157017809788490006
Return to citation in text: [1] -
Campiglia, P.; Aquino, C.; Bertamino, A.; De Simone, N.; Sala, M.; Castellano, S.; Santoriello, M.; Grieco, P.; Novellino, E.; Gomez-Monterrey, I. M. Org. Biomol. Chem. 2010, 8, 622–627. doi:10.1039/b918898c
Return to citation in text: [1] -
Gomez-Monterrey, I.; Campiglia, P.; Aquino, C.; Bertamino, A.; Granata, I.; Carotenuto, A.; Brancaccio, D.; Stiuso, P.; Scognamiglio, I.; Rusciano, M. R.; Maione, A. S.; Illario, M.; Grieco, P.; Maresca, B.; Novellino, E. J. Med. Chem. 2011, 54, 4077–4091. doi:10.1021/jm200094h
Return to citation in text: [1] -
Hamdan, A. J.; Al-Jaroudi, S. Arabian J. Sci. Eng., Sect. A 2003, 28, 51.
Return to citation in text: [1] -
Sartori, M. F. Chem. Rev. 1963, 63, 279–296. doi:10.1021/cr60223a005
Return to citation in text: [1] -
Ueda, K.; Yamashita, M.; Sakaguchi, K.; Tokuda, H.; Iida, A. Chem. Pharm. Bull. 2013, 61, 648–654. doi:10.1248/cpb.c13-00102
Return to citation in text: [1] -
Möhrle, H.; Schulte Herbrüggen, G. Arch. Pharm. 1991, 324, 165–171. doi:10.1002/ardp.19913240307
Return to citation in text: [1] -
Ohta, S.; Hinata, Y.; Yamashita, M.; Kawasaki, I.; Jinda, Y.; Horie, S. Chem. Pharm. Bull. 1994, 42, 1730–1735. doi:10.1248/cpb.42.1730
Return to citation in text: [1] -
Ohta, S.; Hinata, Y.; Yamashita, M.; Kawasaki, I.; Shoji, T.; Yoshikawa, H.; Obana, Y. Chem. Pharm. Bull. 1994, 42, 1185–1190. doi:10.1248/cpb.42.1185
Return to citation in text: [1] -
Reis, M. I. P.; Romeiro, G. A.; Damasceno, R.; da Silva, F. d. C.; Ferreira, V. F. Rev. Virtual Quim. 2013, 5, 283–299. doi:10.5935/1984-6835.20130027
Return to citation in text: [1] -
Barluenga, M.; Bayón, A. M.; Campos, P.; Asensio, G.; Gonzalez-Nuñez, E.; Molina, Y. J. Chem. Soc., Perkin Trans. 1 1988, 1631–1636. doi:10.1039/P19880001631
Return to citation in text: [1] -
Jordão, A. K.; Novais, J.; Leal, B.; Escobar, A. C.; dos Santos Júnior, H. M.; Castro, H. C.; Ferreira, V. F. Eur. J. Med. Chem. 2013, 63, 196–201. doi:10.1016/j.ejmech.2013.01.010
Return to citation in text: [1] -
Guillemin, J. C.; Denis, J. M. J. Chem. Soc., Chem. Commun. 1985, 951–952. doi:10.1039/C39850000951
Return to citation in text: [1] -
Smith, G. S.; Berlin, K. D.; Zisman, S. A.; Holt, E. M.; Green, V. A.; Van Der Helm, D. Phosphorus Sulfur Relat. Elem. 1988, 39, 91–111. doi:10.1080/03086648808072860
Return to citation in text: [1] -
Cremer, D.; Pople, J. A. J. Am. Chem. Soc. 1975, 97, 1354–1358. doi:10.1021/ja00839a011
Return to citation in text: [1]
| 45. | Cremer, D.; Pople, J. A. J. Am. Chem. Soc. 1975, 97, 1354–1358. doi:10.1021/ja00839a011 |
| 43. | Guillemin, J. C.; Denis, J. M. J. Chem. Soc., Chem. Commun. 1985, 951–952. doi:10.1039/C39850000951 |
| 44. | Smith, G. S.; Berlin, K. D.; Zisman, S. A.; Holt, E. M.; Green, V. A.; Van Der Helm, D. Phosphorus Sulfur Relat. Elem. 1988, 39, 91–111. doi:10.1080/03086648808072860 |
| 1. | Patai, S. The chemistry of the quinoid compounds; John Wiley & Sons: London, England, 1974. |
| 2. | Patai, S.; Rappoport, Z. The Chemistry of the Functional Groups. The Chemistry of the Quinonoid Compounds; John Wiley & Sons: New York, USA, 1988. |
| 3. | Thomson, R. H. Naturally Occurring Quinones IV; Blackie Academic & Professional: London, England, 1997. |
| 19. | Tseng, C.-M.; Wu, Y.-L.; Chuang, C.-P. Tetrahedron 2004, 60, 12249–12260. doi:10.1016/j.tet.2004.10.029 |
| 20. | Shvartsberg, M. S.; Kolodina, E. A.; Lebedeva, N. I.; Fedenok, L. G. Tetrahedron Lett. 2009, 50, 6769–6771. doi:10.1016/j.tetlet.2009.09.110 |
| 21. | Inman, M.; Moody, C. J. J. Org. Chem. 2010, 75, 6023–6026. doi:10.1021/jo101071c |
| 22. | Acosta, A.; de la Cruz, P.; De Miguel, P.; Diez-Barra, E.; de la Hoz, A.; Langa, F.; Loupy, A.; Majdoub, M.; Martin, N.; Sanchez, C.; Seoane, C. Tetrahedron Lett. 1995, 36, 2165–2168. doi:10.1016/0040-4039(95)00202-N |
| 23. | Oostveen, E. A.; Speckamp, W. N. Tetrahedron 1987, 43, 255–262. doi:10.1016/S0040-4020(01)89952-X |
| 24. | Comer, E.; Murphy, W. S. ARKIVOC 2003, 7, 286–296. |
| 41. | Barluenga, M.; Bayón, A. M.; Campos, P.; Asensio, G.; Gonzalez-Nuñez, E.; Molina, Y. J. Chem. Soc., Perkin Trans. 1 1988, 1631–1636. doi:10.1039/P19880001631 |
| 15. | Ferreira, S. B.; da Silva, F. d. C.; Pinto, A. C.; Gonzaga, D. T. G.; Ferreira, V. F. J. Heterocycl. Chem. 2009, 46, 1080–1097. doi:10.1002/jhet.232 |
| 16. | da Costa, E. C. B.; Amorim, R.; da Silva, F. C.; Rocha, D. R.; Papa, M. P.; de Arruda, L. B.; Mohana-Borges, R.; Ferreira, V. F.; Tanuri, A.; da Costa, L. J.; Ferreira, S. B. PLoS One 2013, 8, e82504. doi:10.1371/journal.pone.0082504 |
| 17. | da Rocha, D. R.; de Souza, A. C. G.; Resende, J. A. L. C.; Santos, W. C.; dos Santos, E. A.; Pessoa, C.; de Moraes, M. O.; Costa-Lotufo, L. V.; Montenegro, R. C.; Ferreira, V. F. Org. Biomol. Chem. 2011, 9, 4315–4322. doi:10.1039/c1ob05209h |
| 18. | Ferreira, V. F.; Ferreira, S. B.; da Silva, F. d. C. Org. Biomol. Chem. 2010, 8, 4793–4802. doi:10.1039/c0ob00277a |
| 42. | Jordão, A. K.; Novais, J.; Leal, B.; Escobar, A. C.; dos Santos Júnior, H. M.; Castro, H. C.; Ferreira, V. F. Eur. J. Med. Chem. 2013, 63, 196–201. doi:10.1016/j.ejmech.2013.01.010 |
| 5. | Rao, M. M.; Kingston, D. G. I. J. Nat. Prod. 1982, 45, 600–604. doi:10.1021/np50023a014 |
| 6. | Cardoso, M. F. C.; Rodrigues, P. C.; Oliveira, M. E. I. M.; Gama, I. L.; da Silva, I. M. C. B.; Santos, I. O.; Rocha, D. R.; Pinho, R. T.; Ferreira, V. F.; de Souza, M. C. B. V.; da Silva, F. d. C.; Silva, F. P., Jr. Eur. J. Med. Chem. 2014, 84, 708–717. doi:10.1016/j.ejmech.2014.07.079 |
| 7. | Eyong, K. O.; Kumar, P. S.; Kuete, V.; Folefoc, G. N.; Nkengfack, E. A.; Baskaran, S. Bioorg. Med. Chem. Lett. 2008, 18, 5387–5390. doi:10.1016/j.bmcl.2008.09.053 |
| 8. | Ferreira, M. d. P. S. B. C.; Cardoso, M. F. d. C.; da Silva, F. d. C.; Ferreira, V. F.; Lima, E. S.; Souza, J. V. B. Ann. Clin. Microbiol. Antimicrob. 2014, 13, No. 26. doi:10.1186/1476-0711-13-26 |
| 9. | Teimouri, M. B.; Khavasi, H. R. Tetrahedron 2007, 63, 10269–10275. doi:10.1016/j.tet.2007.07.082 |
| 10. | da Silva, A. J. M.; Netto, C. D.; Pacienza-Lima, W.; Torres-Santos, E. C.; Rossi-Bergmann, B.; Maurel, S.; Valentin, A.; Costa, P. R. R. J. Braz. Chem. Soc. 2009, 20, 176–182. doi:10.1590/S0103-50532009000100026 |
| 11. | Ueda, S.; Umemura, T.; Dohguchi, K.; Matsuzaki, T.; Tokuda, H.; Nishino, H.; Iwashima, A. Phytochemistry 1994, 36, 323–325. doi:10.1016/S0031-9422(00)97069-9 |
| 12. | Wu, C.; Johnson, R. K.; Mattern, M. R.; Wong, J. C.; Kingston, D. G. I. J. Nat. Prod. 1999, 62, 963–968. doi:10.1021/np9900019 |
| 13. | Jiménez-Alonso, S.; Guasch, J.; Estévez-Braun, A.; Ratera, I.; Veciana, J.; Ravelo, A. G. J. Org. Chem. 2011, 76, 1634–1643. doi:10.1021/jo102233j |
| 14. | Reichstein, A.; Vortherms, S.; Bannwitz, S.; Tentrop, J.; Prinz, H.; Müller, K. J. Med. Chem. 2012, 55, 7273–7284. doi:10.1021/jm3009597 |
| 38. | Ohta, S.; Hinata, Y.; Yamashita, M.; Kawasaki, I.; Jinda, Y.; Horie, S. Chem. Pharm. Bull. 1994, 42, 1730–1735. doi:10.1248/cpb.42.1730 |
| 39. | Ohta, S.; Hinata, Y.; Yamashita, M.; Kawasaki, I.; Shoji, T.; Yoshikawa, H.; Obana, Y. Chem. Pharm. Bull. 1994, 42, 1185–1190. doi:10.1248/cpb.42.1185 |
| 4. | Ferreira, V. F.; da Rocha, D. R.; da Silva, F. C.; Ferreira, P. G.; Boechat, N. A.; Magalhães, J. L. Expert Opin. Ther. Pat. 2013, 23, 319–331. doi:10.1517/13543776.2013.749862 |
| 40. | Reis, M. I. P.; Romeiro, G. A.; Damasceno, R.; da Silva, F. d. C.; Ferreira, V. F. Rev. Virtual Quim. 2013, 5, 283–299. doi:10.5935/1984-6835.20130027 |
| 36. | Ueda, K.; Yamashita, M.; Sakaguchi, K.; Tokuda, H.; Iida, A. Chem. Pharm. Bull. 2013, 61, 648–654. doi:10.1248/cpb.c13-00102 |
| 27. | Kobayashi, K.; Yoneda, K.; Uchida, M.; Matsuoka, H.; Morikawa, O.; Konishi, H. Heterocycles 2001, 55, 2423–2430. doi:10.3987/COM-01-9358 |
| 28. | Valderrama, J. A.; Astudillo, C.; Tapia, R. A.; Prina, E.; Estrabaud, E.; Mahieux, R.; Fournet, A. Chem. Pharm. Bull. 2002, 50, 1215–1218. doi:10.1248/cpb.50.1215 |
| 29. | Tapia, R. A.; Alegria, L.; Pessoa, C. D.; Salas, C.; Cortés, M. J.; Valderrama, J. A.; Sarciron, M.-E.; Pautet, F.; Walchshofer, N.; Fillion, H. Bioorg. Med. Chem. 2003, 11, 2175–2182. doi:10.1016/S0968-0896(03)00122-6 |
| 30. | Ryu, C.-K.; Choi, I. H.; Lee, J. Y.; Jung, S. H. Heterocycles 2005, 65, 1205–1214. doi:10.3987/COM-05-10341 |
| 31. | Valderrama, J. A.; Espinoza, O.; Rodriguez, J.; Theoduloz, C. Lett. Org. Chem. 2009, 6, 278–281. doi:10.2174/157017809788490006 |
| 32. | Campiglia, P.; Aquino, C.; Bertamino, A.; De Simone, N.; Sala, M.; Castellano, S.; Santoriello, M.; Grieco, P.; Novellino, E.; Gomez-Monterrey, I. M. Org. Biomol. Chem. 2010, 8, 622–627. doi:10.1039/b918898c |
| 33. | Gomez-Monterrey, I.; Campiglia, P.; Aquino, C.; Bertamino, A.; Granata, I.; Carotenuto, A.; Brancaccio, D.; Stiuso, P.; Scognamiglio, I.; Rusciano, M. R.; Maione, A. S.; Illario, M.; Grieco, P.; Maresca, B.; Novellino, E. J. Med. Chem. 2011, 54, 4077–4091. doi:10.1021/jm200094h |
| 37. | Möhrle, H.; Schulte Herbrüggen, G. Arch. Pharm. 1991, 324, 165–171. doi:10.1002/ardp.19913240307 |
| 26. | Inoue, A.; Nomura, Y.; Kuroki, N.; Konishi, K. J. Synth. Org. Chem., Jpn. 1958, 16, 536–540. doi:10.5059/yukigoseikyokaishi.16.536 |
| 25. | Bala, B. D.; Muthusaravanan, S.; Perumal, S. Tetrahedron Lett. 2013, 54, 3735–3739. doi:10.1016/j.tetlet.2013.04.125 |
© 2015 Reis et al; licensee Beilstein-Institut.
This is an Open Access article under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
The license is subject to the Beilstein Journal of Organic Chemistry terms and conditions: (http://www.beilstein-journals.org/bjoc)