Abstract
Electrodes with high conductivity and flexibility are crucial to the development of flexible lithium-ion batteries. In this study, three-dimensional (3D) LiFePO4 and Li4Ti5O12 fiber membrane materials were prepared through electrospinning and directly used as self-standing electrodes for lithium-ion batteries. The structure and morphology of the fibers, and the electrochemical performance of the electrodes and the full battery were characterized. The results show that the LiFePO4 and Li4Ti5O12 fiber membrane electrodes exhibit good rate and cycle performance. In particular, the all-fiber-based gel-state battery composed of LiFePO4 and Li4Ti5O12 fiber membrane electrodes can be charged/discharged for 800 cycles at 1C with a retention capacity of more than 100 mAh·g−1 and a coulombic efficiency close to 100%. The good electrochemical performance is attributed to the high electronic and ionic conductivity provided by the 3D network structure of the self-standing electrodes. This design and preparation method for all-fiber-based lithium-ion batteries provides a novel strategy for the development of high-performance flexible batteries.
Introduction
With the rapid development of renewable energy technologies, electric vehicles and electronic devices, energy storage technology has become a focus of global research [1-7]. The high demand for portable and flexible devices requires the development of efficient power supplies to maintain the normal operation of these devices [8-10]. Flexible lithium-ion batteries play a dominant role in the portable equipment market due to their high energy density, long life and environmental friendliness [11-14]. The electrode materials of conventional lithium-ion batteries (LIBs) are generally based on transition metal oxides containing lithium mixed evenly with conductive agents and adhesives. The electrode materials are then coated on metal current collectors [15-17]. However, the electrodes prepared by this method are easily separated from the collectors during repeated bending. Therefore, the design of electrodes that require no current collectors and are flexible to adapt to repeated bending and folding has become particularly important in the current LIB research. Researchers have found that two-dimensional (2D) or 3D free-standing electrode materials [18-21] can significantly improve the electrochemical performance while also offering light weight and superior mechanical properties [22,23].
LiFePO4 and Li4Ti5O12 have been widely developed and applied in LIBs for electric and hybrid vehicles due to their stable discharge potential, good cycling stability and environmental friendliness [24-26]. However, both of them have the disadvantages of low Li+ diffusion coefficient and poor electronic conductivity, which limit the practical applications in LIBs. Nanostructures of various shapes such as nanofibers [27,28], nanoparticles [29], nanotubes [30], nanowires [31], and nanosheets [32] can greatly shorten the conduction path of Li+, thus improving the Li+ conductivity. In addition, coating or blending with conductive carbon can significantly increase the electronic conductivity [33]. Nanofibers combining active substances with conductive carbon as flexible electrodes not only eliminate the need for current collector and binder, but also save the coating process. Moreover, due to the continuous fiber structure, the electronic conductivity of the material is increased, the utilization ratio of the active material is improved, and the structural stability of the material is enhanced [18,27,28]. Therefore, it is highly desirable to fabricate all-fiber-based batteries to achieve high performance for practical applications [34,35].
Electrospinning is an effective method to prepare long-range continuous nanofibers. By controlling the spinning and sintering process, nanofiber membrane materials can be easily formed with high porosity and stable structure, especially continuous conductive networks can be formed, which are very suitable for self-standing electrodes [18,21,28]. In recent years, an increasing number of reports on the preparation of fiber electrode materials by electrospinning were published [18,21,28,34,35].
In this paper, we describe the preparation of LiFePO4 and Li4Ti5O12 nanofiber membrane materials by a modified electrospinning method. We used the materials in all-fiber-based gel batteries (the battery fabrication process is schematically shown in Figure 1). The electrochemical properties of semi-batteries and full batteries were studied, and the mechanisms leading to the high performance of the batteries were subsequently investigated.
![[2190-4286-10-215-1]](/bjnano/content/figures/2190-4286-10-215-1.png?scale=2.0&max-width=1024&background=FFFFFF)
Figure 1: Fabrication process of all-fiber-based gel-state batteries.
Figure 1: Fabrication process of all-fiber-based gel-state batteries.
Results and Discussion
Morphology and phases of the nanofiber membranes
Nanofiber membranes with high flexibility and stable structures can be successfully prepared by electrospinning and hot-pressing sintering as described in our previous works [36-39]. The specific experimental process for LiFePO4 and Li5Ti4O12 nanofiber membranes is shown in Figure 2. It can be seen that the sintered LiFePO4 nanofiber membrane keeps a stable structure and shows good bending ability. Particularly, adding polymers with different molecular weights to the precursors can adjust the distribution of grains in the fibers.
![[2190-4286-10-215-2]](/bjnano/content/figures/2190-4286-10-215-2.jpg?scale=2.0&max-width=1024&background=FFFFFF)
Figure 2: Digital photos of LiFePO4 nanofiber films. (a, b) The precursor fiber membrane; (c) the pre-treated fiber membrane; (d) the sintered fiber membrane.
Figure 2: Digital photos of LiFePO4 nanofiber films. (a, b) The precursor fiber membrane; (c) the pre-treated...
SEM images of LiFePO4 and Li5Ti4O12 nanofiber membranes are shown in Figure 3. It can be seen that the fiber membranes after heat treatment exhibit a 3D network structure, which is the reason for the high flexibility of the electrode. The high-magnification SEM images show uniform growth of crystal grains on the surface of the fibers for both LiFePO4 and Li5Ti4O12. The fiber diameter is less than 1 μm, and the grain size is between 200 and 300 nm. There are numerous channels between the fibers. This structure is beneficial for the penetration of electrolyte and the contact between active substances and electrolyte. The resistance of Li+ during charging and discharging of the battery decreases, and the internal structure of the material cannot collapse of deform easily. Thus, the structure of the material remains unchanged even after many cycles [40,41].
![[2190-4286-10-215-3]](/bjnano/content/figures/2190-4286-10-215-3.jpg?scale=2.0&max-width=1024&background=FFFFFF)
Figure 3: SEM pictures of LiFePO4 and Li4Ti5O12 nanofiber membranes. (a, b) LiFePO4; (c, d) Li4Ti5O12.
Figure 3: SEM pictures of LiFePO4 and Li4Ti5O12 nanofiber membranes. (a, b) LiFePO4; (c, d) Li4Ti5O12.
TEM images of the LiFePO4 and Li5Ti4O12 fibers in Figure 4 reveals that the active particles are uniformly distributed on the surface of the fibers, in agreement with the SEM images. EDS analysis shows either Fe, C, P, and O or Ti, C, and O on the fibers.
![[2190-4286-10-215-4]](/bjnano/content/figures/2190-4286-10-215-4.jpg?scale=2.0&max-width=1024&background=FFFFFF)
Figure 4: TEM pictures and EDS spectra of LiFePO4 and Li4Ti5O12 fibers. (a)TEM of a LiFePO4 fiber; (b) TEM of Li4Ti5O12 fibers; (c)EDS of LiFePO4 at site 1; (d) EDS of Li4Ti5O12 at site 2.
Figure 4: TEM pictures and EDS spectra of LiFePO4 and Li4Ti5O12 fibers. (a)TEM of a LiFePO4 fiber; (b) TEM of...
The fibers were further examined using XRD and Raman spectroscopy. The XRD patterns (Figure 5) show that the diffraction peaks of sintered LiFePO4 and Li4Ti5O12 fibers coincide with those of the olivine LiFePO4 standard (PDF#40-1499) and the spinel Li4Ti5O12 standard (PDF#49-0207), respectively, which means that the sintered fibers contain the expected phases. Only a small amount of TiO2 was found in the diffraction peaks of Li4Ti5O12 fibers, which may be related to the sintering atmosphere of Li4Ti5O12 fibers. Because the sintering of Li4Ti5O12 fibers was carried out under the pressure of a graphite plate in N2 atmosphere, the sintering atmosphere is a partially reductive inert atmosphere. However, TiO2 itself is a relatively stable anode material [42], so the appearance of such impurities would not affect the performance of the electrode. The Raman spectra of the two fiber materials in Figure 6 show two characteristic peaks at 1350 cm−1 and 1580 cm−1 corresponding to the D and the G band of carbon, respectively. The ratio of the two peaks reflects the degree of graphitization. This kind of composite not only benefits the flexibility of the fibers, but also grants good conductivity to the fibers, which is crucial for the preparation of free-standing electrodes.
![[2190-4286-10-215-5]](/bjnano/content/figures/2190-4286-10-215-5.png?scale=2.0&max-width=1024&background=FFFFFF)
Figure 5: XRD patterns of LiFePO4 and Li4Ti5O12 fiber membranes.
Figure 5: XRD patterns of LiFePO4 and Li4Ti5O12 fiber membranes.
![[2190-4286-10-215-6]](/bjnano/content/figures/2190-4286-10-215-6.png?scale=2.0&max-width=1024&background=FFFFFF)
Figure 6: Raman spectra of LiFePO4 and Li4Ti5O12 fiber membranes.
Figure 6: Raman spectra of LiFePO4 and Li4Ti5O12 fiber membranes.
Electrode performance
The two fiber membranes were directly cut into free-standing electrodes, and coin-cell batteries were assembled for a series of performance tests. Figure 7 are the cyclic voltammetry curves (CV, the second cycle) of the two electrodes. It can be seen that the strong redox peaks of the LiFePO4 cathode appear at 3.62 V and 3.24 V, respectively. This corresponds to the Li+ removal from and intercalation in LiFePO4, i.e., the redox process of Fe3+/Fe2+ [24]. The redox peaks of Li4Ti5O12 at 1.71 V and 1.47 V correspond to the Li+ removal from and intercalation in Li4Ti5O12, i.e., the redox process of Ti4+/Ti3+ [25]. The two small redox peaks at 2.06 V and 1.72 V correspond to the Li+ removal from and intercalation in TiO2.
![[2190-4286-10-215-7]](/bjnano/content/figures/2190-4286-10-215-7.png?scale=2.0&max-width=1024&background=FFFFFF)
Figure 7: CV curves of LiFePO4 and Li4Ti5O12 fiber membrane electrodes.
Figure 7: CV curves of LiFePO4 and Li4Ti5O12 fiber membrane electrodes.
From the charge–discharge curves in Figure 8 (the second cycle), it can be seen that both electrodes have obvious charge–discharge plateaus at about 3.5 V and 1.5 V, which are consistent with the CV curves, corresponding to the processes of Li+ removal from and intercalation in LiFePO4 and Li4Ti5O12, respectively. It is noteworthy that two smaller plateaus for charging and discharging of TiO2 are also found on the charge-discharge curves of Li4Ti5O12, which is in agreement with the redox peaks in the CV curves.
![[2190-4286-10-215-8]](/bjnano/content/figures/2190-4286-10-215-8.png?scale=2.0&max-width=1024&background=FFFFFF)
Figure 8: Charge–discharge curves of LiFePO4 and Li4Ti5O12 fiber membrane electrodes.
Figure 8: Charge–discharge curves of LiFePO4 and Li4Ti5O12 fiber membrane electrodes.
Figure 9 shows the electrical impedance spectroscopy curves of the two kinds of fiber membrane electrodes. It can be seen that there is a regular semicircle in the high-frequency region, which represents the magnitude of the charge transfer resistance Rct. The oblique line in the low-frequency region is larger than 45°, which is the impedance of the Li+ diffusion process in the electrode. Overall, although the electrodes did not use metal collectors, both electrodes showed a smaller charge transfer impedance and a smaller ion transfer impedance, which illustrates the effect of the 3D conductive network and the high porosity on electron and ion transport [40,41].
![[2190-4286-10-215-9]](/bjnano/content/figures/2190-4286-10-215-9.png?scale=2.0&max-width=1024&background=FFFFFF)
Figure 9: EIS curves of LiFePO4 and Li4Ti5O12 fiber membrane electrodes.
Figure 9: EIS curves of LiFePO4 and Li4Ti5O12 fiber membrane electrodes.
Figure 10 shows the rate performance of LiFePO4 and Li4Ti5O12 fiber membrane electrodes. Both electrodes can be charged and discharged normally from 0.5C to 10C. When the current density returns to 1C, the discharge capacity goes back to the initial value, indicating that the electrodes have a good reversibility. It is noteworthy that with the increase of current density, the capacity difference between LiFePO4 and Li4Ti5O12 gradually widens, which may be related to the different grain sizes of active substances in these two electrodes. It is known that nanomerization could improve the rate capability and insertion kinetics of electrode materials. The SEM and TEM images in Figure 3 and Figure 4 show that the grain size of LiFePO4 is larger than that of Li4Ti5O12, which is the possible reason why the capacity of the LiFePO4 electrode decreases more quickly with the increase of rate.
![[2190-4286-10-215-10]](/bjnano/content/figures/2190-4286-10-215-10.png?scale=2.0&max-width=1024&background=FFFFFF)
Figure 10: Rate performance of LiFePO4 and Li4Ti5O12 fiber membrane electrodes.
Figure 10: Rate performance of LiFePO4 and Li4Ti5O12 fiber membrane electrodes.
The cycle performance of the electrodes at 1C was further tested. Figure 11 shows the charge–discharge cycle performance of the two electrodes at 1C. It can be seen that both electrodes can be stably cycled for more than 700 cycles, and the discharge capacity decreases from the initial 135 to 125 mAh·g−1, showing a good stability. Meanwhile, the two kinds of electrodes also show a high coulombic efficiency close to 100%. It further shows that the free-standing electrodes have excellent cycling stability and high charge–discharge reversibility.
![[2190-4286-10-215-11]](/bjnano/content/figures/2190-4286-10-215-11.png?scale=2.0&max-width=1024&background=FFFFFF)
Figure 11: Cycle performance of LiFePO4 and Li4Ti5O12 fiber membrane electrodes.
Figure 11: Cycle performance of LiFePO4 and Li4Ti5O12 fiber membrane electrodes.
Battery performance
The two free-standing electrodes were assembled to a full battery with the prepared electrolyte membrane (Figure S1, Supporting Information File 1), and the rate and the cycling performance of the battery were tested. From the charge–discharge curve in Figure 12, it can be seen that the LiFePO4//Li4Ti5O12 battery has a very flat charge–discharge plateau in the voltage window of 1.5–3.0 V, similar to the conventional LiFePO4//Li4Ti5O12 battery. It shows that LiFePO4 and Li4Ti5O12 fiber membrane electrodes match and can deliver their respective capacities.
![[2190-4286-10-215-12]](/bjnano/content/figures/2190-4286-10-215-12.png?scale=2.0&max-width=1024&background=FFFFFF)
Figure 12: Charge–discharge curves of the LiFePO4//Li4Ti5O12 all-fiber battery at 1C.
Figure 12: Charge–discharge curves of the LiFePO4//Li4Ti5O12 all-fiber battery at 1C.
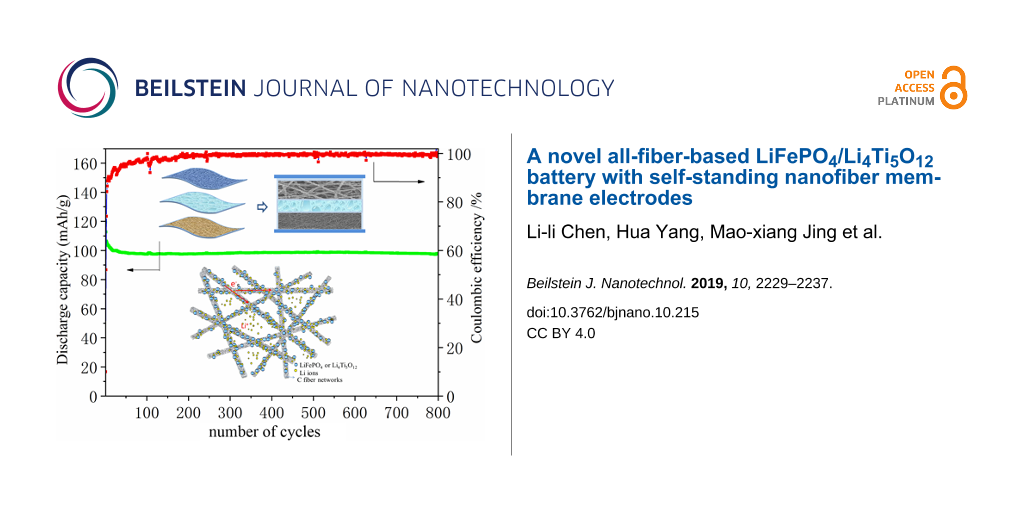
Figure 13 shows the rate performance of the LiFePO4//Li4Ti5O12 battery. The battery can be normally charged and discharged from 0.5C to 10C. The specific discharge capacity at 1C is close to 110 mAh·g−1, and reaches about 70 mAh·g−1 at 5C. When the rate is set to 1C again, the specific capacity is restored to the initial state. The cycling performance of the battery was also tested. As shown in Figure 14, the battery was continuously cycled up to 800 cycles at 1C, and the remaining capacity was over 100 mAh·g−1. The coulombic efficiency is close to 100% except for the first few cycles.
![[2190-4286-10-215-13]](/bjnano/content/figures/2190-4286-10-215-13.png?scale=2.0&max-width=1024&background=FFFFFF)
Figure 13: Rate performance of the LiFePO4//Li4Ti5O12 all-fiber battery.
Figure 13: Rate performance of the LiFePO4//Li4Ti5O12 all-fiber battery.
![[2190-4286-10-215-14]](/bjnano/content/figures/2190-4286-10-215-14.png?scale=2.0&max-width=1024&background=FFFFFF)
Figure 14: Cycle performance of the LiFePO4//Li4Ti5O12 all-fiber battery at 1C.
Figure 14: Cycle performance of the LiFePO4//Li4Ti5O12 all-fiber battery at 1C.
Table S1 in Supporting Information File 1 lists the electrochemical performance obtained in some related works. It can be seen that the flexible self-standing LiFePO4/C fiber membrane cathode and Li4Ti5O12/C fiber membrane anode in this work show a comparable electrochemical performance. In addition, this all-fiber-based LiFePO4//Li4Ti5O12 full battery can be cycled at 1C for 800 times. Figure S2 (Supporting Information File 1) shows a photo and SEM pictures of the LiFePO4 and Li4Ti5O12 fiber membrane electrodes after the charge–discharge cycles. It can be seen that the composite electrodes still keep the 3D network structure after many cycles. TO summarize, the high rate and good cycling performance are mainly attributed to the high electronic and ionic conductivity of the free-standing electrodes with a stable three-dimensional network structure as shown in Figure 15, in which the high porosity, stable structure, and the continuous conductive networks provide the electrodes with fast electronic and ionic transport paths [22,23,34,35]. This design and fabrication of all-fiber-based batteries provides a novel strategy for the development of advanced flexible lithium-ion batteries.
![[2190-4286-10-215-15]](/bjnano/content/figures/2190-4286-10-215-15.jpg?scale=2.0&max-width=1024&background=FFFFFF)
Figure 15: Sketch of 3D network structure for fast electron and ion transport.
Figure 15: Sketch of 3D network structure for fast electron and ion transport.
Conclusion
LiFePO4 and Li4Ti5O12 nanofiber membrane materials were successfully obtained by electrospinning and subsequent heat treatment. The nanofiber membranes were directly used as free-standing electrodes in LIBs and showed superior electrochemical performance. The battery consisting of these two electrodes can be charged and discharged for 800 cycles at 1C with a remaining capacity of 100 mAh·g−1 and a coulombic efficiency close to 100%. The excellent performance is attributed to the high electronic and ionic conductivity of the free-standing electrodes with 3D network structure. This design and fabrication of all-fiber-based LIBs shows a great potential in the development of flexible LIBs.
Experimental
Preparation of nanofiber membrane electrodes
LiFePO4 and Li4Ti5O12 nanofiber membranes were prepared via a modified electrospinning method. The typical preparation processes were as follows:
LiFePO4: 0.007 mol of Fe(NO3)3·9H2O and C2H3O2Li·2H2O, H3PO4 were dissolved in 29 g of N,N-dimethylformamide (DMF) to obtain solution A; 4 g of polyacrylonitrile (PAN) and 2 g of polyvinylpyrrolidone (PVP) were dissolved in 29 g of DMF to obtain solution B. A precursor spinning solution for the LiFePO4 nanofiber membrane is obtained after mixing A and B solutions. From the precursor solution a precursor fiber membrane was formed under a voltage of 25 kV, which was followed by a pre-oxidation at 260 °C for 2 h and then calcination at 800 °C for 10 h in N2 atmosphere (Figure 2c,d).
Li4Ti5O12: 0.01 mol tetra-n-butyl titanate (C16H36O4Ti), 0.9593 g C2H3O2Li·2H2O, and 0.25 mL HNO3 were dissolved in 29 g of N,N-dimethylformamide (DMF) to obtain solution A; 4 g of polyacrylonitrile (PAN) and 2 g of polyvinylpyrrolidone (PVP) were dissolved in 29 g of DMF to obtain solution B. A precursor spinning solution for the Li4Ti5O12 nanofiber membrane was obtained after mixing A and B solutions. From the precursor solution a precursor fiber membrane was formed under a voltage of 25 kV, followed by a pre-oxidation at 260 °C for 2 h and then calcination at 800 °C for 5 h in N2 atmosphere.
General characterization
A Rigaku D/Max2500X-ray diffractometer was used to determine the crystalline structure of the composite fibers. A JSM-5600LV scanning electron microscope and a JEOL JEM2010 transmission electron microscope were used to observe the morphology and microstructures of the fibers.
The electrochemical performance of the electrodes was evaluated in assembled CR2025 coin cells. The nanofiber membranes were directly cut into self-standing electrodes with a diameter of 12 mm. The carbon content in the fiber membrane electrodes is 25–27%, and the active mass of one piece of electrode is 2.5–3.0 mg·cm−2. The coin cells were assembled in an Ar-filled glovebox by using lithium foil as anode and 1 M LiPF6 electrolyte (ethylene carbonate/dimethyl carbonate/ethyl methyl carbonate = 1:1:1). A Celgard 2400 polypropylene membrane was used as separator. The cyclic voltammetry and electrochemical impedance spectroscopy measurements were carried out using a VMP2 electrochemical workstation. EIS was measured in the frequency range of 0.1 Hz to 100 kHz, with a disturbance amplitude of 10 mV. The galvanostatic cycle tests were carried out at different current densities.
Assembly of LFP//LTO all-fibers-based battery
Polyvinylidene fluoride hexafluoropropylene (PVDF-HFP) copolymer was used as the electrolyte membrane matrix in this experiment, and acetone was used as the solvent. The PVDF-HFP solution was prepared by dissolving PVDF-HFP in acetone (PVDF-HFP (mass/g): acetone (volume/mL) = 1:15) through stirring at room temperature. The solution was transferred to a PTFE dish, and dried naturally until the solvent evaporates completely. The obtained electrolyte membrane is shown in Figure S1a (Supporting Information File 1). The optimum thickness of the electrolyte membrane is 60–70 μm.
The prepared electrolyte membrane was then punched into a disk with a diameter of 18 mm (Figure S1b, Supporting Information File 1). Before the assembly of the battery, the cut membrane was first soaked in LiPF6 electrolyte for 2–3 h. A gel-state 2025 type coin-cell battery was then assembled in an Ar-filled glovebox using this membrane as the gel electrolyte, and the prepared LiFePO4 and Li4Ti5O12 nanofiber membranes as cathode and anode respectively. This battery was subsequently subjected to rate and cycling tests.
Supporting Information
A comparison between this work with related literature references, and photographs and SEM pictures of the LiFePO4 and Li4Ti5O12 fiber membrane electrodes after 800 cycles of the battery.
| Supporting Information File 1: Additional experimental data. | ||
| Format: PDF | Size: 330.4 KB | Download |
References
-
Song, F.; Li, W.; Han, G.; Sun, Y. ACS Appl. Energy Mater. 2018, 1, 3–8. doi:10.1021/acsaem.7b00005
Return to citation in text: [1] -
Berger, R.; Grévin, B.; Leclère, P.; Zhang, Y. Beilstein J. Nanotechnol. 2019, 10, 132–134. doi:10.3762/bjnano.10.12
Return to citation in text: [1] -
Song, F.-Z.; Zhu, Q.-L.; Yang, X.; Zhan, W.-W.; Pachfule, P.; Tsumori, N.; Xu, Q. Adv. Energy Mater. 2018, 8, 1701416. doi:10.1002/aenm.201701416
Return to citation in text: [1] -
Mönig, H.; Schmid, M. Beilstein J. Nanotechnol. 2019, 10, 771–773. doi:10.3762/bjnano.10.76
Return to citation in text: [1] -
Song, F.-Z.; Zhu, Q.-L.; Tsumori, N.; Xu, Q. ACS Catal. 2015, 5, 5141–5144. doi:10.1021/acscatal.5b01411
Return to citation in text: [1] -
Schnucklake, M.; Eifert, L.; Schneider, J.; Zeis, R.; Roth, C. Beilstein J. Nanotechnol. 2019, 10, 1131–1139. doi:10.3762/bjnano.10.113
Return to citation in text: [1] -
Song, F.-Z.; Zhu, Q.-L.; Yang, X.-C.; Xu, Q. ChemNanoMat 2016, 2, 942–945. doi:10.1002/cnma.201600198
Return to citation in text: [1] -
Zheng, S.; Liu, D.; Tao, L.; Fan, X.; Liu, K.; Liang, G.; Dou, A.; Su, M.; Liu, Y.; Chu, D. J. Alloys Compd. 2019, 773, 1–10. doi:10.1016/j.jallcom.2018.09.261
Return to citation in text: [1] -
Ostfeld, A. E.; Gaikwad, A. M.; Khan, Y.; Arias, A. C. Sci. Rep. 2016, 6, 26122. doi:10.1038/srep26122
Return to citation in text: [1] -
Liu, D.; Fan, X.; Li, Z.; Liu, T.; Sun, M.; Qian, C.; Ling, M.; Liu, Y.; Liang, C. Nano Energy 2019, 58, 786–796. doi:10.1016/j.nanoen.2019.01.080
Return to citation in text: [1] -
Balogun, M.-S.; Yang, H.; Luo, Y.; Qiu, W.; Huang, Y.; Liu, Z.-Q.; Tong, Y. Energy Environ. Sci. 2018, 11, 1859–1869. doi:10.1039/c8ee00522b
Return to citation in text: [1] -
Amin, K.; Meng, Q.; Ahmad, A.; Cheng, M.; Zhang, M.; Mao, L.; Lu, K.; Wei, Z. Adv. Mater. (Weinheim, Ger.) 2018, 30, 1703868. doi:10.1002/adma.201703868
Return to citation in text: [1] -
Cha, H.; Kim, J.; Lee, Y.; Cho, J.; Park, M. Small 2018, 14, 1702989. doi:10.1002/smll.201702989
Return to citation in text: [1] -
Xia, Q.; Ni, M.; Chen, M.; Xia, H. J. Mater. Chem. A 2019, 7, 6187–6196. doi:10.1039/c9ta00351g
Return to citation in text: [1] -
Rao, D.; Liu, X.; Yang, H.; Zhang, L.; Qiao, G.; Shen, X.; Yan, X.; Wang, G.; Lu, R. J. Mater. Chem. A 2019, 7, 7092–7098. doi:10.1039/c8ta12176a
Return to citation in text: [1] -
Li, L.; Xu, M.; Yao, Q.; Chen, Z.; Song, L.; Zhang, Z.; Gao, C.; Wang, P.; Yu, Z.; Lai, Y. ACS Appl. Mater. Interfaces 2016, 8, 30879–30889. doi:10.1021/acsami.6b09197
Return to citation in text: [1] -
Zhu, L.; Zhu, P.; Fang, Q.; Jing, M.; Shen, X.; Yang, L. Electrochim. Acta 2018, 292, 718–726. doi:10.1016/j.electacta.2018.10.005
Return to citation in text: [1] -
Jing, M.-x.; Li, J.-q.; Pi, Z.-c.; Zhai, H.-a.; Chen, L.-l.; Yao, S.-s.; Xiang, J.; Shen, X.-q.; Xi, X.-m.; Xiao, K.-s. Electrochim. Acta 2016, 212, 898–904. doi:10.1016/j.electacta.2016.07.087
Return to citation in text: [1] [2] [3] [4] -
Feng, Y.; Liu, H. Nanotechnology 2019, 30, 315602. doi:10.1088/1361-6528/ab19e0
Return to citation in text: [1] -
Li, J.; Li, X.; Liu, P.; Zhu, X.; Ali, R. N.; Naz, H.; Yu, Y.; Xiang, B. ACS Appl. Mater. Interfaces 2019, 11, 11442–11450. doi:10.1021/acsami.8b22367
Return to citation in text: [1] -
Kang, T.; Ma, Z.; Zuo, X.; Xiao, X.; Nan, J. Energy Technol. 2019, 7, 1800635. doi:10.1002/ente.201800635
Return to citation in text: [1] [2] [3] -
Wang, Y.; Chen, C.; Xie, H.; Gao, T.; Yao, Y.; Pastel, G.; Han, X.; Li, Y.; Zhao, J.; Fu, K. K.; Hu, L. Adv. Funct. Mater. 2017, 27, 1703140. doi:10.1002/adfm.201703140
Return to citation in text: [1] [2] -
Rao, J.; Liu, N.; Zhang, Z.; Su, J.; Li, L.; Xiong, L.; Gao, Y. Nano Energy 2018, 51, 425–433. doi:10.1016/j.nanoen.2018.06.067
Return to citation in text: [1] [2] -
Wang, X.; Feng, Z.; Huang, J.; Deng, W.; Li, X.; Zhang, H.; Wen, Z. Carbon 2018, 127, 149–157. doi:10.1016/j.carbon.2017.10.101
Return to citation in text: [1] [2] -
Liu, H.; Zhu, Z.; Huang, J.; He, X.; Chen, Y.; Zhang, R.; Lin, R.; Li, Y.; Yu, S.; Xing, X.; Yan, Q.; Li, X.; Frost, M. J.; An, K.; Feng, J.; Kostecki, R.; Xin, H.; Ong, S. P.; Liu, P. ACS Mater. Lett. 2019, 1, 96–102. doi:10.1021/acsmaterialslett.9b00099
Return to citation in text: [1] [2] -
Song, M.-S.; Kim, R.-H.; Baek, S.-W.; Lee, K.-S.; Park, K.; Benayad, A. J. Mater. Chem. A 2014, 2, 631–636. doi:10.1039/c3ta12728a
Return to citation in text: [1] -
Li, W.; Li, M.; Yang, Z.; Xu, J.; Zhong, X.; Wang, J.; Zeng, L.; Liu, X.; Jiang, Y.; Wei, X.; Gu, L.; Yu, Y. Small 2015, 11, 2762–2767. doi:10.1002/smll.201403533
Return to citation in text: [1] [2] -
Shi, X.; Zhou, W.; Ma, D.; Ma, Q.; Bridges, D.; Ma, Y.; Hu, A. J. Nanomater. 2015, 2015, No. 140716. doi:10.1155/2015/140716
Return to citation in text: [1] [2] [3] [4] -
Sun, Y.; Liu, N.; Cui, Y. Nat. Energy 2016, 1, 16071. doi:10.1038/nenergy.2016.71
Return to citation in text: [1] -
Liu, Y.; He, X.; Hanlon, D.; Harvey, A.; Khan, U.; Li, Y.; Coleman, J. N. ACS Nano 2016, 10, 5980–5990. doi:10.1021/acsnano.6b01505
Return to citation in text: [1] -
Cao, K.; Jiao, L.; Liu, Y.; Liu, H.; Wang, Y.; Yuan, H. Adv. Funct. Mater. 2015, 25, 1082–1089. doi:10.1002/adfm.201403111
Return to citation in text: [1] -
Ryu, J.; Hong, D.; Choi, S.; Park, S. ACS Nano 2016, 10, 2843–2851. doi:10.1021/acsnano.5b07977
Return to citation in text: [1] -
Liu, Y.; Fan, X.; Zhang, Z.; Wu, H.-H.; Liu, D.; Dou, A.; Su, M.; Zhang, Q.; Chu, D. ACS Sustainable Chem. Eng. 2019, 7, 2225–2235. doi:10.1021/acssuschemeng.8b04905
Return to citation in text: [1] -
Jayaraman, S.; Aravindan, V.; Suresh Kumar, P.; Chui Ling, W.; Ramakrishna, S.; Madhavi, S. ACS Appl. Mater. Interfaces 2014, 6, 8660–8666. doi:10.1021/am501464d
Return to citation in text: [1] [2] [3] -
Jung, J.-W.; Lee, C.-L.; Yu, S.; Kim, I.-D. J. Mater. Chem. A 2016, 4, 703–750. doi:10.1039/c5ta06844d
Return to citation in text: [1] [2] [3] -
Li, J.-q.; Han, C.; Jing, M.-x.; Yang, H.; Shen, X.-q.; Qin, S.-b. Appl. Phys. A: Mater. Sci. Process. 2018, 124, 450. doi:10.1007/s00339-018-1863-3
Return to citation in text: [1] -
Chen, L.-L.; Shen, X.-Q.; Jing, M.-X.; Zhu, S.-W.; Pi, Z.-C.; Li, J.-Q.; Zhai, H.-A.; Xiao, K.-S. J. Nanosci. Nanotechnol. 2018, 18, 4720–4727. doi:10.1166/jnn.2018.15319
Return to citation in text: [1] -
Jing, M.-x.; Li, J.-q.; Han, C.; Yao, S.-s.; Zhang, J.; Zhai, H.-a.; Chen, L.-l.; Shen, X.-q.; Xiao, K.-s. R. Soc. Open Sci. 2017, 4, 170323. doi:10.1098/rsos.170323
Return to citation in text: [1] -
Jing, M.-x.; Zhang, J.; Han, C.; Yang, H.; Yao, S.-s.; Zhu, L.; Chen, L.-l.; Xie, Q.-l.; Chen, X.; Shen, X.-q.; Qin, S.-b. J. Electrochem. Soc. 2018, 165, A1761–A1769. doi:10.1149/2.0801809jes
Return to citation in text: [1] -
Min, X.; Sun, B.; Chen, S.; Fang, M.; Wu, X.; Liu, Y.; Abdelkader, A.; Huang, Z.; Liu, T.; Xi, K.; Vasant Kumar, R. Energy Storage Mater. 2019, 16, 597–606. doi:10.1016/j.ensm.2018.08.002
Return to citation in text: [1] [2] -
Ni, Q.; Bai, Y.; Li, Y.; Ling, L.; Li, L.; Chen, G.; Wang, Z.; Ren, H.; Wu, F.; Wu, C. Small 2018, 14, 1702864. doi:10.1002/smll.201702864
Return to citation in text: [1] [2] -
Zu, G.; Li, H.; Liu, S.; Li, D.; Wang, J.; Zhao, J. Sustainable Mater. Technol. 2018, 18, e00079. doi:10.1016/j.susmat.2018.e00079
Return to citation in text: [1]
| 40. | Min, X.; Sun, B.; Chen, S.; Fang, M.; Wu, X.; Liu, Y.; Abdelkader, A.; Huang, Z.; Liu, T.; Xi, K.; Vasant Kumar, R. Energy Storage Mater. 2019, 16, 597–606. doi:10.1016/j.ensm.2018.08.002 |
| 41. | Ni, Q.; Bai, Y.; Li, Y.; Ling, L.; Li, L.; Chen, G.; Wang, Z.; Ren, H.; Wu, F.; Wu, C. Small 2018, 14, 1702864. doi:10.1002/smll.201702864 |
| 18. | Jing, M.-x.; Li, J.-q.; Pi, Z.-c.; Zhai, H.-a.; Chen, L.-l.; Yao, S.-s.; Xiang, J.; Shen, X.-q.; Xi, X.-m.; Xiao, K.-s. Electrochim. Acta 2016, 212, 898–904. doi:10.1016/j.electacta.2016.07.087 |
| 21. | Kang, T.; Ma, Z.; Zuo, X.; Xiao, X.; Nan, J. Energy Technol. 2019, 7, 1800635. doi:10.1002/ente.201800635 |
| 28. | Shi, X.; Zhou, W.; Ma, D.; Ma, Q.; Bridges, D.; Ma, Y.; Hu, A. J. Nanomater. 2015, 2015, No. 140716. doi:10.1155/2015/140716 |
| 34. | Jayaraman, S.; Aravindan, V.; Suresh Kumar, P.; Chui Ling, W.; Ramakrishna, S.; Madhavi, S. ACS Appl. Mater. Interfaces 2014, 6, 8660–8666. doi:10.1021/am501464d |
| 35. | Jung, J.-W.; Lee, C.-L.; Yu, S.; Kim, I.-D. J. Mater. Chem. A 2016, 4, 703–750. doi:10.1039/c5ta06844d |
| 36. | Li, J.-q.; Han, C.; Jing, M.-x.; Yang, H.; Shen, X.-q.; Qin, S.-b. Appl. Phys. A: Mater. Sci. Process. 2018, 124, 450. doi:10.1007/s00339-018-1863-3 |
| 37. | Chen, L.-L.; Shen, X.-Q.; Jing, M.-X.; Zhu, S.-W.; Pi, Z.-C.; Li, J.-Q.; Zhai, H.-A.; Xiao, K.-S. J. Nanosci. Nanotechnol. 2018, 18, 4720–4727. doi:10.1166/jnn.2018.15319 |
| 38. | Jing, M.-x.; Li, J.-q.; Han, C.; Yao, S.-s.; Zhang, J.; Zhai, H.-a.; Chen, L.-l.; Shen, X.-q.; Xiao, K.-s. R. Soc. Open Sci. 2017, 4, 170323. doi:10.1098/rsos.170323 |
| 39. | Jing, M.-x.; Zhang, J.; Han, C.; Yang, H.; Yao, S.-s.; Zhu, L.; Chen, L.-l.; Xie, Q.-l.; Chen, X.; Shen, X.-q.; Qin, S.-b. J. Electrochem. Soc. 2018, 165, A1761–A1769. doi:10.1149/2.0801809jes |
| 1. | Song, F.; Li, W.; Han, G.; Sun, Y. ACS Appl. Energy Mater. 2018, 1, 3–8. doi:10.1021/acsaem.7b00005 |
| 2. | Berger, R.; Grévin, B.; Leclère, P.; Zhang, Y. Beilstein J. Nanotechnol. 2019, 10, 132–134. doi:10.3762/bjnano.10.12 |
| 3. | Song, F.-Z.; Zhu, Q.-L.; Yang, X.; Zhan, W.-W.; Pachfule, P.; Tsumori, N.; Xu, Q. Adv. Energy Mater. 2018, 8, 1701416. doi:10.1002/aenm.201701416 |
| 4. | Mönig, H.; Schmid, M. Beilstein J. Nanotechnol. 2019, 10, 771–773. doi:10.3762/bjnano.10.76 |
| 5. | Song, F.-Z.; Zhu, Q.-L.; Tsumori, N.; Xu, Q. ACS Catal. 2015, 5, 5141–5144. doi:10.1021/acscatal.5b01411 |
| 6. | Schnucklake, M.; Eifert, L.; Schneider, J.; Zeis, R.; Roth, C. Beilstein J. Nanotechnol. 2019, 10, 1131–1139. doi:10.3762/bjnano.10.113 |
| 7. | Song, F.-Z.; Zhu, Q.-L.; Yang, X.-C.; Xu, Q. ChemNanoMat 2016, 2, 942–945. doi:10.1002/cnma.201600198 |
| 18. | Jing, M.-x.; Li, J.-q.; Pi, Z.-c.; Zhai, H.-a.; Chen, L.-l.; Yao, S.-s.; Xiang, J.; Shen, X.-q.; Xi, X.-m.; Xiao, K.-s. Electrochim. Acta 2016, 212, 898–904. doi:10.1016/j.electacta.2016.07.087 |
| 19. | Feng, Y.; Liu, H. Nanotechnology 2019, 30, 315602. doi:10.1088/1361-6528/ab19e0 |
| 20. | Li, J.; Li, X.; Liu, P.; Zhu, X.; Ali, R. N.; Naz, H.; Yu, Y.; Xiang, B. ACS Appl. Mater. Interfaces 2019, 11, 11442–11450. doi:10.1021/acsami.8b22367 |
| 21. | Kang, T.; Ma, Z.; Zuo, X.; Xiao, X.; Nan, J. Energy Technol. 2019, 7, 1800635. doi:10.1002/ente.201800635 |
| 34. | Jayaraman, S.; Aravindan, V.; Suresh Kumar, P.; Chui Ling, W.; Ramakrishna, S.; Madhavi, S. ACS Appl. Mater. Interfaces 2014, 6, 8660–8666. doi:10.1021/am501464d |
| 35. | Jung, J.-W.; Lee, C.-L.; Yu, S.; Kim, I.-D. J. Mater. Chem. A 2016, 4, 703–750. doi:10.1039/c5ta06844d |
| 15. | Rao, D.; Liu, X.; Yang, H.; Zhang, L.; Qiao, G.; Shen, X.; Yan, X.; Wang, G.; Lu, R. J. Mater. Chem. A 2019, 7, 7092–7098. doi:10.1039/c8ta12176a |
| 16. | Li, L.; Xu, M.; Yao, Q.; Chen, Z.; Song, L.; Zhang, Z.; Gao, C.; Wang, P.; Yu, Z.; Lai, Y. ACS Appl. Mater. Interfaces 2016, 8, 30879–30889. doi:10.1021/acsami.6b09197 |
| 17. | Zhu, L.; Zhu, P.; Fang, Q.; Jing, M.; Shen, X.; Yang, L. Electrochim. Acta 2018, 292, 718–726. doi:10.1016/j.electacta.2018.10.005 |
| 18. | Jing, M.-x.; Li, J.-q.; Pi, Z.-c.; Zhai, H.-a.; Chen, L.-l.; Yao, S.-s.; Xiang, J.; Shen, X.-q.; Xi, X.-m.; Xiao, K.-s. Electrochim. Acta 2016, 212, 898–904. doi:10.1016/j.electacta.2016.07.087 |
| 21. | Kang, T.; Ma, Z.; Zuo, X.; Xiao, X.; Nan, J. Energy Technol. 2019, 7, 1800635. doi:10.1002/ente.201800635 |
| 28. | Shi, X.; Zhou, W.; Ma, D.; Ma, Q.; Bridges, D.; Ma, Y.; Hu, A. J. Nanomater. 2015, 2015, No. 140716. doi:10.1155/2015/140716 |
| 11. | Balogun, M.-S.; Yang, H.; Luo, Y.; Qiu, W.; Huang, Y.; Liu, Z.-Q.; Tong, Y. Energy Environ. Sci. 2018, 11, 1859–1869. doi:10.1039/c8ee00522b |
| 12. | Amin, K.; Meng, Q.; Ahmad, A.; Cheng, M.; Zhang, M.; Mao, L.; Lu, K.; Wei, Z. Adv. Mater. (Weinheim, Ger.) 2018, 30, 1703868. doi:10.1002/adma.201703868 |
| 13. | Cha, H.; Kim, J.; Lee, Y.; Cho, J.; Park, M. Small 2018, 14, 1702989. doi:10.1002/smll.201702989 |
| 14. | Xia, Q.; Ni, M.; Chen, M.; Xia, H. J. Mater. Chem. A 2019, 7, 6187–6196. doi:10.1039/c9ta00351g |
| 33. | Liu, Y.; Fan, X.; Zhang, Z.; Wu, H.-H.; Liu, D.; Dou, A.; Su, M.; Zhang, Q.; Chu, D. ACS Sustainable Chem. Eng. 2019, 7, 2225–2235. doi:10.1021/acssuschemeng.8b04905 |
| 22. | Wang, Y.; Chen, C.; Xie, H.; Gao, T.; Yao, Y.; Pastel, G.; Han, X.; Li, Y.; Zhao, J.; Fu, K. K.; Hu, L. Adv. Funct. Mater. 2017, 27, 1703140. doi:10.1002/adfm.201703140 |
| 23. | Rao, J.; Liu, N.; Zhang, Z.; Su, J.; Li, L.; Xiong, L.; Gao, Y. Nano Energy 2018, 51, 425–433. doi:10.1016/j.nanoen.2018.06.067 |
| 34. | Jayaraman, S.; Aravindan, V.; Suresh Kumar, P.; Chui Ling, W.; Ramakrishna, S.; Madhavi, S. ACS Appl. Mater. Interfaces 2014, 6, 8660–8666. doi:10.1021/am501464d |
| 35. | Jung, J.-W.; Lee, C.-L.; Yu, S.; Kim, I.-D. J. Mater. Chem. A 2016, 4, 703–750. doi:10.1039/c5ta06844d |
| 8. | Zheng, S.; Liu, D.; Tao, L.; Fan, X.; Liu, K.; Liang, G.; Dou, A.; Su, M.; Liu, Y.; Chu, D. J. Alloys Compd. 2019, 773, 1–10. doi:10.1016/j.jallcom.2018.09.261 |
| 9. | Ostfeld, A. E.; Gaikwad, A. M.; Khan, Y.; Arias, A. C. Sci. Rep. 2016, 6, 26122. doi:10.1038/srep26122 |
| 10. | Liu, D.; Fan, X.; Li, Z.; Liu, T.; Sun, M.; Qian, C.; Ling, M.; Liu, Y.; Liang, C. Nano Energy 2019, 58, 786–796. doi:10.1016/j.nanoen.2019.01.080 |
| 18. | Jing, M.-x.; Li, J.-q.; Pi, Z.-c.; Zhai, H.-a.; Chen, L.-l.; Yao, S.-s.; Xiang, J.; Shen, X.-q.; Xi, X.-m.; Xiao, K.-s. Electrochim. Acta 2016, 212, 898–904. doi:10.1016/j.electacta.2016.07.087 |
| 27. | Li, W.; Li, M.; Yang, Z.; Xu, J.; Zhong, X.; Wang, J.; Zeng, L.; Liu, X.; Jiang, Y.; Wei, X.; Gu, L.; Yu, Y. Small 2015, 11, 2762–2767. doi:10.1002/smll.201403533 |
| 28. | Shi, X.; Zhou, W.; Ma, D.; Ma, Q.; Bridges, D.; Ma, Y.; Hu, A. J. Nanomater. 2015, 2015, No. 140716. doi:10.1155/2015/140716 |
| 29. | Sun, Y.; Liu, N.; Cui, Y. Nat. Energy 2016, 1, 16071. doi:10.1038/nenergy.2016.71 |
| 31. | Cao, K.; Jiao, L.; Liu, Y.; Liu, H.; Wang, Y.; Yuan, H. Adv. Funct. Mater. 2015, 25, 1082–1089. doi:10.1002/adfm.201403111 |
| 25. | Liu, H.; Zhu, Z.; Huang, J.; He, X.; Chen, Y.; Zhang, R.; Lin, R.; Li, Y.; Yu, S.; Xing, X.; Yan, Q.; Li, X.; Frost, M. J.; An, K.; Feng, J.; Kostecki, R.; Xin, H.; Ong, S. P.; Liu, P. ACS Mater. Lett. 2019, 1, 96–102. doi:10.1021/acsmaterialslett.9b00099 |
| 27. | Li, W.; Li, M.; Yang, Z.; Xu, J.; Zhong, X.; Wang, J.; Zeng, L.; Liu, X.; Jiang, Y.; Wei, X.; Gu, L.; Yu, Y. Small 2015, 11, 2762–2767. doi:10.1002/smll.201403533 |
| 28. | Shi, X.; Zhou, W.; Ma, D.; Ma, Q.; Bridges, D.; Ma, Y.; Hu, A. J. Nanomater. 2015, 2015, No. 140716. doi:10.1155/2015/140716 |
| 32. | Ryu, J.; Hong, D.; Choi, S.; Park, S. ACS Nano 2016, 10, 2843–2851. doi:10.1021/acsnano.5b07977 |
| 40. | Min, X.; Sun, B.; Chen, S.; Fang, M.; Wu, X.; Liu, Y.; Abdelkader, A.; Huang, Z.; Liu, T.; Xi, K.; Vasant Kumar, R. Energy Storage Mater. 2019, 16, 597–606. doi:10.1016/j.ensm.2018.08.002 |
| 41. | Ni, Q.; Bai, Y.; Li, Y.; Ling, L.; Li, L.; Chen, G.; Wang, Z.; Ren, H.; Wu, F.; Wu, C. Small 2018, 14, 1702864. doi:10.1002/smll.201702864 |
| 24. | Wang, X.; Feng, Z.; Huang, J.; Deng, W.; Li, X.; Zhang, H.; Wen, Z. Carbon 2018, 127, 149–157. doi:10.1016/j.carbon.2017.10.101 |
| 25. | Liu, H.; Zhu, Z.; Huang, J.; He, X.; Chen, Y.; Zhang, R.; Lin, R.; Li, Y.; Yu, S.; Xing, X.; Yan, Q.; Li, X.; Frost, M. J.; An, K.; Feng, J.; Kostecki, R.; Xin, H.; Ong, S. P.; Liu, P. ACS Mater. Lett. 2019, 1, 96–102. doi:10.1021/acsmaterialslett.9b00099 |
| 26. | Song, M.-S.; Kim, R.-H.; Baek, S.-W.; Lee, K.-S.; Park, K.; Benayad, A. J. Mater. Chem. A 2014, 2, 631–636. doi:10.1039/c3ta12728a |
| 42. | Zu, G.; Li, H.; Liu, S.; Li, D.; Wang, J.; Zhao, J. Sustainable Mater. Technol. 2018, 18, e00079. doi:10.1016/j.susmat.2018.e00079 |
| 22. | Wang, Y.; Chen, C.; Xie, H.; Gao, T.; Yao, Y.; Pastel, G.; Han, X.; Li, Y.; Zhao, J.; Fu, K. K.; Hu, L. Adv. Funct. Mater. 2017, 27, 1703140. doi:10.1002/adfm.201703140 |
| 23. | Rao, J.; Liu, N.; Zhang, Z.; Su, J.; Li, L.; Xiong, L.; Gao, Y. Nano Energy 2018, 51, 425–433. doi:10.1016/j.nanoen.2018.06.067 |
| 30. | Liu, Y.; He, X.; Hanlon, D.; Harvey, A.; Khan, U.; Li, Y.; Coleman, J. N. ACS Nano 2016, 10, 5980–5990. doi:10.1021/acsnano.6b01505 |
| 24. | Wang, X.; Feng, Z.; Huang, J.; Deng, W.; Li, X.; Zhang, H.; Wen, Z. Carbon 2018, 127, 149–157. doi:10.1016/j.carbon.2017.10.101 |
© 2019 Chen et al.; licensee Beilstein-Institut.
This is an Open Access article under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/4.0). Please note that the reuse, redistribution and reproduction in particular requires that the authors and source are credited.
The license is subject to the Beilstein Journal of Nanotechnology terms and conditions: (https://www.beilstein-journals.org/bjnano)