Abstract
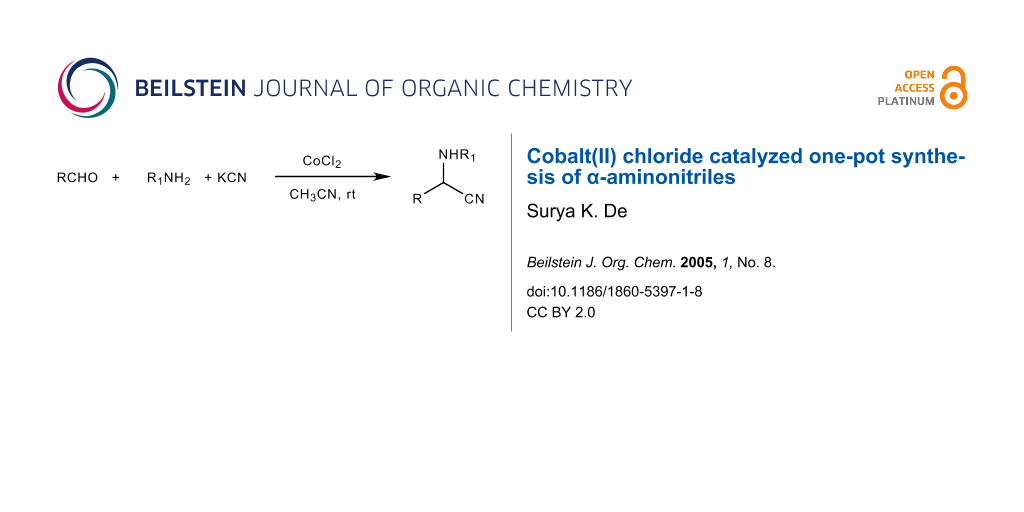
A simple and efficient method has been developed for the synthesis of α-aminonitriles by a one-pot three- component condensation of aldehydes, amines, and potassium cyanide in acetonitrile in the presence of a catalytic amount of CoCl2 at room temperature.
Graphical Abstract
Introduction
The addition of cyanide to imines (the Strecker reaction) [1] provides one of the most direct and viable routes for the synthesis of α-aminonitriles, which are useful intermediates for the synthesis of amino acids and nitrogen-containing heterocycles such as thiadiazoles, imidazoles, etc [2,3]. They are usually prepared by the nucleophilic addition of cyanide anion to imines. Numerous methods describing the preparation of α-aminonitriles have been reported in the literature employing acid catalysts such as InCl3, [4] BiCl3, [5] KSF clay, [6] Sc(OTf)3, [7] Cd(II)-salt, [8] Pt-salt, [9] and other. [10] However, there are still some drawbacks in these catalytic systems including low yields of products, long reaction times, harsh reaction conditions, the requirement for an inert atmosphere, the use of stoichiometric and/or relatively expensive reagents, [4,5,7-9] and also require tedious work-up leading to the generation of a large amount of toxic waste. Therefore, there is a need an efficient and inexpensive catalyst for the synthesis of α-aminonitriles.
In continuation of our work to develop new organic transformations, [11-17] I report herein that cobalt(II) chloride which acts as a mild Lewis acid might be a useful and inexpensive catalyst for the synthesis α-aminonitrile. Although cobalt(II) chloride has been extensively used as a mild catalyst for a plethora of organic transformations, [18-20] there are no examples of the use of cobalt(II) chloride as catalyst for the synthesis of α-aminonitriles.
Results and discussion
The treatment of benzaldehyde and aniline with KCN in the presence of a catalytic amount of CoCl2 afforded the corresponding 2-(N-anilino)-2-phenylacetonitrile in 91% yield. Similarly, a variety of aldehydes were coupled with a wide range of amines and potassium cyanide in a one-pot operation in the presence of a catalytic amount of CoCl2 at room temperature to give the corresponding α-aminonitriles in good to excellent yields (Scheme 1). Both aromatic and aliphatic aldehydes afforded excellent yields whereas ketones did not give any satisfactory yields. On the other hand, all types of primary and secondary amines are readily coupled in good yields. Moreover, acid sensitive aldehyde such as furfuraldehyde reacted in high yield. This method does not require any additives to promote the reaction. The results shown in Table 1 clearly indicate the scope and generality of the reaction with respect to various aldehydes and amines. One reaction was performed for the synthesis of 2-(N-Anilino)-2-phenylacetonitrile (entry 1) using trimethylsilyl cyanide instead of potassium cyanide as cyanide source to give the similar yield. It should be mentioned that both are toxic but potassium cyanide is cheaper than trimethylsilyl cyanide.
Table 1: Cobalt (II) chloride- catalyzed synthesis of α-amino nitriles with potassium cyanide
| Entry | Aldehyde | Amine | Time (h) | Yield a(%) |
|---|---|---|---|---|
| 1 | Benzaldehyde | Aniline | 10 | 91 |
| 2 | 4-Chlorobenzaldehyde | Aniline | 12 | 89 |
| 3 | Decylaldehyde | Aniline | 14 | 76 |
| 4 | 3-Methoxybenzaldehyde | Benzyl amine | 10 | 85 |
| 5 | Furfural | Aniline | 12 | 80 |
| 6 | Thiophene 2-carboxaldehyde | Benzyl amine | 11 | 82 |
| 7 | Benzaldehyde | Morpholine | 12 | 74 |
| 8 | Butyraldehyde | Pyrrolidine | 10 | 80 |
| 9 | 2,4-Dimethoxybenzaldehyde | 3,4,5-Trimethoxyaniline | 11 | 81 |
| 10 | 4-Methoxybenzaldehyde | Aniline | 12 | 83 |
a Yields refer to pure isolated products and were characterized by spectral data.
Conclusion
I have demonstrated a very simple, efficient, and practical method for the synthesis of α-aminonitriles through a one-pot three component coupling of aldehydes, amines, and potassium cyanide using a catalytic amount of cobalt(II) chloride. The major feature of this method is that it is truly a one-pot protocol that does not need a separate step to synthesize an imine for subsequent use. The advantages of this method include (a) operational simplicity, (b) no need any other additive to promote the reaction, (c) short reaction times, (d) the use of relatively cheap commercially available reagents, and (e) high yields of products.
Supporting Information
| Supporting Information File 1: Experimental details. | ||
| Format: DOC | Size: 28.0 KB | Download |
References
-
Shfran, Y. M.; Bakulev, V. S.; Mokrushin, V. S. Russ. Chem. Rev. 1989, 5, 148. doi:10.1070/RC1989v058n02ABEH003432
Return to citation in text: [1] -
Wienstok, L. M.; Davis, P.; Handelsman, B.; Tull, R. J. Org. Chem. 1967, 32, 2823. doi:10.1021/jo01284a040
Return to citation in text: [1] -
Matier, W. L.; Owens, D. A.; Comer, W. T.; Deitchaman, D.; Ferguson, H. C.; Seidehamel, R. J.; Young, J. R. J. Med. Chem. 1973, 16, 901. doi:10.1021/jm00266a008
Return to citation in text: [1] -
Ranu, B. C.; Dey, S. S.; Hajra, A. Tetrahedron 2002, 58, 2529. doi:10.1016/S0040-4020(02)00132-1
Return to citation in text: [1] [2] -
De, S. K.; Gibbs, R. A. Tetrahedron Lett. 2004, 45, 7407. doi:10.1016/j.tetlet.2004.08.071
Return to citation in text: [1] [2] -
Yadav, J. S.; Reddy, B.; Esshwaraih, B.; Srinivas, M. Tetrahedron 2004, 60, 1767. doi:10.1016/j.tet.2003.12.043
Return to citation in text: [1] -
Kobayashi, S.; Basujima, T.; Nagayama, S. Chem. Commun. 1998, 981.
Return to citation in text: [1] [2] -
Ohmuri, O.; Fujita, M. Chem. Commun. 2004, 1586.
Return to citation in text: [1] [2] -
Fossey, J. S.; Richards, C. J. Tetrahedron Lett. 2003, 44, 8773. doi:10.1016/j.tetlet.2003.09.201
Return to citation in text: [1] [2] -
Grover, H. Chem. Rev. 2003, 103, 2795. doi:10.1021/cr020038p
and references cited therein.
Return to citation in text: [1] -
De, S. K. Tetrahedron Lett. 2004, 45, 2339. doi:10.1016/j.tetlet.2004.01.106
Return to citation in text: [1] -
De, S. K. Tetrahedron Lett. 2004, 45, 2919. doi:10.1016/j.tetlet.2004.02.071
Return to citation in text: [1] -
De, S. K.; Gibbs, R. A. Tetrahedron Lett. 2004, 45, 8141. doi:10.1016/j.tetlet.2004.09.060
Return to citation in text: [1] -
De, S. K. Tetrahedron Lett. 2003, 44, 9055. doi:10.1016/j.tetlet.2003.09.208
Return to citation in text: [1] -
De, S. K.; Gibbs, R. A. Tetrahedron Lett. 2005, 46, 1647. doi:10.1016/j.tetlet.2005.01.075
Return to citation in text: [1] -
De, S. K.; Gibbs, R. A. Tetrahedron Lett. 2005, 46, 1811. doi:10.1016/j.tetlet.2005.01.113
Return to citation in text: [1] -
De, S. K. Adv. Synth. Catal. 2005, 347, 673. doi:10.1002/adsc.200404323
Return to citation in text: [1] -
De, S. K. Tetrahedron Lett. 2004, 45, 1035. doi:10.1016/j.tetlet.2003.11.082
Return to citation in text: [1] -
Iqbal, J.; Srivastava, R. R. Tetrahedron Lett. 1991, 32, 1663. doi:10.1016/S0040-4039(00)74299-7
Return to citation in text: [1] -
Velusamy, S.; Borpuzari, S.; Punniyamurthy, T. Tetrahedron 2005, 61, 2001. doi:10.1016/j.tet.2005.01.006
Return to citation in text: [1]
| 1. | Shfran, Y. M.; Bakulev, V. S.; Mokrushin, V. S. Russ. Chem. Rev. 1989, 5, 148. doi:10.1070/RC1989v058n02ABEH003432 |
| 6. | Yadav, J. S.; Reddy, B.; Esshwaraih, B.; Srinivas, M. Tetrahedron 2004, 60, 1767. doi:10.1016/j.tet.2003.12.043 |
| 5. | De, S. K.; Gibbs, R. A. Tetrahedron Lett. 2004, 45, 7407. doi:10.1016/j.tetlet.2004.08.071 |
| 4. | Ranu, B. C.; Dey, S. S.; Hajra, A. Tetrahedron 2002, 58, 2529. doi:10.1016/S0040-4020(02)00132-1 |
| 2. | Wienstok, L. M.; Davis, P.; Handelsman, B.; Tull, R. J. Org. Chem. 1967, 32, 2823. doi:10.1021/jo01284a040 |
| 3. | Matier, W. L.; Owens, D. A.; Comer, W. T.; Deitchaman, D.; Ferguson, H. C.; Seidehamel, R. J.; Young, J. R. J. Med. Chem. 1973, 16, 901. doi:10.1021/jm00266a008 |
| 10. |
Grover, H. Chem. Rev. 2003, 103, 2795. doi:10.1021/cr020038p
and references cited therein. |
| 11. | De, S. K. Tetrahedron Lett. 2004, 45, 2339. doi:10.1016/j.tetlet.2004.01.106 |
| 12. | De, S. K. Tetrahedron Lett. 2004, 45, 2919. doi:10.1016/j.tetlet.2004.02.071 |
| 13. | De, S. K.; Gibbs, R. A. Tetrahedron Lett. 2004, 45, 8141. doi:10.1016/j.tetlet.2004.09.060 |
| 14. | De, S. K. Tetrahedron Lett. 2003, 44, 9055. doi:10.1016/j.tetlet.2003.09.208 |
| 15. | De, S. K.; Gibbs, R. A. Tetrahedron Lett. 2005, 46, 1647. doi:10.1016/j.tetlet.2005.01.075 |
| 16. | De, S. K.; Gibbs, R. A. Tetrahedron Lett. 2005, 46, 1811. doi:10.1016/j.tetlet.2005.01.113 |
| 17. | De, S. K. Adv. Synth. Catal. 2005, 347, 673. doi:10.1002/adsc.200404323 |
| 9. | Fossey, J. S.; Richards, C. J. Tetrahedron Lett. 2003, 44, 8773. doi:10.1016/j.tetlet.2003.09.201 |
| 18. | De, S. K. Tetrahedron Lett. 2004, 45, 1035. doi:10.1016/j.tetlet.2003.11.082 |
| 19. | Iqbal, J.; Srivastava, R. R. Tetrahedron Lett. 1991, 32, 1663. doi:10.1016/S0040-4039(00)74299-7 |
| 20. | Velusamy, S.; Borpuzari, S.; Punniyamurthy, T. Tetrahedron 2005, 61, 2001. doi:10.1016/j.tet.2005.01.006 |
| 4. | Ranu, B. C.; Dey, S. S.; Hajra, A. Tetrahedron 2002, 58, 2529. doi:10.1016/S0040-4020(02)00132-1 |
| 5. | De, S. K.; Gibbs, R. A. Tetrahedron Lett. 2004, 45, 7407. doi:10.1016/j.tetlet.2004.08.071 |
| 7. | Kobayashi, S.; Basujima, T.; Nagayama, S. Chem. Commun. 1998, 981. |
| 8. | Ohmuri, O.; Fujita, M. Chem. Commun. 2004, 1586. |
| 9. | Fossey, J. S.; Richards, C. J. Tetrahedron Lett. 2003, 44, 8773. doi:10.1016/j.tetlet.2003.09.201 |
© 2005 De; licensee Beilstein-Institut.
This is an Open Access article under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
The license is subject to the Beilstein Journal of Organic Chemistry terms and conditions: (http://www.beilstein-journals.org/bjoc)