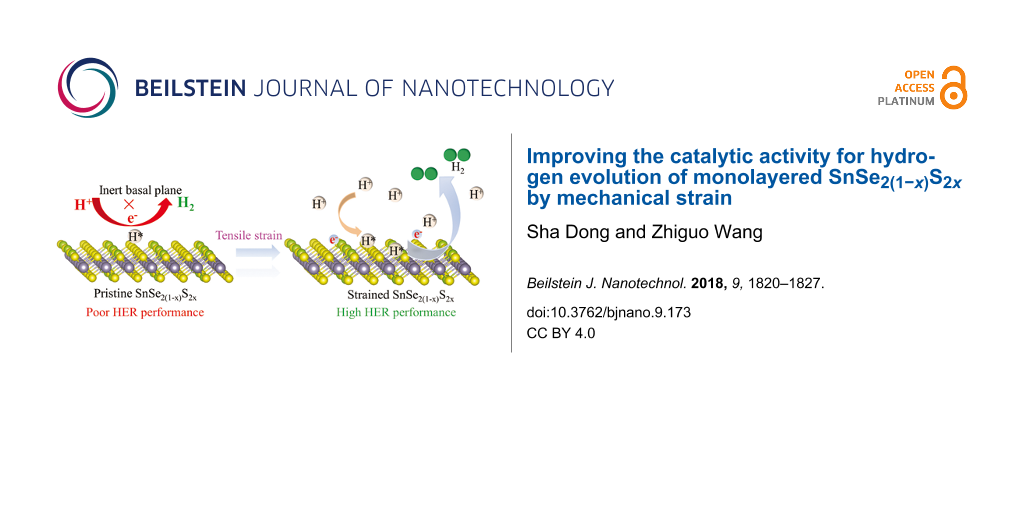
Abstract
Exploring efficient electrocatalysts for hydrogen production with non-noble metals and earth-abundant elements is a promising pathway for achieving practical electrochemical water splitting. In this work, the electronic properties and catalytic activity of monolayer SnSe2(1−x)S2x (x = 0–1) under compressive and tensile strain were investigated using density functional theory (DFT) computations. The results showed SnSe2(1−x)S2x alloys with continuously changing bandgaps from 0.8 eV for SnSe2 to 1.59 eV for SnS2. The band structure of a SnSe2(1−x)S2x monolayer can be further tuned by applied compressive and tensile strain. Moreover, tensile strain provides a direct approach to improve the catalytic activity for the hydrogen evolution reaction (HER) on the basal plane of the SnSe2(1−x)S2x monolayer. SnSeS and SnSe0.5S1.5 monolayers showed the best catalytic activity for HER at a tensile strain of 10%. This work provides a design for improved catalytic activity of the SnSe2(1-x)S2x monolayer.
Introduction
Hydrogen is a clean energy source with outstanding properties such as high specific energy per mass, easy storage and transportation, and ability to reduce harmful emissions [1,2]. Hydrogen is not naturally available as a ready-to-use substance; however, hydrogen can be produced anywhere across the planet through many approaches. Among the approaches used in the mass production of hydrogen, water electrolysis is a clean and “green” approach [3-7]. Efficient electrocatalysts for the hydrogen evolution reaction (HER) with high conversion efficiency are essential for the continuous generation of hydrogen. The platinum (Pt) group materials are regarded as the best electrocatalysts for HER; however, the high cost and limited resources of these types of catalyst restrict their usage in the mass production of hydrogen [8-10]. Therefore, exploring non-noble and earth-abundant elements as catalysts for hydrogen production is one of the most promising pathways for the mass production of hydrogen.
Two-dimensional (2D) atomic layer thin materials, such as monolayer transition-metal dichalcogenides (TMDs) [11-19], have demonstrated many fascinating properties, including the substitution of Pt as an electrocatalyst for HER. 2D MoS2 has been widely studied. Recently, tin dichalcogenides SnX2 (X = S, Se) have also received considerable attention in a variety of fields because of their low cost, use of earth-abundant resources and environmental friendliness [18,20-23]. Monolayer SnX2 has a X–Sn–X sandwich-like structure, which can be easily synthesized by using traditional mechanical exfoliation techniques [24,25] because of the weak van der Waals (vdW) interactions between layers. These mono- and few-layer SnX2 (X = S, Se) compounds are expected to be widely used in the fields of water splitting [26], high-speed photodetection [27], electronics [28], and catalysis [29], as well as in the fabrication of solar cells and film electrodes [30].
A good electro-catalyst for HER should have sufficient active sites for catalysis. Furthermore, because electrons participate in the HER process, an ideal catalyst for HER should have good electronic conductivity. Tuning the band structure of the catalyst is important for improving the HER efficiency. It was reported that the band structure and carrier mobility of monolayer MX2 can be tuned by substitution of M with M' atoms or X with X' atoms to form monolayer MxM'(1−x)X2 or MX2xX'2(1−x) alloys [31-37]. For example, Komsa et al. [34] have investigated the electronic properties of monolayer MoS2xSe2(1−x) and found that the bandgaps can be continuously tuned with the variation of Se composition. Liu et al. [38] have studied Mo1−xWxS2 and observed variations of the direct bandgap between 1.85 and 1.99 eV by varying x from 0 to 1. Other 2D-TMDs alloy nanosheets, such as Mo1−xWxSe2 [39] and WS2(1−x)Se2x [40,41], have also shown tuneable bandgaps and different electrical properties by varying the value of x. Monolayer SnS2 and SnSe2 have indirect bandgaps of 2.1 and 1.1 eV, respectively. Theoretically, alloying SnS2 and SnSe2 may yield tuneable bandgaps [36]. Wang et al. [35] have studied the 2D SnSe2(1−x)S2x alloys that were alloyed using chemical vapour transport (CVT) reactions.
Apart from alloying, strain can also be used to tune the electronic properties of nanomaterials. Yue et al. [42] investigated the electronic properties of monolayer MoS2 under elastic strain and found that the direct-to-indirect transition of MoS2 occurs at a strain of 0.01, and the semiconductor-to-metal transition occurs at a strain of 0.10. Huang et al. [22] reported that both compressive (−11%) and tensile (14%) strain can trigger the semiconductor–metal transition in the SnSe2 monolayer. Furthermore, Scalise et al. [15] showed that the electronic structure of the MoS2 monolayer can be reversibly tuned from direct to indirect by applying strain (ca. 2%).
Much research effort has been devoted in recent decades to the development of inexpensive catalysts for the electrochemical HER. Some of the recent studies have focused on monolayer SnX2 (X = S, Se). Liu et al. [43] investigated SnS2 nanosheets regarding their electrochemical behaviour and electrocatalytic properties for HER by examining trace amounts of Pt nanoparticles interacting with defect-rich SnS2; the results demonstrated that SnS2 may offer new perspectives regarding a utilization in HER. The catalytic activity for HER shows great dependence on the electronic structure of the catalyst. As alloying and strain can be used to tune the electronic properties of the catalyst, they will affect the catalytic behaviour. Several TMD alloy systems have been investigated as catalysts for HER, including Mo1−xWxSe2 nanoflowers [44], WS2(1−x)Se2x nanotubes [45] and MoS2(1−x)Se2x nanobelts [46]. It was reported that alloying provides an opportunity to tune the Gibbs free energy (ΔGH) for hydrogen adsorbed on the monolayer alloys and can be used to enhance HER performance. The HER catalytic properties of the catalyst can also be tuned through strain [42,47-52]. Gao et al. [53] demonstrated that a tensile strain is able to strengthen the hydrogen binding on graphitic carbon nitride (g-C3N4), whereas compressive strain had the opposite effect. Yan et al. [49] showed that large elastic strains influence the catalytic activity of WC for HER.
Very recently, 2D SnSe2(1−x)S2x alloys have been synthesized experimentally [35]. To our knowledge, theoretical studies related to the system of 2D SnSe2(1−x)S2x alloys as catalysts for HER have been reported rarely. Tuning the electronic properties and catalytic behaviour of SnSe2(1−x)S2x monolayers for HER by strain engineering is required for their application in the energy conversion field. In this work, the electronic properties and catalytic behaviour for HER of SnSe2(1−x)S2x (x = 0, 0.125, 0.25, 0.375, 0.5, 0.625, 0.750, 0.875 and 1.0) monolayers were investigated by density functional theory (DFT). It was shown that band gap and catalytic activity of these alloys can be continuously tuned by strain engineering.
Results and Discussion
2D monolayer TMD is a three-atomic thickness structure with one transition-metal atom layer sandwiched by two chalcogen atom layers. A triangular prism or octahedron can be formed as the transition-metal atom is bonded with six chalcogen atoms, and the former and latter structures are often termed as 2H-phase and 1T-phase, respectively. The stable phase for monolayer SnS2 is the 1T-phase. Alloying is an efficient approach to tune the electronic properties of semiconductors. Because of the large difference of bandgaps between SnSe2 and SnS2, the SnSe2(1−x)S2x semiconductor offers wide and continuously fine-tuneable bandgaps when varying the alloy fractions. All the simulations were performed in a 2 × 2 supper cell, which includes 8 S atoms. As 0–8 S atoms are substituted by Se atoms, the monolayer SnSe2(1−x)S2x alloys with x = 0–1 are formed. For each given S content, all possible configurations of the substitution of S by Se atoms in monolayer SnS2 are tested; the configuration with the lowest energy was used to model the monolayer SnSe2(1−x)S2x, which is different from random structure used in [36]. The electronic properties and catalytic behaviour for HER of the SnSe2(1−x)S2x monolayer are all performed for the energy-stable configuration. The cross and side views of monolayer SnSeS are shown in Figure 1a and Figure 1b, respectively. Sn, S, and Se atoms are presented by brown, yellow, and green balls, respectively. For the monolayer SnSeS, S and Se atoms are preferably distributed orderly. As the size of the S atom is different from that of Se atom, the lattice constant of SnSe2(1−x)S2x monolayer changes with the Se content. Figure 1c shows the variation of the lattice constant as a function of the Se content as x increases from 0 to 1. The lattice constant decreases from 3.87 Å for SnSe2 to 3.70 Å for SnS2. The calculated lattice constants of 3.87 and 3.70 Å agree with the reported values of 3.89 Å for SnSe2 and 3.70 Å for SnS2 [35]. The lattice constant shows a linear variation with Se content, which obeys Vegard′s law [54] and agrees well with previous results [36].
![[2190-4286-9-173-1]](/bjnano/content/figures/2190-4286-9-173-1.png?scale=2.0&max-width=1024&background=FFFFFF)
Figure 1: (a) Top view and (b) side view of monolayer SnSeS. (c) Variation of the lattice constant as a function of Se content in monolayer SnSe2(1−x)S2x. (d) Band gaps as a function of Se content in monolayer SnSe2(1−x)S2x. (e–i) The band structures of monolayer SnSe2(1−x)S2x with x equal to (e) 1.0, (f) 0.75, (g) 0.50, (h) 0.25 and (i) 0.0. The arrows indicate the indirect band gap for a given system.
Figure 1: (a) Top view and (b) side view of monolayer SnSeS. (c) Variation of the lattice constant as a funct...
SnS2 and SnSe2 monolayers are indirect-bandgap semiconductors, as highlighted in their band structures shown in Figure 1e and Figure 1i, respectively. The valence-band maximum (VBM) is located at the M-point, whereas the conduction-band minimum (CBM) is located at the Γ-point. The band gaps are 1.59 eV for the SnS2 monolayer and 0.80 eV for the SnSe2 monolayer, in agreement with the previous reported values of 1.60 eV and 0.81 eV, respectively [35]. The band structures of monolayer SnSe2(1−x)S2x with x equal to 0.75, 0.50 and 0.25 are shown in Figure 1f, Figure 1g and Figure 1h, respectively. The substitution of S with Se does not affect the indirect bandgap semiconducting characteristics; however, the band gap is tuned with changing the content of Se, as shown in Figure 1d with the indirect band gap decreasing with increasing Se content. These results agree with the reported by Huang et al. [36] with random SnSe2(1−x)S2x alloys. The bandgap evolution of monolayer SnSe2(1−x)S2x shows a band-bowing effect [36].
There are three possible adsorption sites for hydrogen on pristine SnS2 and SnSe2 monolayers, i.e., the top of the Sn site, the centre of a hexagonal site, and the top of S/Se atoms. Because of the symmetry breaking in SnSe2(1−x)S2x monolayers, the number of possible adsorption sites is increased. For example, there are six adsorption sites for hydrogen on the SnSeS monolayer, as shown in Figure 2, i.e., the top of S (TS) and Se (TSe) atoms, two tops of the Sn sites (T1 and T2), and two centres of the hexagonal sites (H1 and H2). The hydrogen is bounded to two S atoms and one Se atom at the T1 and H1 sites, whereas it is bounded to one S atom and two Se atoms at the T2 and H2 sites. After full relaxation, the T and H sites are not energetically stable adsorption sites for hydrogen, which will be relaxed to near the S or Se atoms. Thus, we considered the sites on top of the S and Se atoms as adsorption sites for hydrogen in the following part of this paper.
![[2190-4286-9-173-2]](/bjnano/content/figures/2190-4286-9-173-2.png?scale=2.0&max-width=1024&background=FFFFFF)
Figure 2: Atom configuration of possible adsorption sites for hydrogen on the SnSeS monolayer.
Figure 2: Atom configuration of possible adsorption sites for hydrogen on the SnSeS monolayer.
The Gibbs free energies of hydrogen adsorption on the basal plane of the monolayer of SnS2 and SnSe2 are 1.08 and 0.96 eV, respectively. Although the Gibbs free energies of hydrogen adsorption at on top of Se and S atoms decrease with increasing the Se content in the SnSe2(1−x)S2x monolayer, the values of ΔGH are 0.99 and 0.61 eV on top of Se and S atoms, respectively, in the SnSe1.75S0.25 monolayer. The large positive values of ΔGH indicate that hydrogen has a weak interaction with the substrate and is not easily adsorbed on the catalyst. Thus, the basal plane of SnSe2(1−x)S2x monolayer remains chemically inert for HER.
As shown in Figure 3a, biaxial strains ranging from −5% to 10% were applied in the SnSe2(1−x)S2x monolayer to modify the catalytic performance. Strained SnSe2(1−x)S2x monolayer are obtained by varying the lattice value with strain ε (ε = −0.05, −0.03, −0.01, 0.02, 0.03, 0.05, 0.06 and 1.00), i.e., the lattice constant is a = a0(1 + ε), where a0 is the lattice constant without strain. The value of ΔGH for hydrogen absorbed on SnSe2(1−x)S2x monolayers increases with compressive strains. The values of ΔGH were calculated to be 0.84 and 1.16 eV in the SnSeS monolayer for hydrogen adsorbed on top of S and Se atoms, respectively, and these values increased to 1.28 and 1.63 eV, respectively, under a compressive strain of −5%. Thus, compressive strain is not helpful for improving the catalytic performance of the SnSe2(1−x)S2x monolayers. With tensile strain applied, the value of ΔGH decreases with increasing the tensile strain. The values of ΔGH decrease from 0.84 to −0.05 eV and from 1.16 to 0.18 eV for hydrogen adsorbed on top of the S atom and the Se atom, respectively, in the SnSeS monolayer as the tensile strain increases from 0% to 10%. For the SnSe2(1−x)S2x monolayer with x in the range between 0.25 and 1.00, the values of ΔGH for hydrogen adsorbed the top of S are close to zero at a tensile strain of 10%; thus, strain can be used to improve the catalytic activity for HER.
![[2190-4286-9-173-3]](/bjnano/content/figures/2190-4286-9-173-3.png?scale=2.0&max-width=1024&background=FFFFFF)
Figure 3: (a) A schematic diagram showing strain applied to SnSe2(1−x)S2x monolayer. (b) Evolution of ΔGH for the SnSe2(1−x)S2x monolayer with mechanical strain. The black dashed line indicates a Gibbs free energy of zero.
Figure 3: (a) A schematic diagram showing strain applied to SnSe2(1−x)S2x monolayer. (b) Evolution of ΔGH for...
As shown in Figure 4, also the band gaps show great dependence on the applied strain. The band gap decreases from 1.59 to 1.48 eV for the SnS2 monolayer as the compressive strain increases from 0% to −5%, whereas it decreases from 1.59 to 0.90 eV as the tensile strain increases from 0% to 10%. The results indicates that the strain can be used to tune the band gaps of the SnSe2(1−x)S2x monolayers.
![[2190-4286-9-173-4]](/bjnano/content/figures/2190-4286-9-173-4.png?scale=2.0&max-width=1024&background=FFFFFF)
Figure 4: Band gaps of the SnSe2(1−x)S2x monolayers as a function of mechanical strain.
Figure 4: Band gaps of the SnSe2(1−x)S2x monolayers as a function of mechanical strain.
These effects may create new opportunities in some specific applications, such as bandgap engineering and device structures. It was reported that the catalytic activity for HER of the monolayer 1T'-MX2 (M = Mo, W; X = S, Se, Te) can be enhanced by applying tensile strain [55,56]. For 1T'-MoS2, it was found that the strong hybridization between Mo d-orbitals and S p-orbitals increases upon application of tensile strain because the valence band and conduction band move upward and downward in energy, respectively. Previous reports showed that H adsorption depends on the density of states near the Fermi energy level [57,58], with the adsorption being enhanced as the d-band centre moves closer to the Fermi level. The number of electronic states near the Fermi energy increased with the increasing of tensile mechanical strain, thereby boosting the supply of electrons to the adsorption sites and thus improving the catalytic activity [59]. Figure 5 shows the band structures for SnSeS and SnSe0.5S1.5 monolayers with strains of −5%, −3%, 2%, 6% and 10%. As seen from Figure 5, there are fewer valence states near the Fermi energy when compressive strain is applied, and the number of electronic states increases with increasing tensile strain. A higher density of electronic states near the valence-band maximum appears with tensile strain. Moreover, tensile strain caused the valence band to shift upward and the conduction band to shift downward in energy. The results can enhance the interaction between hydrogen and the catalyst. Therefore, the hydrogen-adsorption free energies were decreased from 0.84 to −0.05 eV and from 0.90 to −0.06 eV for H atoms adsorbed on SnSeS and SnSe0.5S1.5 at a tensile strain of 10%, respectively. Therefore, applying a suitable tensile strain can efficiently improve the HER efficiency of SnSe2(1−x)S2x monolayers. The tensile strain can be realized by mechanically bending the monolayer SnSe2(1−x)S2x [56].
![[2190-4286-9-173-5]](/bjnano/content/figures/2190-4286-9-173-5.png?scale=2.0&max-width=1024&background=FFFFFF)
Figure 5: Band structures for SnSeS and SnSe0.5S1.5 monolayers with strain of −5%, −3%, 2%, 6% and 10%.
Figure 5: Band structures for SnSeS and SnSe0.5S1.5 monolayers with strain of −5%, −3%, 2%, 6% and 10%.
Conclusion
In conclusion, the electronic properties and catalytic behaviour for HER of SnSe2(1−x)S2x monolayers were investigated using DFT calculations. The band gap of the SnSe2(1−x)S2x monolayer can be continuously tuned from 0.8 eV for SnSe2 to 1.59 eV for SnS2. The band gap of a SnSe2(1−x)S2x monolayer can be further tuned by applying mechanical strain. Although the basal plane of SnSe2(1−x)S2x monolayer is inert for HER, the mechanical tensile strain provides a direct means to improve the catalytic activity for hydrogen evolution reaction of SnSe2(1−x)S2x monolayer. SnSeS and SnSe0.5S1.5 monolayers show the best catalytic activity for HER at a tensile strain of 10%. This work provides a method of improvement of the catalytic activity of SnSe2(1−x)S2x monolayers.
Simulation Details
All calculations were performed based on spin-polarized DFT as implemented in the Vienna ab initio simulation package (VASP) [60,61] within the framework of the projector augmented wave method [62]. Electron exchange–correlation was described with the generalized gradient approximation (GGA) within the Perdew–Burke–Ernzerhof (PBE) functional [63]. An energy cutoff of 520 eV was used for the plane-wave basis sets to converge the relevant quantities. The atomic positions and geometric structures were freely relaxed using the conjugate gradient approximation (CG) until the residual force on each atom is less than 0.02 eV·Å−1. A vacuum of 20 Å perpendicular to the monolayer was used to avoid the periodic image interactions.
The HER can be described by Equation 1 under the conditions pH = 0 and p(H2) = 1bar [64]:
The first step of HER is that the H atom is bound to the active site of the catalyst; this step is the rate-determining step [65]. The Gibbs free energy of hydrogen adsorption (ΔGH) of this step is a key quantity to describe the HER activity of the catalyst; a value of ΔGH for a good catalyst should be close to zero [66-68]. ΔGH can be calculated by using Equation 2,
where ΔEZPE is the zero-point energy difference between the adsorbed state and the gas phase of hydrogen; because this quantity has a small contribution to ΔGH, it was neglected in this work. T is the temperature. ΔSH is the entropy contribution to ΔGH, which can be approximated as: ΔSH = 1/2SH2, where SH2 is the entropy of H2 in the gas phase under standard conditions (300 K, 1 bar) [69]. The approximate value of ΔEZPE − TΔSH is 0.38 eV [70,71]. ΔEH is the hydrogen chemisorption energy and was calculated using Equation 3,
where EM+H, EM, and EH2 are the total energy of the catalyst with an adsorbed hydrogen atom, the total energy of the catalyst without adsorption of hydrogen, and the total energy of a molecule of hydrogen, respectively. Using the above values, the Gibbs free energy can be calculated using Equation 4,
Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (11474047) and the Fundamental Research Funds for the Central Universities (ZYGX2016J202). This work was conducted at the National Supercomputer Center in Tianjin, and the calculations were performed on TianHe-1(A).
References
-
Dincer, I.; Acar, C. Int. J. Hydrogen Energy 2015, 40, 11094–11111. doi:10.1016/j.ijhydene.2014.12.035
Return to citation in text: [1] -
Burton, L. A.; Whittles, T. J.; Hesp, D.; Linhart, W. M.; Skelton, J. M.; Hou, B.; Webster, R. F.; O'Dowd, G.; Reece, C.; Cherns, D.; Fermin, D. J.; Veal, T. D.; Dhanak, V. R.; Walsh, A. J. Mater. Chem. A 2016, 4, 1312–1318. doi:10.1039/c5ta08214e
Return to citation in text: [1] -
Xu, Z.; Ao, Z.; Chu, D.; Younis, A.; Li, C. M.; Li, S. Sci. Rep. 2014, 4, 6450. doi:10.1038/srep06450
Return to citation in text: [1] -
Kudo, A.; Miseki, Y. Chem. Soc. Rev. 2009, 38, 253–278. doi:10.1039/B800489G
Return to citation in text: [1] -
Khan, S. U. M.; Al-Shahry, M.; Ingler, W. B., Jr. Science 2002, 297, 2243–2245. doi:10.1126/science.1075035
Return to citation in text: [1] -
Chen, W.-F.; Iyer, S.; Iyer, S.; Sasaki, K.; Wang, C.-H.; Zhu, Y.; Muckerman, J. T.; Fujita, E. Energy Environ. Sci. 2013, 6, 1818–1826. doi:10.1039/C3EE40596F
Return to citation in text: [1] -
Chen, W.-F.; Muckerman, J. T.; Fujita, E. Chem. Commun. 2013, 49, 8896. doi:10.1039/c3cc44076a
Return to citation in text: [1] -
Bockris, J. O. M.; Ammar, I. A.; Huq, A. K. M. S. J. Phys. Chem. 1957, 61, 879–886. doi:10.1021/j150553a008
Return to citation in text: [1] -
Parsons, R. Trans. Faraday Soc. 1960, 56, 1340–1350. doi:10.1039/TF9605601340
Return to citation in text: [1] -
Walter, M. G.; Warren, E. L.; Mckone, J. R.; Boettcher, S. W.; Mi, Q.; Santori, E. A.; Lewis, N. S. Chem. Rev. 2010, 110, 6446. doi:10.1021/cr1002326
Return to citation in text: [1] -
Novoselov, K. S.; Jiang, D.; Schedin, F.; Booth, T. J.; Khotkevich, V. V.; Morozov, S. V.; Geim, A. K. Proc. Natl. Acad. Sci. U. S. A. 2005, 102, 10451–10453. doi:10.1073/pnas.0502848102
Return to citation in text: [1] -
Li, H.; Pan, L.; Lu, T.; Zhan, Y.; Nie, C.; Sun, Z. J. Electroanal. Chem. 2011, 653, 40–44. doi:10.1016/j.jelechem.2011.01.012
Return to citation in text: [1] -
Novoselov, K. S.; Geim, A. K.; Morozov, S. V.; Jiang, D.; Zhang, Y.; Dubonos, S. V.; Grigorieva, I. V.; Firsov, A. A. Science 2004, 306, 666. doi:10.1126/science.1102896
Return to citation in text: [1] -
Yang, L.; Majumdar, K.; Liu, H.; Du, Y.; Wu, H.; Hatzistergos, M.; Hung, P. Y.; Tieckelmann, R.; Tsai, W.; Hobbs, C.; Ye, P. D. Nano Lett. 2014, 14, 6275. doi:10.1021/nl502603d
Return to citation in text: [1] -
Scalise, E.; Houssa, M.; Pourtois, G.; Afanas’ev, V.; Stesmans, A. Nano Res. 2012, 5, 43–48. doi:10.1007/s12274-011-0183-0
Return to citation in text: [1] [2] -
Perea-López, N.; Elías, A. L.; Berkdemir, A.; CastroBeltran, A.; Gutiérrez, H. R.; Feng, S.; Lv, R.; Hayashi, T.; López-Urías, F.; Ghosh, S.; Muchharla, B.; Talapatra, S.; Terrones, H.; Terrones, M. Adv. Funct. Mater. 2013, 23, 5511–5517. doi:10.1002/adfm.201300760
Return to citation in text: [1] -
Gao, Y.; Liu, Z.; Sun, D.-M.; Huang, L.; Ma, L.-P.; Yin, L.-C.; Ma, T.; Zhang, Z.; Ma, X.-L.; Peng, L.-M.; Cheng, H.-M.; Ren, W. c. Nat. Commun. 2015, 6, 8569. doi:10.1038/ncomms9569
Return to citation in text: [1] -
Huang, Y.; Sutter, E.; Sadowski, J. T.; Cotlet, M.; Monti, O. L. A.; Racke, D. A.; Neupane, M. R.; Wickramaratne, D.; Lake, R. K.; Parkinson, B. A.; Sutter, P. ACS Nano 2014, 8, 10743. doi:10.1021/nn504481r
Return to citation in text: [1] [2] -
Ramakrishna Matte, H. S. S.; Gomathi, A.; Manna, A. K.; Late, D. J.; Datta, R.; Pati, S. K.; Rao, C. N. R. Angew. Chem., Int. Ed. 2010, 49, 4059–4062. doi:10.1002/anie.201000009
Return to citation in text: [1] -
Zhang, H.; Xia, C.; Zhao, X.; Wang, T.; Li, J. Appl. Surf. Sci. 2015, 356, 1200–1206. doi:10.1016/j.apsusc.2015.08.213
Return to citation in text: [1] -
Huang, Y.; Ling, C.; Chen, X.; Zhou, D.; Wang, S. RSC Adv. 2015, 5, 32505–32510. doi:10.1039/C5RA01211B
Return to citation in text: [1] -
Huang, Y.; Ling, C.; Liu, H.; Wang, S.; Geng, B. J. Phys. Chem. C 2014, 118, 9251–9260. doi:10.1021/jp5013158
Return to citation in text: [1] [2] -
Seo, J.-w.; Jang, J.-t.; Park, S.-w.; Kim, C.; Park, B.; Cheon, J. Adv. Mater. 2008, 20, 4269–4273. doi:10.1002/adma.200703122
Return to citation in text: [1] -
Schlaf, R.; Armstrong, N. R.; Parkinson, B. A.; Pettenkofer, C.; Jaegermann, W. Surf. Sci. 1997, 385, 1–14. doi:10.1016/S0039-6028(97)00066-6
Return to citation in text: [1] -
Su, Y.; Ebrish, M. A.; Olson, E. J.; Koester, S. J. Appl. Phys. Lett. 2013, 103, 8983. doi:10.1063/1.4857495
Return to citation in text: [1] -
Sun, Y.; Cheng, H.; Gao, S.; Sun, Z.; Liu, Q.; Liu, Q.; Lei, F.; Yao, T.; He, J.; Wei, S.; Xie, Y. Angew. Chem., Int. Ed. 2012, 51, 8727. doi:10.1002/ange.201205557
Return to citation in text: [1] -
Su, G.; Hadjiev, V. G.; Loya, P. E.; Zhang, J.; Lei, S.; Maharjan, S.; Dong, P.; Ajayan, P. M.; Lou, J.; Peng, H. Nano Lett. 2015, 15, 506. doi:10.1021/nl503857r
Return to citation in text: [1] -
Pan, T. S.; De, D.; Manongdo, J.; Guloy, A. M.; Hadjiev, V. G.; Lin, Y.; Peng, H. B. Appl. Phys. Lett. 2013, 103, 666. doi:10.1063/1.4819072
Return to citation in text: [1] -
Zhang, Y. C.; Li, J.; Zhang, M.; Dionysiou, D. D. Environ. Sci. Technol. 2011, 45, 9324. doi:10.1021/es202012b
Return to citation in text: [1] -
Choi, J.; Jin, J.; Jung, I. G.; Kim, J. M.; Kim, H. J.; Son, S. U. Chem. Commun. 2011, 47, 5241–5243. doi:10.1039/c1cc10317b
Return to citation in text: [1] -
Zhang, M.; Wu, J.; Zhu, Y.; Dumcenco, D. O.; Hong, J.; Mao, N.; Deng, S.; Chen, Y.; Yang, Y.; Jin, C.; Chaki, S. H.; Huang, Y.-S.; Zhang, J.; Xie, L. ACS Nano 2014, 8, 7130–7137. doi:10.1021/nn5020566
Return to citation in text: [1] -
Xia, C.; An, J.; Wei, S.; Jia, Y.; Zhang, Q. Comput. Mater. Sci. 2014, 95, 712–717. doi:10.1016/j.commatsci.2014.07.002
Return to citation in text: [1] -
Su, S.-H.; Hsu, W.-T.; Hsu, C.-L.; Chen, C.-H.; Chiu, M.-H.; Lin, Y.-C.; Chang, W.-H.; Suenaga, K.; He, J.-H.; Li, L.-J. Front. Energy Res. 2014, 2, 27. doi:10.3389/fenrg.2014.00027
Return to citation in text: [1] -
Komsa, H.-P.; Krasheninnikov, A. V. J. Phys. Chem. Lett. 2012, 3, 3652. doi:10.1021/jz301673x
Return to citation in text: [1] [2] -
Wang, Y.; Huang, L.; Li, B.; Shang, J.; Xia, C.; Fan, C.; Deng, H. X.; Wei, Z.; Li, J. J. Mater. Chem. C 2017, 5, 84–90. doi:10.1039/C6TC03751H
Return to citation in text: [1] [2] [3] [4] [5] -
Huang, Y.; Chen, X.; Zhou, D.; Liu, H.; Wang, C.; Du, J.; Ning, L.; Wang, S. J. Phys. Chem. C 2016, 120, 5839–5847. doi:10.1021/acs.jpcc.6b00794
Return to citation in text: [1] [2] [3] [4] [5] [6] -
Pei, T.; Bao, L.; Wang, G.; Ma, R.; Yang, H.; Li, J.; Gu, C.; Pantelides, S.; Du, S.; Gao, H.-j. Appl. Phys. Lett. 2016, 108, 10451. doi:10.1063/1.4941394
Return to citation in text: [1] -
Liu, H.; Antwi, K. K. A.; Chua, S.; Chi, D. Nanoscale 2014, 6, 624. doi:10.1039/c3nr04515c
Return to citation in text: [1] -
Tongay, S.; Narang, D. S.; Kang, J.; Fan, W.; Ko, C.; Luce, A. V.; Wang, K. X.; Suh, J.; Patel, K. D.; Pathak, V. M.; Li, J.; Wu, J. Appl. Phys. Lett. 2014, 104, 012101. doi:10.1063/1.4834358
Return to citation in text: [1] -
Duan, X.; Wang, C.; Zheng, F.; Hao, G.; Kou, L.; Halim, U.; Li, H.; Wu, X.; Wang, Y.; Jiang, J.; Pan, A.; Huang, Y.; Yu, R.; Duan, X. Nano Lett. 2016, 16, 264. doi:10.1021/acs.nanolett.5b03662
Return to citation in text: [1] -
Fu, Q.; Yang, L.; Wang, W.; Han, A.; Huang, J.; Du, P.; Fan, Z.; Zhang, J.; Xiang, B. Adv. Mater. 2015, 27, 4732–4738. doi:10.1002/adma.201500368
Return to citation in text: [1] -
Yue, Q.; Kang, J.; Shao, Z.; Zhang, X.; Chang, S.; Wang, G.; Qin, S.; Li, J. Phys. Lett. A 2012, 376, 1166–1170. doi:10.1016/j.physleta.2012.02.029
Return to citation in text: [1] [2] -
Liu, G.; Qiu, Y.; Wang, Z.; Zhang, J.; Chen, X.; Dai, M.; Jia, D.; Zhou, Y.; Li, Z.; Hu, P. ACS Appl. Mater. Interfaces 2017, 9, 37750–37759. doi:10.1021/acsami.7b11413
Return to citation in text: [1] -
Meiron, O. E.; Kuraganti, V.; Hod, I.; Bar-Ziv, R.; Bar-Sadan, M. Nanoscale 2017, 9, 13998–14005. doi:10.1039/c7nr04922f
Return to citation in text: [1] -
Xu, K.; Wang, F.; Wang, Z.; Zhan, X.; Wang, Q.; Cheng, Z.; Safdar, M.; He, J. ACS Nano 2014, 8, 8468–8476. doi:10.1021/nn503027k
Return to citation in text: [1] -
Yang, L.; Wang, W.; Fu, Q.; Zhang, J.; Xiang, B. Electrochim. Acta 2015, 185, 236–241. doi:10.1016/j.electacta.2015.10.153
Return to citation in text: [1] -
Topsakal, M.; Cahangirov, S.; Ciraci, S. Appl. Phys. Lett. 2010, 96, 666. doi:10.1063/1.3353968
Return to citation in text: [1] -
Benson, E. E.; Miller, E. M.; Nanayakkara, S. U.; Svedruzic, D.; Ferrere, S.; Neale, N. R.; van de Lagemaat, J.; Gregg, B. A. Chem. Mater. 2017, 29, 2173–2179. doi:10.1021/acs.chemmater.6b04881
Return to citation in text: [1] -
Yan, K.; Kim, S. K.; Khorshidi, A.; Guduru, P. R.; Peterson, A. A. J. Phys. Chem. C 2017, 121, 6177–6183. doi:10.1021/acs.jpcc.7b00281
Return to citation in text: [1] [2] -
Yan, K.; Maark, T. A.; Khorshidi, A.; Sethuraman, V. A.; Peterson, A. A.; Guduru, P. R. Angew. Chem., Int. Ed. 2016, 55, 6175–6181. doi:10.1002/anie.201508613
Return to citation in text: [1] -
Liang, D.; Namburu, R. R.; O’Regan, T. P.; Dubey, M.; Dongare, A. M. J. Mater. Sci. 2014, 49, 6762–6771. doi:10.1007/s10853-014-8370-5
Return to citation in text: [1] -
Yue, Q.; Chang, S.; Kang, J.; Qin, S.; Li, J. Int. J. Photoenergy 2013, 117, 14804–14811. doi:10.1021/jp4021189
Return to citation in text: [1] -
Gao, G.; Jiao, Y.; Ma, F.; Jiao, Y.; Waclawik, E.; Du, A. J. Catal. 2015, 332, 149–155. doi:10.1016/j.jcat.2015.10.006
Return to citation in text: [1] -
Denton, A. R.; Ashcroft, N. W. Phys. Rev. A 1991, 43, 3161. doi:10.1103/PhysRevA.43.3161
Return to citation in text: [1] -
Putungan, D. B.; Lin, S.-H.; Kuo, J.-L. Phys. Chem. Chem. Phys. 2015, 17, 21702–21708. doi:10.1039/c5cp03799a
Return to citation in text: [1] -
Shi, W.; Wang, Z.; Fu, Y. Q. J. Nanopart. Res. 2017, 19, 296. doi:10.1007/s11051-017-3996-2
Return to citation in text: [1] [2] -
Ouyang, Y.; Ling, C.; Chen, Q.; Wang, Z.; Shi, L.; Wang, J. Chem. Mater. 2016, 28, 4390–4396. doi:10.1021/acs.chemmater.6b01395
Return to citation in text: [1] -
Lin, S.-H.; Kuo, J.-L. Phys. Chem. Chem. Phys. 2015, 17, 29305–29310. doi:10.1039/c5cp04760a
Return to citation in text: [1] -
Li, H.; Tsai, C.; Koh, A. L.; Cai, L.; Contryman, A. W.; Fragapane, A. H.; Zhao, J.; Han, H. S.; Manoharan, H. C.; Abild-Pedersen, F.; Norskov, J. K.; Zheng, X. Nat. Mater. 2016, 15, 364. doi:10.1038/nmat4564
Return to citation in text: [1] -
Kresse, G.; Hafner, J. Phys. Rev. B 1993, 47, 558. doi:10.1103/PhysRevB.47.558
Return to citation in text: [1] -
Kresse, G.; Furthmüller, J. Comput. Mater. Sci. 1996, 6, 15–50. doi:10.1016/0927-0256(96)00008-0
Return to citation in text: [1] -
Blöchl, P. E. Phys. Rev. B 1994, 50, 17953. doi:10.1103/PhysRevB.50.17953
Return to citation in text: [1] -
Perdew, J. P.; Chevary, J. A.; Vosko, S. H.; Jackson, K. A.; Pederson, M. R.; Singh, D. J.; Fiolhais, C. Phys. Rev. B 1992, 46, 6671. doi:10.1103/PhysRevB.46.6671
Return to citation in text: [1] -
Gao, G.; Sun, Q.; Du, A. J. Phys. Chem. C 2016, 120, 16761–16766. doi:10.1021/acs.jpcc.6b04692
Return to citation in text: [1] -
Gao, M.-R.; Liang, J.-X.; Zheng, Y.-R.; Xu, Y.-F.; Jiang, J.; Gao, Q.; Li, J.; Yu, S.-H. Nat. Commun. 2015, 6, 5982. doi:10.1038/ncomms6982
Return to citation in text: [1] -
Jiao, Y.; Zheng, Y.; Jaroniec, M.; Qiao, S. Z. Chem. Soc. Rev. 2015, 44, 2060–2086. doi:10.1039/C4CS00470A
Return to citation in text: [1] -
Schmickler, W.; Trasatti, S. J. Electrochem. Soc. 2006, 153, L31. doi:10.1149/1.2358294
Return to citation in text: [1] -
Greeley, J.; Jaramillo, T. F.; Bonde, J.; Chorkendorff, I. B.; Nørskov, J. K. Nat. Mater. 2006, 5, 909–913. doi:10.1038/nmat1752
Return to citation in text: [1] -
Voiry, D.; Yamaguchi, H.; Li, J.; Silva, R.; Alves, D. C.; Fujita, T.; Chen, M.; Asefa, T.; Shenoy, V. B.; Eda, G.; Chhowalla, M. Nat. Mater. 2013, 12, 850–855. doi:10.1038/nmat3700
Return to citation in text: [1] -
Pandey, M.; Vojvodic, A.; Thygesen, K. S.; Jacobsen, K. W. J. Phys. Chem. Lett. 2015, 6, 1577. doi:10.1021/acs.jpclett.5b00353
Return to citation in text: [1] -
Seo, B.; Jung, G. Y.; Sa, Y. J.; Jeong, H. Y.; Cheon, J. Y.; Lee, J. H.; Kim, H. Y.; Kim, J. C.; Shin, H. S.; Kwak, S. K.; Joo, S. H. ACS Nano 2015, 9, 3728. doi:10.1021/acsnano.5b00786
Return to citation in text: [1]
| 49. | Yan, K.; Kim, S. K.; Khorshidi, A.; Guduru, P. R.; Peterson, A. A. J. Phys. Chem. C 2017, 121, 6177–6183. doi:10.1021/acs.jpcc.7b00281 |
| 35. | Wang, Y.; Huang, L.; Li, B.; Shang, J.; Xia, C.; Fan, C.; Deng, H. X.; Wei, Z.; Li, J. J. Mater. Chem. C 2017, 5, 84–90. doi:10.1039/C6TC03751H |
| 36. | Huang, Y.; Chen, X.; Zhou, D.; Liu, H.; Wang, C.; Du, J.; Ning, L.; Wang, S. J. Phys. Chem. C 2016, 120, 5839–5847. doi:10.1021/acs.jpcc.6b00794 |
| 1. | Dincer, I.; Acar, C. Int. J. Hydrogen Energy 2015, 40, 11094–11111. doi:10.1016/j.ijhydene.2014.12.035 |
| 2. | Burton, L. A.; Whittles, T. J.; Hesp, D.; Linhart, W. M.; Skelton, J. M.; Hou, B.; Webster, R. F.; O'Dowd, G.; Reece, C.; Cherns, D.; Fermin, D. J.; Veal, T. D.; Dhanak, V. R.; Walsh, A. J. Mater. Chem. A 2016, 4, 1312–1318. doi:10.1039/c5ta08214e |
| 18. | Huang, Y.; Sutter, E.; Sadowski, J. T.; Cotlet, M.; Monti, O. L. A.; Racke, D. A.; Neupane, M. R.; Wickramaratne, D.; Lake, R. K.; Parkinson, B. A.; Sutter, P. ACS Nano 2014, 8, 10743. doi:10.1021/nn504481r |
| 20. | Zhang, H.; Xia, C.; Zhao, X.; Wang, T.; Li, J. Appl. Surf. Sci. 2015, 356, 1200–1206. doi:10.1016/j.apsusc.2015.08.213 |
| 21. | Huang, Y.; Ling, C.; Chen, X.; Zhou, D.; Wang, S. RSC Adv. 2015, 5, 32505–32510. doi:10.1039/C5RA01211B |
| 22. | Huang, Y.; Ling, C.; Liu, H.; Wang, S.; Geng, B. J. Phys. Chem. C 2014, 118, 9251–9260. doi:10.1021/jp5013158 |
| 23. | Seo, J.-w.; Jang, J.-t.; Park, S.-w.; Kim, C.; Park, B.; Cheon, J. Adv. Mater. 2008, 20, 4269–4273. doi:10.1002/adma.200703122 |
| 39. | Tongay, S.; Narang, D. S.; Kang, J.; Fan, W.; Ko, C.; Luce, A. V.; Wang, K. X.; Suh, J.; Patel, K. D.; Pathak, V. M.; Li, J.; Wu, J. Appl. Phys. Lett. 2014, 104, 012101. doi:10.1063/1.4834358 |
| 55. | Putungan, D. B.; Lin, S.-H.; Kuo, J.-L. Phys. Chem. Chem. Phys. 2015, 17, 21702–21708. doi:10.1039/c5cp03799a |
| 56. | Shi, W.; Wang, Z.; Fu, Y. Q. J. Nanopart. Res. 2017, 19, 296. doi:10.1007/s11051-017-3996-2 |
| 11. | Novoselov, K. S.; Jiang, D.; Schedin, F.; Booth, T. J.; Khotkevich, V. V.; Morozov, S. V.; Geim, A. K. Proc. Natl. Acad. Sci. U. S. A. 2005, 102, 10451–10453. doi:10.1073/pnas.0502848102 |
| 12. | Li, H.; Pan, L.; Lu, T.; Zhan, Y.; Nie, C.; Sun, Z. J. Electroanal. Chem. 2011, 653, 40–44. doi:10.1016/j.jelechem.2011.01.012 |
| 13. | Novoselov, K. S.; Geim, A. K.; Morozov, S. V.; Jiang, D.; Zhang, Y.; Dubonos, S. V.; Grigorieva, I. V.; Firsov, A. A. Science 2004, 306, 666. doi:10.1126/science.1102896 |
| 14. | Yang, L.; Majumdar, K.; Liu, H.; Du, Y.; Wu, H.; Hatzistergos, M.; Hung, P. Y.; Tieckelmann, R.; Tsai, W.; Hobbs, C.; Ye, P. D. Nano Lett. 2014, 14, 6275. doi:10.1021/nl502603d |
| 15. | Scalise, E.; Houssa, M.; Pourtois, G.; Afanas’ev, V.; Stesmans, A. Nano Res. 2012, 5, 43–48. doi:10.1007/s12274-011-0183-0 |
| 16. | Perea-López, N.; Elías, A. L.; Berkdemir, A.; CastroBeltran, A.; Gutiérrez, H. R.; Feng, S.; Lv, R.; Hayashi, T.; López-Urías, F.; Ghosh, S.; Muchharla, B.; Talapatra, S.; Terrones, H.; Terrones, M. Adv. Funct. Mater. 2013, 23, 5511–5517. doi:10.1002/adfm.201300760 |
| 17. | Gao, Y.; Liu, Z.; Sun, D.-M.; Huang, L.; Ma, L.-P.; Yin, L.-C.; Ma, T.; Zhang, Z.; Ma, X.-L.; Peng, L.-M.; Cheng, H.-M.; Ren, W. c. Nat. Commun. 2015, 6, 8569. doi:10.1038/ncomms9569 |
| 18. | Huang, Y.; Sutter, E.; Sadowski, J. T.; Cotlet, M.; Monti, O. L. A.; Racke, D. A.; Neupane, M. R.; Wickramaratne, D.; Lake, R. K.; Parkinson, B. A.; Sutter, P. ACS Nano 2014, 8, 10743. doi:10.1021/nn504481r |
| 19. | Ramakrishna Matte, H. S. S.; Gomathi, A.; Manna, A. K.; Late, D. J.; Datta, R.; Pati, S. K.; Rao, C. N. R. Angew. Chem., Int. Ed. 2010, 49, 4059–4062. doi:10.1002/anie.201000009 |
| 40. | Duan, X.; Wang, C.; Zheng, F.; Hao, G.; Kou, L.; Halim, U.; Li, H.; Wu, X.; Wang, Y.; Jiang, J.; Pan, A.; Huang, Y.; Yu, R.; Duan, X. Nano Lett. 2016, 16, 264. doi:10.1021/acs.nanolett.5b03662 |
| 41. | Fu, Q.; Yang, L.; Wang, W.; Han, A.; Huang, J.; Du, P.; Fan, Z.; Zhang, J.; Xiang, B. Adv. Mater. 2015, 27, 4732–4738. doi:10.1002/adma.201500368 |
| 57. | Ouyang, Y.; Ling, C.; Chen, Q.; Wang, Z.; Shi, L.; Wang, J. Chem. Mater. 2016, 28, 4390–4396. doi:10.1021/acs.chemmater.6b01395 |
| 58. | Lin, S.-H.; Kuo, J.-L. Phys. Chem. Chem. Phys. 2015, 17, 29305–29310. doi:10.1039/c5cp04760a |
| 8. | Bockris, J. O. M.; Ammar, I. A.; Huq, A. K. M. S. J. Phys. Chem. 1957, 61, 879–886. doi:10.1021/j150553a008 |
| 9. | Parsons, R. Trans. Faraday Soc. 1960, 56, 1340–1350. doi:10.1039/TF9605601340 |
| 10. | Walter, M. G.; Warren, E. L.; Mckone, J. R.; Boettcher, S. W.; Mi, Q.; Santori, E. A.; Lewis, N. S. Chem. Rev. 2010, 110, 6446. doi:10.1021/cr1002326 |
| 34. | Komsa, H.-P.; Krasheninnikov, A. V. J. Phys. Chem. Lett. 2012, 3, 3652. doi:10.1021/jz301673x |
| 36. | Huang, Y.; Chen, X.; Zhou, D.; Liu, H.; Wang, C.; Du, J.; Ning, L.; Wang, S. J. Phys. Chem. C 2016, 120, 5839–5847. doi:10.1021/acs.jpcc.6b00794 |
| 3. | Xu, Z.; Ao, Z.; Chu, D.; Younis, A.; Li, C. M.; Li, S. Sci. Rep. 2014, 4, 6450. doi:10.1038/srep06450 |
| 4. | Kudo, A.; Miseki, Y. Chem. Soc. Rev. 2009, 38, 253–278. doi:10.1039/B800489G |
| 5. | Khan, S. U. M.; Al-Shahry, M.; Ingler, W. B., Jr. Science 2002, 297, 2243–2245. doi:10.1126/science.1075035 |
| 6. | Chen, W.-F.; Iyer, S.; Iyer, S.; Sasaki, K.; Wang, C.-H.; Zhu, Y.; Muckerman, J. T.; Fujita, E. Energy Environ. Sci. 2013, 6, 1818–1826. doi:10.1039/C3EE40596F |
| 7. | Chen, W.-F.; Muckerman, J. T.; Fujita, E. Chem. Commun. 2013, 49, 8896. doi:10.1039/c3cc44076a |
| 38. | Liu, H.; Antwi, K. K. A.; Chua, S.; Chi, D. Nanoscale 2014, 6, 624. doi:10.1039/c3nr04515c |
| 36. | Huang, Y.; Chen, X.; Zhou, D.; Liu, H.; Wang, C.; Du, J.; Ning, L.; Wang, S. J. Phys. Chem. C 2016, 120, 5839–5847. doi:10.1021/acs.jpcc.6b00794 |
| 28. | Pan, T. S.; De, D.; Manongdo, J.; Guloy, A. M.; Hadjiev, V. G.; Lin, Y.; Peng, H. B. Appl. Phys. Lett. 2013, 103, 666. doi:10.1063/1.4819072 |
| 30. | Choi, J.; Jin, J.; Jung, I. G.; Kim, J. M.; Kim, H. J.; Son, S. U. Chem. Commun. 2011, 47, 5241–5243. doi:10.1039/c1cc10317b |
| 36. | Huang, Y.; Chen, X.; Zhou, D.; Liu, H.; Wang, C.; Du, J.; Ning, L.; Wang, S. J. Phys. Chem. C 2016, 120, 5839–5847. doi:10.1021/acs.jpcc.6b00794 |
| 27. | Su, G.; Hadjiev, V. G.; Loya, P. E.; Zhang, J.; Lei, S.; Maharjan, S.; Dong, P.; Ajayan, P. M.; Lou, J.; Peng, H. Nano Lett. 2015, 15, 506. doi:10.1021/nl503857r |
| 31. | Zhang, M.; Wu, J.; Zhu, Y.; Dumcenco, D. O.; Hong, J.; Mao, N.; Deng, S.; Chen, Y.; Yang, Y.; Jin, C.; Chaki, S. H.; Huang, Y.-S.; Zhang, J.; Xie, L. ACS Nano 2014, 8, 7130–7137. doi:10.1021/nn5020566 |
| 32. | Xia, C.; An, J.; Wei, S.; Jia, Y.; Zhang, Q. Comput. Mater. Sci. 2014, 95, 712–717. doi:10.1016/j.commatsci.2014.07.002 |
| 33. | Su, S.-H.; Hsu, W.-T.; Hsu, C.-L.; Chen, C.-H.; Chiu, M.-H.; Lin, Y.-C.; Chang, W.-H.; Suenaga, K.; He, J.-H.; Li, L.-J. Front. Energy Res. 2014, 2, 27. doi:10.3389/fenrg.2014.00027 |
| 34. | Komsa, H.-P.; Krasheninnikov, A. V. J. Phys. Chem. Lett. 2012, 3, 3652. doi:10.1021/jz301673x |
| 35. | Wang, Y.; Huang, L.; Li, B.; Shang, J.; Xia, C.; Fan, C.; Deng, H. X.; Wei, Z.; Li, J. J. Mater. Chem. C 2017, 5, 84–90. doi:10.1039/C6TC03751H |
| 36. | Huang, Y.; Chen, X.; Zhou, D.; Liu, H.; Wang, C.; Du, J.; Ning, L.; Wang, S. J. Phys. Chem. C 2016, 120, 5839–5847. doi:10.1021/acs.jpcc.6b00794 |
| 37. | Pei, T.; Bao, L.; Wang, G.; Ma, R.; Yang, H.; Li, J.; Gu, C.; Pantelides, S.; Du, S.; Gao, H.-j. Appl. Phys. Lett. 2016, 108, 10451. doi:10.1063/1.4941394 |
| 35. | Wang, Y.; Huang, L.; Li, B.; Shang, J.; Xia, C.; Fan, C.; Deng, H. X.; Wei, Z.; Li, J. J. Mater. Chem. C 2017, 5, 84–90. doi:10.1039/C6TC03751H |
| 26. | Sun, Y.; Cheng, H.; Gao, S.; Sun, Z.; Liu, Q.; Liu, Q.; Lei, F.; Yao, T.; He, J.; Wei, S.; Xie, Y. Angew. Chem., Int. Ed. 2012, 51, 8727. doi:10.1002/ange.201205557 |
| 35. | Wang, Y.; Huang, L.; Li, B.; Shang, J.; Xia, C.; Fan, C.; Deng, H. X.; Wei, Z.; Li, J. J. Mater. Chem. C 2017, 5, 84–90. doi:10.1039/C6TC03751H |
| 24. | Schlaf, R.; Armstrong, N. R.; Parkinson, B. A.; Pettenkofer, C.; Jaegermann, W. Surf. Sci. 1997, 385, 1–14. doi:10.1016/S0039-6028(97)00066-6 |
| 25. | Su, Y.; Ebrish, M. A.; Olson, E. J.; Koester, S. J. Appl. Phys. Lett. 2013, 103, 8983. doi:10.1063/1.4857495 |
| 29. | Zhang, Y. C.; Li, J.; Zhang, M.; Dionysiou, D. D. Environ. Sci. Technol. 2011, 45, 9324. doi:10.1021/es202012b |
| 54. | Denton, A. R.; Ashcroft, N. W. Phys. Rev. A 1991, 43, 3161. doi:10.1103/PhysRevA.43.3161 |
| 42. | Yue, Q.; Kang, J.; Shao, Z.; Zhang, X.; Chang, S.; Wang, G.; Qin, S.; Li, J. Phys. Lett. A 2012, 376, 1166–1170. doi:10.1016/j.physleta.2012.02.029 |
| 36. | Huang, Y.; Chen, X.; Zhou, D.; Liu, H.; Wang, C.; Du, J.; Ning, L.; Wang, S. J. Phys. Chem. C 2016, 120, 5839–5847. doi:10.1021/acs.jpcc.6b00794 |
| 59. | Li, H.; Tsai, C.; Koh, A. L.; Cai, L.; Contryman, A. W.; Fragapane, A. H.; Zhao, J.; Han, H. S.; Manoharan, H. C.; Abild-Pedersen, F.; Norskov, J. K.; Zheng, X. Nat. Mater. 2016, 15, 364. doi:10.1038/nmat4564 |
| 35. | Wang, Y.; Huang, L.; Li, B.; Shang, J.; Xia, C.; Fan, C.; Deng, H. X.; Wei, Z.; Li, J. J. Mater. Chem. C 2017, 5, 84–90. doi:10.1039/C6TC03751H |
| 56. | Shi, W.; Wang, Z.; Fu, Y. Q. J. Nanopart. Res. 2017, 19, 296. doi:10.1007/s11051-017-3996-2 |
| 60. | Kresse, G.; Hafner, J. Phys. Rev. B 1993, 47, 558. doi:10.1103/PhysRevB.47.558 |
| 61. | Kresse, G.; Furthmüller, J. Comput. Mater. Sci. 1996, 6, 15–50. doi:10.1016/0927-0256(96)00008-0 |
| 42. | Yue, Q.; Kang, J.; Shao, Z.; Zhang, X.; Chang, S.; Wang, G.; Qin, S.; Li, J. Phys. Lett. A 2012, 376, 1166–1170. doi:10.1016/j.physleta.2012.02.029 |
| 47. | Topsakal, M.; Cahangirov, S.; Ciraci, S. Appl. Phys. Lett. 2010, 96, 666. doi:10.1063/1.3353968 |
| 48. | Benson, E. E.; Miller, E. M.; Nanayakkara, S. U.; Svedruzic, D.; Ferrere, S.; Neale, N. R.; van de Lagemaat, J.; Gregg, B. A. Chem. Mater. 2017, 29, 2173–2179. doi:10.1021/acs.chemmater.6b04881 |
| 49. | Yan, K.; Kim, S. K.; Khorshidi, A.; Guduru, P. R.; Peterson, A. A. J. Phys. Chem. C 2017, 121, 6177–6183. doi:10.1021/acs.jpcc.7b00281 |
| 50. | Yan, K.; Maark, T. A.; Khorshidi, A.; Sethuraman, V. A.; Peterson, A. A.; Guduru, P. R. Angew. Chem., Int. Ed. 2016, 55, 6175–6181. doi:10.1002/anie.201508613 |
| 51. | Liang, D.; Namburu, R. R.; O’Regan, T. P.; Dubey, M.; Dongare, A. M. J. Mater. Sci. 2014, 49, 6762–6771. doi:10.1007/s10853-014-8370-5 |
| 52. | Yue, Q.; Chang, S.; Kang, J.; Qin, S.; Li, J. Int. J. Photoenergy 2013, 117, 14804–14811. doi:10.1021/jp4021189 |
| 70. | Pandey, M.; Vojvodic, A.; Thygesen, K. S.; Jacobsen, K. W. J. Phys. Chem. Lett. 2015, 6, 1577. doi:10.1021/acs.jpclett.5b00353 |
| 71. | Seo, B.; Jung, G. Y.; Sa, Y. J.; Jeong, H. Y.; Cheon, J. Y.; Lee, J. H.; Kim, H. Y.; Kim, J. C.; Shin, H. S.; Kwak, S. K.; Joo, S. H. ACS Nano 2015, 9, 3728. doi:10.1021/acsnano.5b00786 |
| 53. | Gao, G.; Jiao, Y.; Ma, F.; Jiao, Y.; Waclawik, E.; Du, A. J. Catal. 2015, 332, 149–155. doi:10.1016/j.jcat.2015.10.006 |
| 45. | Xu, K.; Wang, F.; Wang, Z.; Zhan, X.; Wang, Q.; Cheng, Z.; Safdar, M.; He, J. ACS Nano 2014, 8, 8468–8476. doi:10.1021/nn503027k |
| 66. | Jiao, Y.; Zheng, Y.; Jaroniec, M.; Qiao, S. Z. Chem. Soc. Rev. 2015, 44, 2060–2086. doi:10.1039/C4CS00470A |
| 67. | Schmickler, W.; Trasatti, S. J. Electrochem. Soc. 2006, 153, L31. doi:10.1149/1.2358294 |
| 68. | Greeley, J.; Jaramillo, T. F.; Bonde, J.; Chorkendorff, I. B.; Nørskov, J. K. Nat. Mater. 2006, 5, 909–913. doi:10.1038/nmat1752 |
| 46. | Yang, L.; Wang, W.; Fu, Q.; Zhang, J.; Xiang, B. Electrochim. Acta 2015, 185, 236–241. doi:10.1016/j.electacta.2015.10.153 |
| 69. | Voiry, D.; Yamaguchi, H.; Li, J.; Silva, R.; Alves, D. C.; Fujita, T.; Chen, M.; Asefa, T.; Shenoy, V. B.; Eda, G.; Chhowalla, M. Nat. Mater. 2013, 12, 850–855. doi:10.1038/nmat3700 |
| 43. | Liu, G.; Qiu, Y.; Wang, Z.; Zhang, J.; Chen, X.; Dai, M.; Jia, D.; Zhou, Y.; Li, Z.; Hu, P. ACS Appl. Mater. Interfaces 2017, 9, 37750–37759. doi:10.1021/acsami.7b11413 |
| 64. | Gao, G.; Sun, Q.; Du, A. J. Phys. Chem. C 2016, 120, 16761–16766. doi:10.1021/acs.jpcc.6b04692 |
| 44. | Meiron, O. E.; Kuraganti, V.; Hod, I.; Bar-Ziv, R.; Bar-Sadan, M. Nanoscale 2017, 9, 13998–14005. doi:10.1039/c7nr04922f |
| 65. | Gao, M.-R.; Liang, J.-X.; Zheng, Y.-R.; Xu, Y.-F.; Jiang, J.; Gao, Q.; Li, J.; Yu, S.-H. Nat. Commun. 2015, 6, 5982. doi:10.1038/ncomms6982 |
| 22. | Huang, Y.; Ling, C.; Liu, H.; Wang, S.; Geng, B. J. Phys. Chem. C 2014, 118, 9251–9260. doi:10.1021/jp5013158 |
| 15. | Scalise, E.; Houssa, M.; Pourtois, G.; Afanas’ev, V.; Stesmans, A. Nano Res. 2012, 5, 43–48. doi:10.1007/s12274-011-0183-0 |
| 63. | Perdew, J. P.; Chevary, J. A.; Vosko, S. H.; Jackson, K. A.; Pederson, M. R.; Singh, D. J.; Fiolhais, C. Phys. Rev. B 1992, 46, 6671. doi:10.1103/PhysRevB.46.6671 |
© 2018 Dong and Wang; licensee Beilstein-Institut.
This is an Open Access article under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
The license is subject to the Beilstein Journal of Nanotechnology terms and conditions: (https://www.beilstein-journals.org/bjnano)