Abstract
Introducing anchoring materials into cathodes for Li–S batteries has been demonstrated as an effective way to overcome the shuttle effect and enhance the cycling stability. In this work, the anchoring effects of 2H-MoS2 and 1T'-MoS2 monolayers for Li–S batteries were investigated by using density functional theory calculations. It was found that the binding energies of Li2Sx absorbed on 1T'-MoS2 monolayer are in the range of 0.31–2.94 eV, which is much higher than on the 2H-phase. The 1T'-MoS2 monolayer shows stronger trapping ability for Li2Sx than the 2H-MoS2 monolayer. The 1T'-MoS2 monolayer can be used as effective anchoring material in cathodes for Li–S batteries.
Introduction
To satisfy the increasing demand for high-capacity energy storage systems, rechargeable lithium–sulfur (Li–S) batteries have attracted much attention in recent years due to a high theoretical specific energy density of 2567 Wh/kg, a high theoretical capacity of 1672 mAh/g, low cost, non-toxicity, and the abundance of sulfur [1]. The energy density of a Li–S battery is six times higher than that of current commercially used lithium-ion batteries (387 Wh/kg) [2-5]. Typically, a rechargeable Li–S battery is composed of a sulfur cathode and a metallic Li anode, with an organic liquid electrolyte as the ionic conductor, and a porous separator. The Li–S batteries undergo the reaction of 16Li + S8 → 8Li2S, with a simplified reaction sequence of S8 → Li2S8 → Li2S6/Li2S4 → Li2S2/Li2S. Low coulombic efficiency, active material loss, and rapid capacity fading hinder the practical application of Li–S batteries [6]. The insulating nature of sulfur and its lithiation products, Li2S2 and Li2S, leads to low electrical conductivity of the cathode and low rate capability. Dissolved higher-order lithium polysulfides (LPSs) (Li2Sx, x = 4–8) in the organic electrolyte solvent will migrate and react with the lithium anode, which results in capacity fading and low coulombic efficiency [7,8]. The major issue is the complex diffusion of LPSs, which in combination with the subsequent redox reactions is known as the shuttle effect. The shuttle effect aggravates the cyclic performance of the Li–S battery.
During recent years, many approaches have been devoted to suppressing the shuttle effect and improving the conductivity. Physical confinement of LPSs within host materials with large surface area, such as carbon nanotubes and porous materials, has been a common strategy to minimize the leakage of LPSs. However, the function of physical confinement is limited, and it slows down diffusion for ionic transport [9]. The addition of anchoring materials into the cathodes with a strong binding affinity to LPSs was thought as an efficient approach to improve the electrochemical performance. Due to the polarity of LPSs species, the interaction between LPSs and anchoring materials can be enhanced through polar–polar interactions. Graphene-based materials have been considered as anchoring materials due to their high electrical conductivity [10]. However, the adsorption of polarized LPSs on non-polarized graphene is weak; heteroatom doping is necessary for improving the anchoring effect. Nitrogen doping has been used to modify the anchoring behavior of graphene, and the N-doped graphene showed improved anchoring of Li2Sx [11]. Polar materials were explored to trap LPSs, such as metal oxide [12,13] and metal-carbide nanoparticles [14]. Many two-dimensional (2D) materials, such as borophene [15], silicene [16], phosphorene [17], Mxene [18] and MoS2 [8], have been investigated as anchoring materials due to their large surface-to-volume ratio. An ideal anchoring material should display a binding energy with LPSs in the range from 0.8 to 2.0 eV in order to effectively trap Li2Sx [8], and good electrical and ionic conductivity.
Nanostructured MoS2 used as an electrode material for lithium-ion batteries shows a higher specific capacity. Nanoflower MoS2/reduced graphene oxides composites exhibited a high specific capacity (1225 mAh/g) and an excellent cycling performance (680 mAh/g) after 250 cycles [19]. MoS2 nanoparticles have been used as a starting material for the synthesis of Li–S battery cathodes, since Li2S and metallic Mo are formed when MoS2 is fully lithiated [20,21]. 2D MoS2 (ca. 10 nm thick) can also be used as a protective layer for Li metal anodes to suppress the dendrite formation in Li–S batteries. A threefold improvement in cycle life was shown for protected Li metal compared to bare Li metal, which greatly improved the performance of Li–S batteries [22]. MoS2 has been used as anchoring material for LPSs to enhance the performance of Li–S batteries, when it is embedded into a sulfur-rich matrix cathode [23]. However, density functional theory (DFT) calculations showed that the LPSs are weakly bound to 2H-MoS2 [8]. The enhanced performance of Li–S batteries using MoS2 as anchoring material [23] may be attributed to the strong binding at the edge and terrace sites [24].
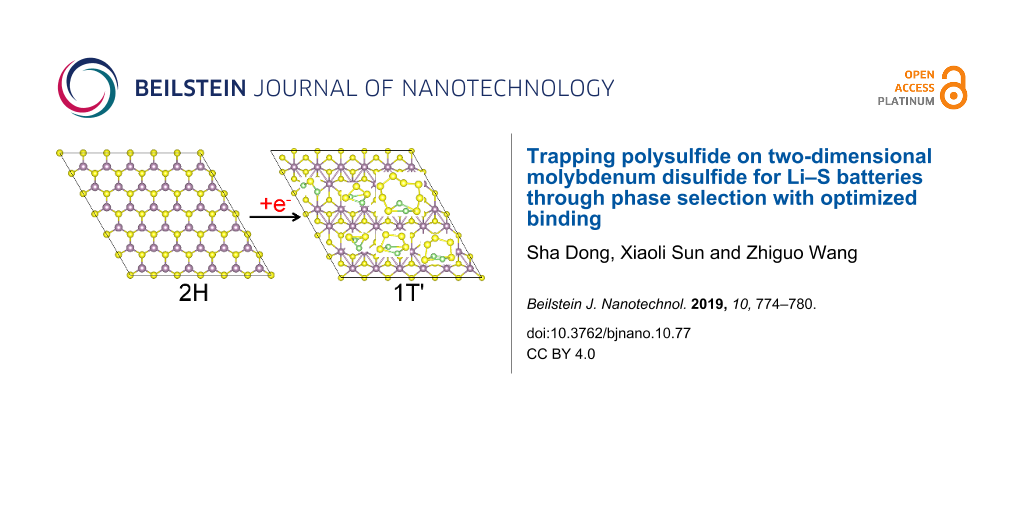
2D MoS2 shows different polymorphs [25], differing in the position of the S atoms on each side of the Mo atomic layer. 2H-MoS2 is the energetically stable phase with semiconductor characteristics, in which the S atoms are located in the lattice positions of a hexagonal close-packed structure. 1T'-MoS2 is a meta-stable phase with narrow bandgap, in which each Mo atom is octahedrally coordinated with six S atoms. The phase transformation of 2H→1T' has been widely studied [26-28]. The fundamental mechanisms of this structural transformation are governed by electron transfer [26], so the phase transition can be initiated by treatment with n-butyllithium (n-BuLi) [27], intercalation of alkali-metal ions [29,30], substitution of Mo by Re atoms [28], electron-beam irradiation [31] and hot-electron injection [32]. Recently, it was reported that MoS2/reduced graphene oxide (rGO)/S cathodes for Li–S batteries exhibit outstanding performance. X-ray photoelectron spectroscopy and Raman spectroscopy showed that few-layered MoS2 is composed of 1T'-phases and 2H-phases [33]. The composites of 1T'-MoS2 with other active materials, such as graphene [34], carbon nanotubes [35], Mxene [36], and SnO2 [37], have received much attention regarding the use as cathodes for Li–S batteries. The electrochemical performance including the capacity, rate capability and stability can be greatly improved by using these cathodes.
The phase structure has a profound influence on physical and chemical properties such as electron conductivity and catalytic behavior [38]. The mechanism of 1T MoS2 enhancing the electrochemical behavior is not well understood. In this study, we systematically investigated the adsorption of LPSs on 2H-MoS2 and 1T'-MoS2 monolayers with DFT calculation. Our results show that the 1T'-MoS2 monolayer interacts strongly with Li2Sx, which will hinder the shuttle effect. Taking into account the better conductivity, 1T'-MoS2 monolayers can be used as a conductive anchoring material to design advanced Li–S batteries.
Results and Discussion
Both 2H-MoS2 and 1T'-MoS2 monolayers exhibit a structure with three atomic layers, in which the Mo atomic layer is sandwiched by two S atomic layers. The 6 × 6 supercells for 2H-MoS2 and 1T'-MoS2 monolayers used in the work are shown in Figure 1a and Figure 1b, respectively. The electronic band structures along high-symmetry points are shown in Figure 1c and Figure 1d, respectively. The 2H-MoS2 monolayer is a semiconductor with a direct bandgap of 1.67 eV, both the conduction band minimum (CBM) and valence band maximum (VBM) are located at the K point, which is consistent with previous DFT calculations [39]. 1T'-MoS2 is a narrow-bandgap semiconductor with a bandgap of 0.15 eV.
![[2190-4286-10-77-1]](/bjnano/content/figures/2190-4286-10-77-1.jpg?scale=2.0&max-width=1024&background=FFFFFF)
Figure 1: Cross-section and side views of (a) 2H-MoS2 and (b) 1T'-MoS2 monolayers. Orbital-decomposed band structures along high-symmetry points of (c) 2H-MoS2 and (d) 1T'-MoS2 monolayers. (e) The optimized atomic configurations of Li2Sx (x = 1–8) and S8 in their ground states.
Figure 1: Cross-section and side views of (a) 2H-MoS2 and (b) 1T'-MoS2 monolayers. Orbital-decomposed band st...
Various intermediates, Li2Sx (x = 1–8), of LPSs were observed in Li–S batteries [40]. The optimized atomic configurations of LPSs in the ground state are shown in Figure 1e. All Li2Sx compounds exhibit a nonplanar shape instead of sulfur chains with terminal Li atoms. The shortest bond length of Li–S increases with the increase of x for x < 5. For higher x, there is no linear dependence. The bond -length values are 2.082, 2.213, 2.307, 2.333, 2.359, 2.354, and 2.330 Å for Li2S, Li2S2, Li2S3, Li2S4, Li2S5, Li2S6, and Li2S8, respectively. Li2S and Li2S2 have C2v symmetry, Li2Sx (x = 3–8) has C2 symmetry, and S8 with a puckered ring structure has a D4d symmetry.
The trapping of Li2Sx on 2H-MoS2 and 1T'-MoS2 monolayers was evaluated by the calculation of the binding energy (Eb) with Equation 1
where and
are the total energy of the MoS2 monolayer with and without Li2Sx adsorption.
is the energy of an isolated Li2Sx molecule in a cubic lattice with a cell length of 30 Å. A more negative binding energy indicates a stronger adsorption interaction between the MoS2 monolayer and the Li2Sx molecule. The calculated binding energy is shown in Figure 2. The binding energy of Li2Sx absorbed on a 2H-MoS2 monolayer decreases from 0.90 to 0.08 eV as x increases from 1 to 8. For Li2Sx absorbed on a 1T'-MoS2 monolayer, the binding energy decreases from 2.94 to 0.64 eV. The 1T'-MoS2 monolayer shows stronger trapping ability for Li2Sx than the 2H-MoS2 monolayer. The orbital-decomposed band structures of 2H-MoS2 and 1T'-MoS2 monolayers are shown in Figure 1c and Figure 1d, respectively. The unoccupied lowest conduction band of the 2H-MoS2 monolayer at the K point is dominated by the
orbital, whereas that of 1T'-MoS2 monolayer is dominated by the dxy and
orbitals. As the Li2Sx absorbed on the monolayer, electron transfers from Li2Sx to the unoccupied lowest states of the monolayer. The dxy and
orbitals of the 1T'-MoS2 monolayer are lower in energy than the
orbital of 2H-MoS2, thus leading to a stronger binding. Both 2H-MoS2 and 1T'-MoS2 monolayers show less trapping of S8 with a binding energy of 0.04 eV. The binding energies of Li2Sx on borophene are in the range from −1.00 to −3.00 eV [15] and on Ni-doped graphene are in the range from 1.13 to 1.40 eV [11]. The 1T'-MoS2 monolayer can be used as a conductive anchoring material to design advanced Li–S batteries.
![[2190-4286-10-77-2]](/bjnano/content/figures/2190-4286-10-77-2.png?scale=2.0&max-width=1024&background=FFFFFF)
Figure 2: The calculated binding energies of Li2Sx on 2H-MoS2 and 1T'-MoS2 monolayers.
Figure 2: The calculated binding energies of Li2Sx on 2H-MoS2 and 1T'-MoS2 monolayers.
Detailed analysis of the atomic structure shows that the Li atom of Li2Sx is energetically favorable to bind with the 2H-MoS2 monolayer. The Li atom prefers to locate at the top position above the Mo atom, which is similar to the Li adsorption on 2H-MoS2 monolayers [41]. The distance between the Li atom and the plane of S atoms increases from 2.027 to 3.511 Å as x increases from 1 to 8 in Li2Sx. The increased distance results in the weak trapping of Li2Sx by the 2H-MoS2 monolayer. As the LPSs are absorbed on the 1T'-MoS2 monolayer, except for Li absorbed on the S plane, one of the S atoms in Li2Sx (x = 1–3) is also bound to the S plane, thus increasing the anchoring behavior. The distance between the Li atoms in Li2Sx and the plane of S atoms of the 1T'-MoS2 monolayer is much shorter than that to the 2H-MoS2 monolayer. Hence, the 1T'-MoS2 monolayer exhibits a stronger trapping of Li2Sx than the 2H-MoS2 monolayer. The puckered ring structure S8 prefers to align parallel to the surface of both 2H-MoS2 and 1T'-MoS2 monolayer with distances of 3.99 and 3.67 Å, respectively. The large distance and the small binding strength of S8 on 2H-MoS2 and 1T'-MoS2 monolayers indicate that the interaction mainly originates from van der Waals interactions.
To understand the binding between LPSs and the 2HMoS2- and 1T'-MoS2 monolayers, the charge-density difference was calculated using Equation 2:
where
and
are the charge densities of the adsorbed system, the MoS2 monolayer, and the Li2Sx molecule, respectively. The differences of charge densities for Li2Sx absorbed on a 2H-MoS2 monolayer are shown in Figure 3. The red and green surfaces correspond to charge gain and loss, respectively. It can be observed that there is charge depletion in Li2Sx and the 2H-MoS2 monolayer, and that charge accumulates between the Li atoms and the S atoms of 2H-MoS2, suggesting a chemical Li–S bond between Li2Sx and the 2H-MoS2 monolayer. With increasing x the charge redistribution becomes less pronounced, and there is almost no charge exchange between Li2S8 and the 2H-MoS2 monolayer, causing only weak adsorption. This agrees with the fact that the binding energy of Li2Sx absorbed on the 2H-MoS2 monolayer decreases as x increases from 1 to 8. The charge-density differences for Li2Sx absorbed on a 1T'-MoS2 monolayer are shown in Figure 4. In contrast to the case of Li2Sx absorbed on the 2H-MoS2 monolayer, the charge redistribution is more apparent, indicating the strong trapping ability for Li2Sx. For both 2H-MoS2 and 1T'-MoS2 monolayers, there is no charge migration between the monolayer and the puckered ring structure S8, which is consistent with the corresponding small binding energies.
![[2190-4286-10-77-3]](/bjnano/content/figures/2190-4286-10-77-3.jpg?scale=2.0&max-width=1024&background=FFFFFF)
Figure 3: Isosurface (0.0005 e/Å3) of the charge distributions of Li2Sx absorbed on 2H-MoS2 monolayer. The red and green surfaces correspond to charge gain and loss, respectively.
Figure 3: Isosurface (0.0005 e/Å3) of the charge distributions of Li2Sx absorbed on 2H-MoS2 monolayer. The re...
![[2190-4286-10-77-4]](/bjnano/content/figures/2190-4286-10-77-4.jpg?scale=2.0&max-width=1024&background=FFFFFF)
Figure 4: Isosurface (0.0005 e/Å3) of the charge distributions of Li2Sx absorbed on 1T'-MoS2 monolayer. The red and green surfaces correspond to charge gain and loss, respectively.
Figure 4: Isosurface (0.0005 e/Å3) of the charge distributions of Li2Sx absorbed on 1T'-MoS2 monolayer. The r...
The working mechanism of Li–S batteries includes the following steps, octasulfur is reduced to long chains of LPSs, Li2Sx (6 < x ≤ 8), and further to lower-order LPSs, Li2Sx (2 < x ≤ 6), the final product of Li2S is formed upon discharging; and the charging process occurs through the reverse reactions [40]. The insulating nature of sulfur and the lithiation products (Li2S2 and Li2S), and the dissolution of higher-order Li2Sx (x = 4–8) are the main challenges in the application of Li–S batteries [7,8]. An ideal anchoring material with binding energies with LPSs in the range from 0.8 to 2.0 eV [15], and with good electrical and ionic conductivity is desirable for improving the electrochemical performance of Li–S batteries. Although the 2H-MoS2 monolayer shows good Li conductivity, it is a semiconductor with weak binding energies for LiPSs. Through phase engineering of the 2H-MoS2 to the 1T'-MoS2 monolayer, the anchoring behavior can be greatly improved. 1T'-MoS2 also exhibits good electrical and ionic conductivity. Hence, the electrochemical performance of Li–S batteries is improved by using 1T'-MoS2 as additive in the cathodes [33-37].
Conclusion
In conclusion, the anchoring effects of 2H-MoS2 and 1T'-MoS2 monolayers for Li–S batteries were investigated by using DFT calculations. It was found that the binding energies of Li2Sx absorbed on the 1T'-MoS2 monolayer are in the range of 0.31–2.94 eV, whereas they are in the range of 0.08–0.90 eV for Li2Sx absorbed on the 2H-MoS2 monolayer. The 1T'-MoS2 monolayer shows a stronger trapping of Li2Sx than the 2H-MoS2 monolayer. The 1T'-MoS2 monolayer can be employed as effective anchoring material in cathodes for Li–S batteries.
Simulation Details
The same simulation method and models of [26] were used in the present work. All spin-polarized DFT calculations were performed with the Vienna ab initio simulation package (VASP) [42] plane-wave simulations. Electron–ion interaction and electron exchange–correlation were described using the projector augmented wave (PAW) method [43] and the generalized gradient approximation (GGA) was described using the Perdew–Burke–Ernzerhof (PBE) function, respectively. The energy cutoff for the plane-wave basis expansion was chosen to be 520 eV. To avoid the interaction between the periodic images, a 6 × 6 supercell of a MoS2 monolayer, which contains 36 Mo and 72 S atoms, was used to investigate the adsorption of Li2Sx. A 25 Å vacuum space was constructed perpendicular to the monolayers. The Brillouin zone was integrated using the Monkhorst–Pack scheme [44] with a 3 × 3 × 1 k-grid for the geometry optimization. All atomic positions and cell parameters were relaxed until the force on each atom is less than 0.02 eV/Å.
Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (11474047) and the Fundamental Research Funds for the Central Universities (ZYGX2016J202). This work was conducted at the National Supercomputer Center in Tianjin, and the calculations were performed on TianHe-1(A).
References
-
Yin, Y.-X.; Xin, S.; Guo, Y.-G.; Wan, L.-J. Angew. Chem., Int. Ed. 2013, 52, 13186–13200. doi:10.1002/anie.201304762
Return to citation in text: [1] -
Seh, Z. W.; Sun, Y.; Zhang, Q.; Cui, Y. Chem. Soc. Rev. 2016, 45, 5605–5634. doi:10.1039/c5cs00410a
Return to citation in text: [1] -
Liu, X.; Huang, J.-Q.; Zhang, Q.; Mai, L. Adv. Mater. (Weinheim, Ger.) 2017, 29, 1601759. doi:10.1002/adma.201601759
Return to citation in text: [1] -
Cheon, S.-E.; Ko, K.-S.; Cho, J.-H.; Kim, S.-W.; Chin, E.-Y.; Kim, H.-T. J. Electrochem. Soc. 2003, 150, A800–A805. doi:10.1149/1.1571533
Return to citation in text: [1] -
Evers, S.; Nazar, L. F. Acc. Chem. Res. 2013, 46, 1135–1143. doi:10.1021/ar3001348
Return to citation in text: [1] -
Bruce, P. G.; Freunberger, S. A.; Hardwick, L. J.; Tarascon, J.-M. Nat. Mater. 2012, 11, 19–29. doi:10.1038/nmat3191
Return to citation in text: [1] -
Yang, Y.; Zheng, G.; Cui, Y. Chem. Soc. Rev. 2013, 42, 3018–3032. doi:10.1039/c2cs35256g
Return to citation in text: [1] [2] -
Zhang, Q.; Wang, Y.; Seh, Z. W.; Fu, Z.; Zhang, R.; Cui, Y. Nano Lett. 2015, 15, 3780–3786. doi:10.1021/acs.nanolett.5b00367
Return to citation in text: [1] [2] [3] [4] [5] -
Peng, H.-J.; Zhang, Q. Angew. Chem., Int. Ed. 2015, 54, 11018–11020. doi:10.1002/anie.201505444
Return to citation in text: [1] -
Zhao, W.; Chen, P.; Tang, P.; Li, Y.; Wu, J.; Duan, W. Appl. Phys. Lett. 2014, 104, 043901. doi:10.1063/1.4862983
Return to citation in text: [1] -
Yi, G. S.; Sim, E. S.; Chung, Y.-C. Phys. Chem. Chem. Phys. 2017, 19, 28189–28194. doi:10.1039/c7cp04507g
Return to citation in text: [1] [2] -
Zhou, L.; Ding, N.; Yang, J.; Yang, L.; Zong, Y.; Liu, Z.; Yu, A. ACS Sustainable Chem. Eng. 2016, 4, 3679–3687. doi:10.1021/acssuschemeng.6b00206
Return to citation in text: [1] -
Liang, X.; Kwok, C. Y.; Lodi-Marzano, F.; Pang, Q.; Cuisinier, M.; Huang, H.; Hart, C. J.; Houtarde, D.; Kaup, K.; Sommer, H.; Brezesinski, T.; Janek, J.; Nazar, L. F. Adv. Energy Mater. 2016, 6, 1501636. doi:10.1002/aenm.201501636
Return to citation in text: [1] -
Zhou, F.; Li, Z.; Luo, X.; Wu, T.; Jiang, B.; Lu, L.-L.; Yao, H.-B.; Antonietti, M.; Yu, S.-H. Nano Lett. 2018, 18, 1035–1043. doi:10.1021/acs.nanolett.7b04505
Return to citation in text: [1] -
Zhang, L.; Liang, P.; Shu, H.-b.; Man, X.-l.; Li, F.; Huang, J.; Dong, Q.-m.; Chao, D.-l. J. Phys. Chem. C 2017, 121, 15549–15555. doi:10.1021/acs.jpcc.7b03741
Return to citation in text: [1] [2] [3] -
Liu, Z.; Balbuena, P. B.; Mukherjee, P. P. J. Coord. Chem. 2016, 69, 2090–2105. doi:10.1080/00958972.2016.1184265
Return to citation in text: [1] -
Zhao, J.; Yang, Y.; Katiyar, R. S.; Chen, Z. J. Mater. Chem. A 2016, 4, 6124–6130. doi:10.1039/c6ta00871b
Return to citation in text: [1] -
Zhao, Y.; Zhao, J. Appl. Surf. Sci. 2017, 412, 591–598. doi:10.1016/j.apsusc.2017.04.013
Return to citation in text: [1] -
Xiong, F.; Cai, Z.; Qu, L.; Zhang, P.; Yuan, Z.; Asare, O. K.; Xu, W.; Lin, C.; Mai, L. ACS Appl. Mater. Interfaces 2015, 7, 12625–12630. doi:10.1021/acsami.5b02978
Return to citation in text: [1] -
Fang, X.; Guo, X.; Mao, Y.; Hua, C.; Shen, L.; Hu, Y.; Wang, Z.; Wu, F.; Chen, L. Chem. – Asian J. 2012, 7, 1013–1017. doi:10.1002/asia.201100796
Return to citation in text: [1] -
Balach, J.; Jaumann, T.; Giebeler, L. Energy Storage Mater. 2017, 8, 209–216. doi:10.1016/j.ensm.2017.03.013
Return to citation in text: [1] -
Cha, E.; Patel, M. D.; Park, J.; Hwang, J.; Prasad, V.; Cho, K.; Choi, W. Nat. Nanotechnol. 2018, 13, 337–344. doi:10.1038/s41565-018-0061-y
Return to citation in text: [1] -
Dirlam, P. T.; Park, J.; Simmonds, A. G.; Domanik, K.; Arrington, C. B.; Schaefer, J. L.; Oleshko, V. P.; Kleine, T. S.; Char, K.; Glass, R. S.; Soles, C. L.; Kim, C.; Pinna, N.; Sung, Y.-E.; Pyun, J. ACS Appl. Mater. Interfaces 2016, 8, 13437–13448. doi:10.1021/acsami.6b03200
Return to citation in text: [1] [2] -
Wang, H.; Zhang, Q.; Yao, H.; Liang, Z.; Lee, H.-W.; Hsu, P.-C.; Zheng, G.; Cui, Y. Nano Lett. 2014, 14, 7138–7144. doi:10.1021/nl503730c
Return to citation in text: [1] -
Ataca, C.; Şahin, H.; Ciraci, S. J. Phys. Chem. C 2012, 116, 8983–8999. doi:10.1021/jp212558p
Return to citation in text: [1] -
Sun, X.; Wang, Z.; Li, Z.; Fu, Y. Q. Sci. Rep. 2016, 6, 26666. doi:10.1038/srep26666
Return to citation in text: [1] [2] [3] -
Sun, L.; Yan, X.; Zheng, J.; Yu, H.; Lu, Z.; Gao, S.-p.; Liu, L.; Pan, X.; Wang, D.; Wang, Z.; Wang, P.; Jiao, L. Nano Lett. 2018, 18, 3435–3440. doi:10.1021/acs.nanolett.8b00452
Return to citation in text: [1] [2] -
Enyashin, A. N.; Yadgarov, L.; Houben, L.; Popov, I.; Weidenbach, M.; Tenne, R.; Bar-Sadan, M.; Seifert, G. J. Phys. Chem. C 2011, 115, 24586–24591. doi:10.1021/jp2076325
Return to citation in text: [1] [2] -
Enyashin, A. N.; Seifert, G. Comput. Theor. Chem. 2012, 999, 13–20. doi:10.1016/j.comptc.2012.08.005
Return to citation in text: [1] -
Nasr Esfahani, D.; Leenaerts, O.; Sahin, H.; Partoens, B.; Peeters, F. M. J. Phys. Chem. C 2015, 119, 10602–10609. doi:10.1021/jp510083w
Return to citation in text: [1] -
Lin, Y.-C.; Dumcenco, D. O.; Huang, Y.-S.; Suenaga, K. Nat. Nanotechnol. 2014, 9, 391–396. doi:10.1038/nnano.2014.64
Return to citation in text: [1] -
Kang, Y.; Najmaei, S.; Liu, Z.; Bao, Y.; Wang, Y.; Zhu, X.; Halas, N. J.; Nordlander, P.; Ajayan, P. M.; Lou, J.; Fang, Z. Adv. Mater. (Weinheim, Ger.) 2014, 26, 6467–6471. doi:10.1002/adma.201401802
Return to citation in text: [1] -
You, Y.; Ye, Y.; Wei, M.; Sun, W.; Tang, Q.; Zhang, J.; Chen, X.; Li, H.; Xu, J. Chem. Eng. J. 2019, 355, 671–678. doi:10.1016/j.cej.2018.08.176
Return to citation in text: [1] [2] -
He, J.; Hartmann, G.; Lee, M.; Hwang, G. S.; Chen, Y.; Manthiram, A. Energy Environ. Sci. 2019, 12, 344–350. doi:10.1039/c8ee03252a
Return to citation in text: [1] [2] -
Jeong, Y. C.; Kim, J. H.; Kwon, S. H.; Oh, J. Y.; Park, J.; Jung, Y.; Lee, S. G.; Yang, S. J.; Park, C. R. J. Mater. Chem. A 2017, 5, 23909–23918. doi:10.1039/c7ta08153g
Return to citation in text: [1] [2] -
Zhang, Y.; Mu, Z.; Yang, C.; Xu, Z.; Zhang, S.; Zhang, X.; Li, Y.; Lai, J.; Sun, Z.; Yang, Y.; Chao, Y.; Li, C.; Ge, X.; Yang, W.; Guo, S. Adv. Funct. Mater. 2018, 28, 1707578. doi:10.1002/adfm.201707578
Return to citation in text: [1] [2] -
Wang, M.; Fan, L.; Tian, D.; Wu, X.; Qiu, Y.; Zhao, C.; Guan, B.; Wang, Y.; Zhang, N.; Sun, K. ACS Energy Lett. 2018, 3, 1627–1633. doi:10.1021/acsenergylett.8b00856
Return to citation in text: [1] [2] -
Yu, Y.; Nam, G.-H.; He, Q.; Wu, X.-J.; Zhang, K.; Yang, Z.; Chen, J.; Ma, Q.; Zhao, M.; Liu, Z.; Ran, F.-R.; Wang, X.; Li, H.; Huang, X.; Li, B.; Xiong, Q.; Zhang, Q.; Liu, Z.; Gu, L.; Du, Y.; Huang, W.; Zhang, H. Nat. Chem. 2018, 10, 638–643. doi:10.1038/s41557-018-0035-6
Return to citation in text: [1] -
Chang, C.-H.; Fan, X.; Lin, S.-H.; Kuo, J.-L. Phys. Rev. B 2013, 88, 195420. doi:10.1103/physrevb.88.195420
Return to citation in text: [1] -
Manthiram, A.; Fu, Y.; Chung, S.-H.; Zu, C.; Su, Y.-S. Chem. Rev. 2014, 114, 11751–11787. doi:10.1021/cr500062v
Return to citation in text: [1] [2] -
Sun, X.; Wang, Z. Beilstein J. Nanotechnol. 2017, 8, 2711–2718. doi:10.3762/bjnano.8.270
Return to citation in text: [1] -
Kresse, G.; Furthmüller, J. Comput. Mater. Sci. 1996, 6, 15–50. doi:10.1016/0927-0256(96)00008-0
Return to citation in text: [1] -
Kresse, G.; Joubert, D. Phys. Rev. B 1999, 59, 1758–1775. doi:10.1103/physrevb.59.1758
Return to citation in text: [1] -
Pack, J. D.; Monkhorst, H. J. Phys. Rev. B 1977, 16, 1748–1749. doi:10.1103/physrevb.16.1748
Return to citation in text: [1]
| 28. | Enyashin, A. N.; Yadgarov, L.; Houben, L.; Popov, I.; Weidenbach, M.; Tenne, R.; Bar-Sadan, M.; Seifert, G. J. Phys. Chem. C 2011, 115, 24586–24591. doi:10.1021/jp2076325 |
| 31. | Lin, Y.-C.; Dumcenco, D. O.; Huang, Y.-S.; Suenaga, K. Nat. Nanotechnol. 2014, 9, 391–396. doi:10.1038/nnano.2014.64 |
| 32. | Kang, Y.; Najmaei, S.; Liu, Z.; Bao, Y.; Wang, Y.; Zhu, X.; Halas, N. J.; Nordlander, P.; Ajayan, P. M.; Lou, J.; Fang, Z. Adv. Mater. (Weinheim, Ger.) 2014, 26, 6467–6471. doi:10.1002/adma.201401802 |
| 39. | Chang, C.-H.; Fan, X.; Lin, S.-H.; Kuo, J.-L. Phys. Rev. B 2013, 88, 195420. doi:10.1103/physrevb.88.195420 |
| 40. | Manthiram, A.; Fu, Y.; Chung, S.-H.; Zu, C.; Su, Y.-S. Chem. Rev. 2014, 114, 11751–11787. doi:10.1021/cr500062v |
| 37. | Wang, M.; Fan, L.; Tian, D.; Wu, X.; Qiu, Y.; Zhao, C.; Guan, B.; Wang, Y.; Zhang, N.; Sun, K. ACS Energy Lett. 2018, 3, 1627–1633. doi:10.1021/acsenergylett.8b00856 |
| 38. | Yu, Y.; Nam, G.-H.; He, Q.; Wu, X.-J.; Zhang, K.; Yang, Z.; Chen, J.; Ma, Q.; Zhao, M.; Liu, Z.; Ran, F.-R.; Wang, X.; Li, H.; Huang, X.; Li, B.; Xiong, Q.; Zhang, Q.; Liu, Z.; Gu, L.; Du, Y.; Huang, W.; Zhang, H. Nat. Chem. 2018, 10, 638–643. doi:10.1038/s41557-018-0035-6 |
| 35. | Jeong, Y. C.; Kim, J. H.; Kwon, S. H.; Oh, J. Y.; Park, J.; Jung, Y.; Lee, S. G.; Yang, S. J.; Park, C. R. J. Mater. Chem. A 2017, 5, 23909–23918. doi:10.1039/c7ta08153g |
| 36. | Zhang, Y.; Mu, Z.; Yang, C.; Xu, Z.; Zhang, S.; Zhang, X.; Li, Y.; Lai, J.; Sun, Z.; Yang, Y.; Chao, Y.; Li, C.; Ge, X.; Yang, W.; Guo, S. Adv. Funct. Mater. 2018, 28, 1707578. doi:10.1002/adfm.201707578 |
| 33. | You, Y.; Ye, Y.; Wei, M.; Sun, W.; Tang, Q.; Zhang, J.; Chen, X.; Li, H.; Xu, J. Chem. Eng. J. 2019, 355, 671–678. doi:10.1016/j.cej.2018.08.176 |
| 34. | He, J.; Hartmann, G.; Lee, M.; Hwang, G. S.; Chen, Y.; Manthiram, A. Energy Environ. Sci. 2019, 12, 344–350. doi:10.1039/c8ee03252a |
| 15. | Zhang, L.; Liang, P.; Shu, H.-b.; Man, X.-l.; Li, F.; Huang, J.; Dong, Q.-m.; Chao, D.-l. J. Phys. Chem. C 2017, 121, 15549–15555. doi:10.1021/acs.jpcc.7b03741 |
| 11. | Yi, G. S.; Sim, E. S.; Chung, Y.-C. Phys. Chem. Chem. Phys. 2017, 19, 28189–28194. doi:10.1039/c7cp04507g |
| 41. | Sun, X.; Wang, Z. Beilstein J. Nanotechnol. 2017, 8, 2711–2718. doi:10.3762/bjnano.8.270 |
| 43. | Kresse, G.; Joubert, D. Phys. Rev. B 1999, 59, 1758–1775. doi:10.1103/physrevb.59.1758 |
| 44. | Pack, J. D.; Monkhorst, H. J. Phys. Rev. B 1977, 16, 1748–1749. doi:10.1103/physrevb.16.1748 |
| 26. | Sun, X.; Wang, Z.; Li, Z.; Fu, Y. Q. Sci. Rep. 2016, 6, 26666. doi:10.1038/srep26666 |
| 42. | Kresse, G.; Furthmüller, J. Comput. Mater. Sci. 1996, 6, 15–50. doi:10.1016/0927-0256(96)00008-0 |
| 15. | Zhang, L.; Liang, P.; Shu, H.-b.; Man, X.-l.; Li, F.; Huang, J.; Dong, Q.-m.; Chao, D.-l. J. Phys. Chem. C 2017, 121, 15549–15555. doi:10.1021/acs.jpcc.7b03741 |
| 33. | You, Y.; Ye, Y.; Wei, M.; Sun, W.; Tang, Q.; Zhang, J.; Chen, X.; Li, H.; Xu, J. Chem. Eng. J. 2019, 355, 671–678. doi:10.1016/j.cej.2018.08.176 |
| 34. | He, J.; Hartmann, G.; Lee, M.; Hwang, G. S.; Chen, Y.; Manthiram, A. Energy Environ. Sci. 2019, 12, 344–350. doi:10.1039/c8ee03252a |
| 35. | Jeong, Y. C.; Kim, J. H.; Kwon, S. H.; Oh, J. Y.; Park, J.; Jung, Y.; Lee, S. G.; Yang, S. J.; Park, C. R. J. Mater. Chem. A 2017, 5, 23909–23918. doi:10.1039/c7ta08153g |
| 36. | Zhang, Y.; Mu, Z.; Yang, C.; Xu, Z.; Zhang, S.; Zhang, X.; Li, Y.; Lai, J.; Sun, Z.; Yang, Y.; Chao, Y.; Li, C.; Ge, X.; Yang, W.; Guo, S. Adv. Funct. Mater. 2018, 28, 1707578. doi:10.1002/adfm.201707578 |
| 37. | Wang, M.; Fan, L.; Tian, D.; Wu, X.; Qiu, Y.; Zhao, C.; Guan, B.; Wang, Y.; Zhang, N.; Sun, K. ACS Energy Lett. 2018, 3, 1627–1633. doi:10.1021/acsenergylett.8b00856 |
| 40. | Manthiram, A.; Fu, Y.; Chung, S.-H.; Zu, C.; Su, Y.-S. Chem. Rev. 2014, 114, 11751–11787. doi:10.1021/cr500062v |
| 7. | Yang, Y.; Zheng, G.; Cui, Y. Chem. Soc. Rev. 2013, 42, 3018–3032. doi:10.1039/c2cs35256g |
| 8. | Zhang, Q.; Wang, Y.; Seh, Z. W.; Fu, Z.; Zhang, R.; Cui, Y. Nano Lett. 2015, 15, 3780–3786. doi:10.1021/acs.nanolett.5b00367 |
| 1. | Yin, Y.-X.; Xin, S.; Guo, Y.-G.; Wan, L.-J. Angew. Chem., Int. Ed. 2013, 52, 13186–13200. doi:10.1002/anie.201304762 |
| 9. | Peng, H.-J.; Zhang, Q. Angew. Chem., Int. Ed. 2015, 54, 11018–11020. doi:10.1002/anie.201505444 |
| 8. | Zhang, Q.; Wang, Y.; Seh, Z. W.; Fu, Z.; Zhang, R.; Cui, Y. Nano Lett. 2015, 15, 3780–3786. doi:10.1021/acs.nanolett.5b00367 |
| 7. | Yang, Y.; Zheng, G.; Cui, Y. Chem. Soc. Rev. 2013, 42, 3018–3032. doi:10.1039/c2cs35256g |
| 8. | Zhang, Q.; Wang, Y.; Seh, Z. W.; Fu, Z.; Zhang, R.; Cui, Y. Nano Lett. 2015, 15, 3780–3786. doi:10.1021/acs.nanolett.5b00367 |
| 19. | Xiong, F.; Cai, Z.; Qu, L.; Zhang, P.; Yuan, Z.; Asare, O. K.; Xu, W.; Lin, C.; Mai, L. ACS Appl. Mater. Interfaces 2015, 7, 12625–12630. doi:10.1021/acsami.5b02978 |
| 6. | Bruce, P. G.; Freunberger, S. A.; Hardwick, L. J.; Tarascon, J.-M. Nat. Mater. 2012, 11, 19–29. doi:10.1038/nmat3191 |
| 18. | Zhao, Y.; Zhao, J. Appl. Surf. Sci. 2017, 412, 591–598. doi:10.1016/j.apsusc.2017.04.013 |
| 2. | Seh, Z. W.; Sun, Y.; Zhang, Q.; Cui, Y. Chem. Soc. Rev. 2016, 45, 5605–5634. doi:10.1039/c5cs00410a |
| 3. | Liu, X.; Huang, J.-Q.; Zhang, Q.; Mai, L. Adv. Mater. (Weinheim, Ger.) 2017, 29, 1601759. doi:10.1002/adma.201601759 |
| 4. | Cheon, S.-E.; Ko, K.-S.; Cho, J.-H.; Kim, S.-W.; Chin, E.-Y.; Kim, H.-T. J. Electrochem. Soc. 2003, 150, A800–A805. doi:10.1149/1.1571533 |
| 5. | Evers, S.; Nazar, L. F. Acc. Chem. Res. 2013, 46, 1135–1143. doi:10.1021/ar3001348 |
| 8. | Zhang, Q.; Wang, Y.; Seh, Z. W.; Fu, Z.; Zhang, R.; Cui, Y. Nano Lett. 2015, 15, 3780–3786. doi:10.1021/acs.nanolett.5b00367 |
| 14. | Zhou, F.; Li, Z.; Luo, X.; Wu, T.; Jiang, B.; Lu, L.-L.; Yao, H.-B.; Antonietti, M.; Yu, S.-H. Nano Lett. 2018, 18, 1035–1043. doi:10.1021/acs.nanolett.7b04505 |
| 16. | Liu, Z.; Balbuena, P. B.; Mukherjee, P. P. J. Coord. Chem. 2016, 69, 2090–2105. doi:10.1080/00958972.2016.1184265 |
| 12. | Zhou, L.; Ding, N.; Yang, J.; Yang, L.; Zong, Y.; Liu, Z.; Yu, A. ACS Sustainable Chem. Eng. 2016, 4, 3679–3687. doi:10.1021/acssuschemeng.6b00206 |
| 13. | Liang, X.; Kwok, C. Y.; Lodi-Marzano, F.; Pang, Q.; Cuisinier, M.; Huang, H.; Hart, C. J.; Houtarde, D.; Kaup, K.; Sommer, H.; Brezesinski, T.; Janek, J.; Nazar, L. F. Adv. Energy Mater. 2016, 6, 1501636. doi:10.1002/aenm.201501636 |
| 17. | Zhao, J.; Yang, Y.; Katiyar, R. S.; Chen, Z. J. Mater. Chem. A 2016, 4, 6124–6130. doi:10.1039/c6ta00871b |
| 11. | Yi, G. S.; Sim, E. S.; Chung, Y.-C. Phys. Chem. Chem. Phys. 2017, 19, 28189–28194. doi:10.1039/c7cp04507g |
| 10. | Zhao, W.; Chen, P.; Tang, P.; Li, Y.; Wu, J.; Duan, W. Appl. Phys. Lett. 2014, 104, 043901. doi:10.1063/1.4862983 |
| 15. | Zhang, L.; Liang, P.; Shu, H.-b.; Man, X.-l.; Li, F.; Huang, J.; Dong, Q.-m.; Chao, D.-l. J. Phys. Chem. C 2017, 121, 15549–15555. doi:10.1021/acs.jpcc.7b03741 |
| 23. | Dirlam, P. T.; Park, J.; Simmonds, A. G.; Domanik, K.; Arrington, C. B.; Schaefer, J. L.; Oleshko, V. P.; Kleine, T. S.; Char, K.; Glass, R. S.; Soles, C. L.; Kim, C.; Pinna, N.; Sung, Y.-E.; Pyun, J. ACS Appl. Mater. Interfaces 2016, 8, 13437–13448. doi:10.1021/acsami.6b03200 |
| 20. | Fang, X.; Guo, X.; Mao, Y.; Hua, C.; Shen, L.; Hu, Y.; Wang, Z.; Wu, F.; Chen, L. Chem. – Asian J. 2012, 7, 1013–1017. doi:10.1002/asia.201100796 |
| 21. | Balach, J.; Jaumann, T.; Giebeler, L. Energy Storage Mater. 2017, 8, 209–216. doi:10.1016/j.ensm.2017.03.013 |
| 22. | Cha, E.; Patel, M. D.; Park, J.; Hwang, J.; Prasad, V.; Cho, K.; Choi, W. Nat. Nanotechnol. 2018, 13, 337–344. doi:10.1038/s41565-018-0061-y |
| 27. | Sun, L.; Yan, X.; Zheng, J.; Yu, H.; Lu, Z.; Gao, S.-p.; Liu, L.; Pan, X.; Wang, D.; Wang, Z.; Wang, P.; Jiao, L. Nano Lett. 2018, 18, 3435–3440. doi:10.1021/acs.nanolett.8b00452 |
| 29. | Enyashin, A. N.; Seifert, G. Comput. Theor. Chem. 2012, 999, 13–20. doi:10.1016/j.comptc.2012.08.005 |
| 30. | Nasr Esfahani, D.; Leenaerts, O.; Sahin, H.; Partoens, B.; Peeters, F. M. J. Phys. Chem. C 2015, 119, 10602–10609. doi:10.1021/jp510083w |
| 26. | Sun, X.; Wang, Z.; Li, Z.; Fu, Y. Q. Sci. Rep. 2016, 6, 26666. doi:10.1038/srep26666 |
| 27. | Sun, L.; Yan, X.; Zheng, J.; Yu, H.; Lu, Z.; Gao, S.-p.; Liu, L.; Pan, X.; Wang, D.; Wang, Z.; Wang, P.; Jiao, L. Nano Lett. 2018, 18, 3435–3440. doi:10.1021/acs.nanolett.8b00452 |
| 28. | Enyashin, A. N.; Yadgarov, L.; Houben, L.; Popov, I.; Weidenbach, M.; Tenne, R.; Bar-Sadan, M.; Seifert, G. J. Phys. Chem. C 2011, 115, 24586–24591. doi:10.1021/jp2076325 |
| 26. | Sun, X.; Wang, Z.; Li, Z.; Fu, Y. Q. Sci. Rep. 2016, 6, 26666. doi:10.1038/srep26666 |
| 24. | Wang, H.; Zhang, Q.; Yao, H.; Liang, Z.; Lee, H.-W.; Hsu, P.-C.; Zheng, G.; Cui, Y. Nano Lett. 2014, 14, 7138–7144. doi:10.1021/nl503730c |
| 25. | Ataca, C.; Şahin, H.; Ciraci, S. J. Phys. Chem. C 2012, 116, 8983–8999. doi:10.1021/jp212558p |
| 8. | Zhang, Q.; Wang, Y.; Seh, Z. W.; Fu, Z.; Zhang, R.; Cui, Y. Nano Lett. 2015, 15, 3780–3786. doi:10.1021/acs.nanolett.5b00367 |
| 23. | Dirlam, P. T.; Park, J.; Simmonds, A. G.; Domanik, K.; Arrington, C. B.; Schaefer, J. L.; Oleshko, V. P.; Kleine, T. S.; Char, K.; Glass, R. S.; Soles, C. L.; Kim, C.; Pinna, N.; Sung, Y.-E.; Pyun, J. ACS Appl. Mater. Interfaces 2016, 8, 13437–13448. doi:10.1021/acsami.6b03200 |
© 2019 Dong et al.; licensee Beilstein-Institut.
This is an Open Access article under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/4.0). Please note that the reuse, redistribution and reproduction in particular requires that the authors and source are credited.
The license is subject to the Beilstein Journal of Nanotechnology terms and conditions: (https://www.beilstein-journals.org/bjnano)