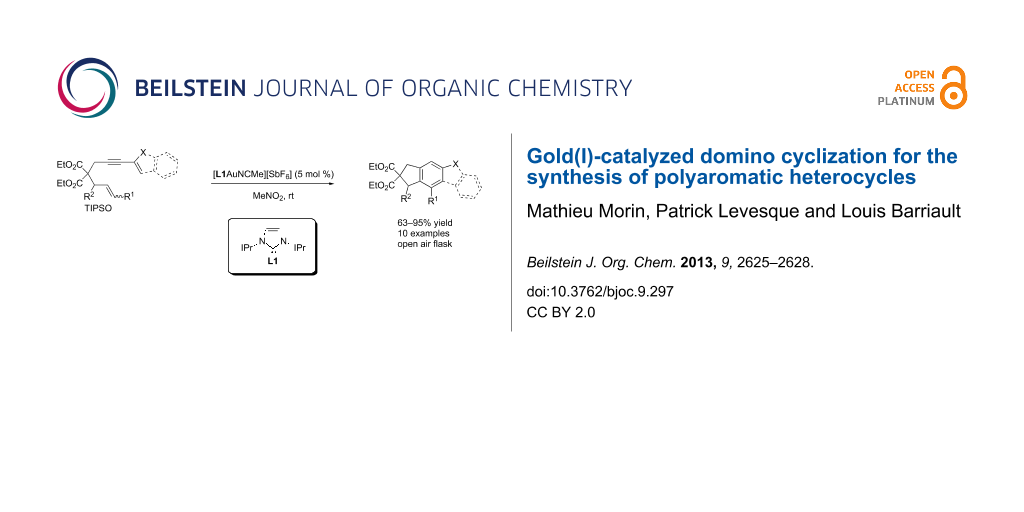
Abstract
Gold(I) complexes have emerged as powerful and useful catalysts for the formation of new C–C, C–O and C–N bonds. Taking advantage of the specificity of [IPrAuNCMe][SbF6] complexes to favor the 5-exo-dig cyclization over the 6-endo-dig pathway, we report a high yielding and efficient method to generate substituted polyaromatic heterocycles under remarkably mild reaction conditions.
Graphical Abstract
Introduction
In the last decade, phosphino and NHC–gold complexes have become prominent catalysts for the addition of nucleophiles to alkenes, alkynes and allenes [1-11]. Owing to the high affinity of gold(I) complexes to C–C π-systems in the presence of other functional groups combined by its predictable reactivity pattern, the gold(I)-catalyzed reaction provides tremendous opportunities for the discovery of new and useful reactions [12]. Recently, we [13] and other groups [14-17] reported that divergent pathway can be obtained by modulating the steric and electronic properties of the gold(I) catalyst (Scheme 1). The ancillary ligand plays a direct role in the regioselectivity for the first bond formation rather than via a common intermediate created after an initial bond formation [18]. Indeed, the cyclization of cyclic enol ether 1 using σ-donor ligands such as IPr (L1) [19] was exceptionally selective for the 5-exo-dig pathway (1→2) whereas bulky Me4XPhos (L2) [12] gave mainly 6-endo-dig-cyclized product 3.
Scheme 1: Gold(I)-catalyzed carbocyclization.
Scheme 1: Gold(I)-catalyzed carbocyclization.
Results and Discussion
During the course of our investigation, we examined the cyclization of non-cyclic enol ethers. As expected, the cyclization of enol ether 4 using [L2AuNCMe][SbF6] in dichloromethane afforded the cyclohexene 5 in 79% yield (Scheme 2). However, the anticipated 5-exo-dig product 6 was not observed when the catalyst [L1AuNCMe][SbF6] was utilized. Instead, the benzothiophene 7 was isolated in 89% yield. The formation of this unforeseen product can be explained by the proposed mechanism illustrated in Scheme 2. The gold(I) complexation of alkyne 4 triggers the 5-exo-dig cyclization to produce intermediate 9. At this point, a nucleophilic addition of the thiophene unit to the carboxonium provides the sulfonium 10 which upon protodeauration and aromatization gives 7 [20-25]. Other polar solvents such as acetone and dichloromethane were employed without much success. One might consider that the high polarity of nitromethane helps to alleviate the charge build-up at the cationic cyclization transition state.
Scheme 2: Proposed mechanism for the gold(I)-catalyzed cyclization.
Scheme 2: Proposed mechanism for the gold(I)-catalyzed cyclization.
Substituted aromatic compounds have a fundamental importance in organic and medicinal chemistry as well as in materials. Although there are many methods to functionalize aromatic rings, one can recognize that the above transformation represents an attractive and complementary approach for the synthesis of substituted aromatic rings. Taking advantage of the high regiospecificity of [L1AuNCMe][SbF6] associated with alkyne activation, we examined the scope of the reaction using various substituted alkynes (Scheme 3). Gold(I)-catalyzed cyclizations of the enol ether 11a (R1 = p-BrC6H4, R2 = H) gave the corresponding benzothiophene 12a in 83% yield. The use of electron-poor silyl enol ether 11b (R1 = p-NO2C6H4, R2 = H) gave the desired product 12b, albeit in lower yield of 63%. Di- and trisubstituted silyl enol ethers 11c (R1 and R2 = H) and 11d (R1 = H and R2 = Me) were converted to benzothiophenes 12c and 12d in 82% and 95% yield, respectively. The synthesis of substituted hydrindene 12e was also achieved in 85% yield from monosubstituted enyne 11e (R1 = Ph, R2 = H). Substituted enynes bearing heterocycles such as indole 11f (R1 and R2 = H) and furan 11g (R1 = Ph and R2 = H) were effectively transformed to the desired carbazole 12f and benzofuran 12g in 95% yields. It can be noticed that large substituents at R1 and R2 did not affect the efficiency of the reaction. The gold(I)-catalyzed cyclization of 11h (R1 = R2 = Ph) and 11i (R1 = Ph and R2 = Me) provided the corresponding benzothiophenes 12h and 12i in 91% and 87% yield, respectively.
Scheme 3: Gold-catalyzed 5-exo-dig carbocyclization cascade.
Scheme 3: Gold-catalyzed 5-exo-dig carbocyclization cascade.
Conclusion
In summary, we have developed a mild and efficient gold(I)-catalyzed 5-exo-dig polycyclization cascade to prepare an array of substituted aromatic compounds such as benzofuran, benzothiophene, carbazole and hydrindene in high yields. The use of σ-donor ligands such as IPr (L1) was exceptionally selective for the 5-exo-dig pathway. This Au(I)-catalyzed cyclization occurring in cascade provides a direct access to synthetically useful motifs commonly found in natural products and important medicinally compounds. The application of this method in the total syntheses of senaequidolide (13) [26] and ellipticine (14) [27,28] are currently underway and will be reported in due course (Figure 1).
Figure 1: Structure of senaequidolide (13) and ellipticine (14).
Figure 1: Structure of senaequidolide (13) and ellipticine (14).
Experimental
General experimental procedure for the Au(I)-catalyzed cyclization: In a flask equipped with a magnetic stirrer was added the silyl enol ether 11 (0.101 mmol) followed by nitromethane (1 mL) and [L1AuNCMe][SbF6] (0.005 mmol). After stirring overnight, the reaction mixture was concentrated in vacuo and the crude mixture was purified by flash chromatography (1–5% ethyl acetate/hexanes) to give the desired cyclized product 12.
Supporting Information
| Supporting Information File 1: Materials and methods, experimental procedures for 4 and 11a–i, characterization data for 5, 7 and 12a–i, 1H and 13C NMR spectra for all cyclized compounds. | ||
| Format: PDF | Size: 1.4 MB | Download |
References
-
Gorin, D. J.; Toste, F. D. Nature 2007, 446, 395. doi:10.1038/nature05592
Return to citation in text: [1] -
Jiménez-Núñez, E.; Echavarren, A. M. Chem. Commun. 2007, 333. doi:10.1039/B612008C
Return to citation in text: [1] -
Fürtsner, A.; Davies, P. W. Angew. Chem., Int. Ed. 2007, 46, 3410. doi:10.1002/anie.200604335
Return to citation in text: [1] -
Hashmi, A. S. K. Chem. Rev. 2007, 107, 3180. doi:10.1021/cr000436x
Return to citation in text: [1] -
Gorin, D. J.; Sherry, B. D.; Toste, F. D. Chem. Rev. 2008, 108, 3351. doi:10.1021/cr068430g
Return to citation in text: [1] -
Li, Z.; Brouwer, C.; He, C. Chem. Rev. 2008, 108, 3239. doi:10.1021/cr068434l
Return to citation in text: [1] -
Arcadi, A. Chem. Rev. 2008, 108, 3266. doi:10.1021/cr068435d
Return to citation in text: [1] -
Skouta, R.; Li, C.-J. Tetrahedron 2008, 64, 4917. doi:10.1016/j.tet.2008.03.083
Return to citation in text: [1] -
Shapiro, N. D.; Toste, F. D. Synlett 2010, 5, 675. doi:10.1055/s-0029-1219369
Return to citation in text: [1] -
Wagner, W. A.; Auzias, M. Angew. Chem., Int. Ed. 2011, 50, 8236. doi:10.1002/anie.201101603
Return to citation in text: [1] -
Watson, I. D. G.; Toste, F. D. Chem. Sci. 2012, 3, 2899. doi:10.1039/c2sc20542d
Return to citation in text: [1] -
Hashmi, A. S. K.; Rudolph, M. Chem. Soc. Rev. 2008, 37, 1766. doi:10.1039/b615629k
Return to citation in text: [1] [2] -
Barabé, F.; Levesque, P.; Korobkov, I.; Barriault, L. Org. Lett. 2011, 13, 5580. doi:10.1021/ol202314q
Return to citation in text: [1] -
Mauleón, P.; Zeldin, R. M.; González, A. Z.; Toste, F. D. J. Am. Chem. Soc. 2009, 131, 6348. doi:10.1021/ja901649s
Return to citation in text: [1] -
Benitez, D.; Shapiro, N. D.; Tkatchouk, E.; Wang, Y.; Goddard, W. A., III; Toste, F. D. Nat. Chem. 2009, 1, 482. doi:10.1038/nchem.331
Return to citation in text: [1] -
Alcarazo, M.; Stork, T.; Anoop, A.; Thiel, W.; Fürstner, A. Angew. Chem., Int. Ed. 2010, 49, 2542. doi:10.1002/anie.200907194
Return to citation in text: [1] -
Benitez, D.; Tkatchouk, E.; Gonzalez, A. Z.; Goddard, W. A., III; Toste, F. D. Org. Lett. 2009, 11, 4798. doi:10.1021/ol9018002
Return to citation in text: [1] -
Barabé, F.; Levesque, P.; Sow, B.; Bellavance, G.; Bétournay, G.; Barriault, L. Pure Appl. Chem. 2013, 85, 1161. doi:10.1351/pac-con-13-01-02
Return to citation in text: [1] -
de Frémont, P.; Scott, N. M.; Stevens, E. D.; Nolan, S. P. Organometallics 2005, 24, 2411. doi:10.1021/om050111c
Return to citation in text: [1] -
Nieto-Oberhuber, C.; López, S.; Echavarren, A. M. J. Am. Chem. Soc. 2005, 127, 6178. doi:10.1021/ja042257t
Return to citation in text: [1] -
Nieto-Oberhuber, C.; Pérez-Galán, P.; Herrero-Gómez, E.; Lauterbach, T.; Rodriguez, C.; López, S.; Bour, C.; Rosellón, A.; Cárdenas, D. J.; Echavarren, A. M. J. Am. Chem. Soc. 2008, 130, 269. doi:10.1021/ja075794x
Return to citation in text: [1] -
Yeh, M.-C. P.; Tsao, W.-C.; Lee, B.-J.; Lin, T.-L. Organometallics 2008, 27, 5326. doi:10.1021/om8005917
Return to citation in text: [1] -
Pérez-Galán, P.; Martin, N. J. A.; Campaña, A.; Cárdenas, D. J.; Echavarren, A. M. Chem.–Asian J. 2011, 6, 482. doi:10.1002/asia.201000557
Return to citation in text: [1] -
Dateer, R. B.; Shaibu, B. S.; Liu, R.-S. Angew. Chem., Int. Ed. 2012, 51, 113. doi:10.1002/anie.201105921
Return to citation in text: [1] -
Brazeau, J.-F.; Zhang, S.; Colomer, I.; Corkey, B. K.; Toste, F. D. J. Am. Chem. Soc. 2012, 134, 2742. doi:10.1021/ja210388g
Return to citation in text: [1] -
Bohlmann, F.; Ziesche, J.; King, R. M.; Robinson, H. Phytochemistry 1980, 19, 2675. doi:10.1016/S0031-9422(00)83942-4
Return to citation in text: [1] -
Goodwin, S.; Smith, A. F.; Horning, E. C. J. Am. Chem. Soc. 1959, 81, 1903. doi:10.1021/ja01517a031
Return to citation in text: [1] -
Miller, M. C.; McCarthy, F. O. RSC Adv. 2012, 2, 8883. doi:10.1039/c2ra20584j
Return to citation in text: [1]
| 1. | Gorin, D. J.; Toste, F. D. Nature 2007, 446, 395. doi:10.1038/nature05592 |
| 2. | Jiménez-Núñez, E.; Echavarren, A. M. Chem. Commun. 2007, 333. doi:10.1039/B612008C |
| 3. | Fürtsner, A.; Davies, P. W. Angew. Chem., Int. Ed. 2007, 46, 3410. doi:10.1002/anie.200604335 |
| 4. | Hashmi, A. S. K. Chem. Rev. 2007, 107, 3180. doi:10.1021/cr000436x |
| 5. | Gorin, D. J.; Sherry, B. D.; Toste, F. D. Chem. Rev. 2008, 108, 3351. doi:10.1021/cr068430g |
| 6. | Li, Z.; Brouwer, C.; He, C. Chem. Rev. 2008, 108, 3239. doi:10.1021/cr068434l |
| 7. | Arcadi, A. Chem. Rev. 2008, 108, 3266. doi:10.1021/cr068435d |
| 8. | Skouta, R.; Li, C.-J. Tetrahedron 2008, 64, 4917. doi:10.1016/j.tet.2008.03.083 |
| 9. | Shapiro, N. D.; Toste, F. D. Synlett 2010, 5, 675. doi:10.1055/s-0029-1219369 |
| 10. | Wagner, W. A.; Auzias, M. Angew. Chem., Int. Ed. 2011, 50, 8236. doi:10.1002/anie.201101603 |
| 11. | Watson, I. D. G.; Toste, F. D. Chem. Sci. 2012, 3, 2899. doi:10.1039/c2sc20542d |
| 18. | Barabé, F.; Levesque, P.; Sow, B.; Bellavance, G.; Bétournay, G.; Barriault, L. Pure Appl. Chem. 2013, 85, 1161. doi:10.1351/pac-con-13-01-02 |
| 14. | Mauleón, P.; Zeldin, R. M.; González, A. Z.; Toste, F. D. J. Am. Chem. Soc. 2009, 131, 6348. doi:10.1021/ja901649s |
| 15. | Benitez, D.; Shapiro, N. D.; Tkatchouk, E.; Wang, Y.; Goddard, W. A., III; Toste, F. D. Nat. Chem. 2009, 1, 482. doi:10.1038/nchem.331 |
| 16. | Alcarazo, M.; Stork, T.; Anoop, A.; Thiel, W.; Fürstner, A. Angew. Chem., Int. Ed. 2010, 49, 2542. doi:10.1002/anie.200907194 |
| 17. | Benitez, D.; Tkatchouk, E.; Gonzalez, A. Z.; Goddard, W. A., III; Toste, F. D. Org. Lett. 2009, 11, 4798. doi:10.1021/ol9018002 |
| 13. | Barabé, F.; Levesque, P.; Korobkov, I.; Barriault, L. Org. Lett. 2011, 13, 5580. doi:10.1021/ol202314q |
| 12. | Hashmi, A. S. K.; Rudolph, M. Chem. Soc. Rev. 2008, 37, 1766. doi:10.1039/b615629k |
| 26. | Bohlmann, F.; Ziesche, J.; King, R. M.; Robinson, H. Phytochemistry 1980, 19, 2675. doi:10.1016/S0031-9422(00)83942-4 |
| 20. | Nieto-Oberhuber, C.; López, S.; Echavarren, A. M. J. Am. Chem. Soc. 2005, 127, 6178. doi:10.1021/ja042257t |
| 21. | Nieto-Oberhuber, C.; Pérez-Galán, P.; Herrero-Gómez, E.; Lauterbach, T.; Rodriguez, C.; López, S.; Bour, C.; Rosellón, A.; Cárdenas, D. J.; Echavarren, A. M. J. Am. Chem. Soc. 2008, 130, 269. doi:10.1021/ja075794x |
| 22. | Yeh, M.-C. P.; Tsao, W.-C.; Lee, B.-J.; Lin, T.-L. Organometallics 2008, 27, 5326. doi:10.1021/om8005917 |
| 23. | Pérez-Galán, P.; Martin, N. J. A.; Campaña, A.; Cárdenas, D. J.; Echavarren, A. M. Chem.–Asian J. 2011, 6, 482. doi:10.1002/asia.201000557 |
| 24. | Dateer, R. B.; Shaibu, B. S.; Liu, R.-S. Angew. Chem., Int. Ed. 2012, 51, 113. doi:10.1002/anie.201105921 |
| 25. | Brazeau, J.-F.; Zhang, S.; Colomer, I.; Corkey, B. K.; Toste, F. D. J. Am. Chem. Soc. 2012, 134, 2742. doi:10.1021/ja210388g |
| 12. | Hashmi, A. S. K.; Rudolph, M. Chem. Soc. Rev. 2008, 37, 1766. doi:10.1039/b615629k |
| 19. | de Frémont, P.; Scott, N. M.; Stevens, E. D.; Nolan, S. P. Organometallics 2005, 24, 2411. doi:10.1021/om050111c |
| 27. | Goodwin, S.; Smith, A. F.; Horning, E. C. J. Am. Chem. Soc. 1959, 81, 1903. doi:10.1021/ja01517a031 |
| 28. | Miller, M. C.; McCarthy, F. O. RSC Adv. 2012, 2, 8883. doi:10.1039/c2ra20584j |
© 2013 Morin et al; licensee Beilstein-Institut.
This is an Open Access article under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
The license is subject to the Beilstein Journal of Organic Chemistry terms and conditions: (http://www.beilstein-journals.org/bjoc)