Abstract
The synthesis of six cyclic depsipeptoids inspired by the natural depsipeptide sansalvamide A is described. An efficient and fast synthetic strategy was developed using a combination of consecutive isocyanide-based multicomponent reactions (Ugi and Passerini reactions). This methodology can be used to access a variety of cyclic oligodepsipeptoids.
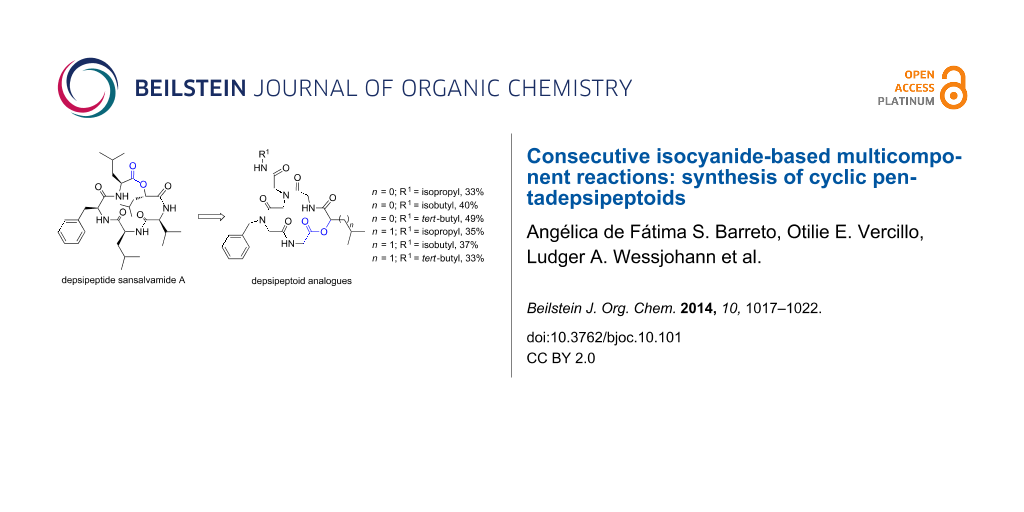
Graphical Abstract
Introduction
Peptoids are an interesting class of non-natural compounds that have recently received much attention due to their wide range of biological activities, which makes them attractive candidates for drug discovery [1-7]. This family of oligomers comprising poly-N-substituted glycines mimics the primary natural structure of peptides and exhibits greater proteolytic stability and increased cellular permeabilities in comparison to peptides [5-7]. A powerful synthetic tool for the preparation of a peptoid backbone is the Ugi four-component reaction (U-4CR) [8-14]. It has been demonstrated that the combination of multicomponent reactions with the use of microwave irradiation is able to efficiently produce complex molecules with a reduced number of steps and short reaction times [15-18].
Depsipeptides are polymeric natural compounds, analogues of peptides, being formed by amino acids and hydroxy acids linked together by amide and ester bonds. These natural products show promising biological activities, especially regarding their therapeutic potential in cancer treatment [19]. An example of a cyclic depsipeptide is sansalvamide A (San A, Figure 1) [20-29], which was isolated from a marine fungus (Fusarium spp.) [20] and exhibits antitumor activity against multiple cancer cell lines. It is cytotoxic against colon (HCT-116) [20,23,25,26], pancreatic (S2-013 and AsPC-1) [22,28,29], prostate (PC-3), breast (MDA-MB231) and melanoma cancers (WM-115) [24]. It has been reported that substitution of an ester group by an amide in the structure of a peptide provides an efficient way to evaluate the role of protein hydrogen bonding [30-34]. Recent works have discovered that some analogues of San A inhibit Hsp 90, a key protein that enables many proteins involved in tumor progression [35-39].
Figure 1: Sansalvamide A (1) and its depsipeptoid analogues (2).
Figure 1: Sansalvamide A (1) and its depsipeptoid analogues (2).
The Passerini three-component reaction (P-3CR) allows an easy access to depsipeptides using a convergent approach. It has become a powerful tool in combinatorial synthesis [40-43] and can be used strategically for the synthesis of depsipeptoids. By analogy to peptides and peptoids, a depsipeptoid would be a peptoid bearing an ester group instead of an amide group. Differences between peptide, peptoid, depsipeptide and depsipeptoid structures are outlined in Figure 2.
Figure 2: Generic structures of (a) peptide, (b) peptoid, (c) depsipeptide and (d) depsipeptoid.
Figure 2: Generic structures of (a) peptide, (b) peptoid, (c) depsipeptide and (d) depsipeptoid.
Results and Discussion
In continuing our research on the synthesis of peptoids with potential pharmacological activity [12,17,18,44,45] and using a fast and efficient microwave-assisted synthesis of peptoids [15,17,18], we decided to carry out the synthesis of depsipeptoid analogues of San A based on a strategy developed in our groups for the synthesis of cyclic RGD pentapeptoids [44]. This strategy was adapted by a combination of microwave-assisted Ugi and Passerini reactions. It is also important to highlight that the synthesis of cyclic depsipeptoids had not been explored yet. In this paper, we describe the synthesis of six pentadepsipeptoid analogues of San A (Figure 3).
Figure 3: Structures of six pentadepsipeptoid analogues of San A.
Figure 3: Structures of six pentadepsipeptoid analogues of San A.
The synthetic route for the synthesis of cyclic pentadepsipeptoids via consecutive Ugi reactions allows only three side chains connected to three nitrogen atoms. The pentapeptide of San A has in its structure five side chains attached to the α carbon atoms: one isopropyl, one benzyl and three isobutyl groups. To generate the pentapeptoid analogues of San A, the side chain groups isobutyl, isopropyl and benzyl linked to the α carbon atom present in the peptide were moved to the nitrogen atoms. It was decided to keep at least one benzyl group in the structure of the peptoids and vary the isopropyl and isobutyl groups, thus maintaining a greater similarity with the structure of the San A depsipeptide.
The retrosynthetic analysis of the depsipeptoids (Scheme 1) shows that the proposed compounds can be achieved using a strategy based on: (a) formation of a peptoid via Ugi reaction; (b) ester hydrolysis; (c) formation of an acyclic depsipeptoid scaffold through a Passerini reaction; (d) deprotection of the amine/acid groups and (e) a macrocyclization step via an intramolecular Ugi reaction.
Scheme 1: Retrosynthetic analysis of the cyclic depsipeptoids.
Scheme 1: Retrosynthetic analysis of the cyclic depsipeptoids.
The general strategy for the synthesis of cyclic pentadepsipeptoids is depicted in Scheme 2. The synthesis of analogues 2 was initiated by an Ugi 4-component reaction (U-4CR) using methyl isocyanoacetate (3a), paraformaldehyde (4), benzylamine (5) and N-Boc-glycine (6) in MeOH (Scheme 2) in a microwave (MW) reactor (3 min at 80 °C) to provide the peptoid 7 in 77–87% yield. Peptoid 7 was subjected to hydrolysis in the presence of LiOH (THF/H2O, 0 °C, 2.5 h) followed by treatment with 2 M NaHSO4 providing the corresponding acid 8 in 92–100% yield. Acid 8 was then employed in a Passerini reaction with isobutyraldehyde (9a) or isovaleraldehyde (9b) and tert-butyl isocyanoacetate (3b), in a MW reactor (20 min at 80 °C) in THF, affording the acyclic depsipeptoids 10a and 10b in 70% and 66% yield, respectively. Removal of the Boc protecting group and ester hydrolysis were achieved after treatment of the acyclic depsipeptoids 10a,b with TFA in CH2Cl2, giving the corresponding amino acids 11a,b as TFA salt in quantitative yields.
Scheme 2: Synthesis of acyclic depsipeptoids 11a,b.
Scheme 2: Synthesis of acyclic depsipeptoids 11a,b.
In the last step, the depsipeptoid amino acid salts 11a,b were subjected to an Ugi three-component four-center reaction (U-3C4CR) (Scheme 3). Compounds 11a,b were added under pseudo-high dilution conditions (addition rate: 0.6 mL/h; addition time: 83 h; concentration: 0.80 mmol/L) to a suspension of paraformaldehyde 4, triethylamine, sodium sulfate and isopropyl/isobutyl/tert-butyl isocyanide 12a–c in methanol to yield the target cyclic pentadepsipeptoids 2a–f after purification by column chromatography (yields ranged from 33–49% depending on the substrate). The structures of the final products obtained are shown in Figure 3.
Conclusion
In summary, the approach developed herein allows the synthesis of a wide range of cyclic depsipeptoids. Different structures can be obtained by changes in the amine component in the first Ugi reaction, in the carbonyl component in the Passerini reaction or in the isocyanide and carbonyl components in the macrocyclization step. The general route and procedure developed allows an easy access to complex molecules with a significantly reduced number of steps in short reaction times, and high yields in most of the steps. The strategic combination of two isocyanide-based multicomponent reactions and microwave irradiation makes this a very useful and attractive protocol. The obtained depsipeptoids will be tested for different biological activities.
Supporting Information
| Supporting Information File 1: General procedures, NMR and mass spectra of all compounds. | ||
| Format: PDF | Size: 2.4 MB | Download |
References
-
Wessjohann, L. A.; Andrade, C. K. Z.; Vercillo, O. E.; Rivera, D. G. Targets Heterocycl. Syst. 2006, 10, 24–53.
Return to citation in text: [1] -
Wessjohann, L. A.; Rhoden, C. R. B.; Rivera, D. G.; Vercillo, O. E. Top. Heterocycl. Chem. 2010, 23, 199–226. doi:10.1007/7081_2009_25
Return to citation in text: [1] -
Messeguer, J.; Masip, I.; Montolio, M.; del Rio, J. A.; Soriano, E.; Messeguer, A. Tetrahedron 2010, 66, 2444–2454. doi:10.1016/j.tet.2010.01.090
Return to citation in text: [1] -
Fowler, S. A.; Blackwell, H. E. Org. Biomol. Chem. 2009, 7, 1508–1524. doi:10.1039/b817980h
Return to citation in text: [1] -
Miller, S. M.; Simon, R. J.; Ng, S.; Zuckermann, R. N.; Kerr, J. M.; Moos, W. H. Bioorg. Med. Chem. Lett. 1994, 4, 2657–2662. doi:10.1016/S0960-894X(01)80691-0
Return to citation in text: [1] [2] -
Wender, P. A.; Mitchell, D. J.; Pattabiraman, K.; Pelkey, E. T.; Steinman, L.; Rothbard, J. B. Proc. Natl. Acad. Sci. U. S. A. 2000, 97, 13003–13008. doi:10.1073/pnas.97.24.13003
Return to citation in text: [1] [2] -
Kwon, Y.-U.; Kodadek, T. J. Am. Chem. Soc. 2007, 129, 1508–1509. doi:10.1021/ja0668623
Return to citation in text: [1] [2] -
Wessjohann, L. A.; Rivera, D. G.; Vercillo, O. E. Chem. Rev. 2009, 109, 796–814. doi:10.1021/cr8003407
Return to citation in text: [1] -
Dömling, A. Chem. Rev. 2006, 106, 17–89. doi:10.1021/cr0505728
Return to citation in text: [1] -
Dömling, A.; Wang, W.; Wang, K. Chem. Rev. 2012, 112, 3083–3135. doi:10.1021/cr100233r
Return to citation in text: [1] -
Dömling, A.; Beck, B.; Eichelberger, U.; Sakamuri, S.; Menon, S.; Chen, Q.-Z.; Lu, Y.; Wessjohann, L. A. Angew. Chem., Int. Ed. 2006, 45, 7235–7239. doi:10.1002/anie.200601259
Erratum: Angew. Chem., Int. Ed. 2007, 46, 2347–2348. doi:10.1002/anie.200790053
Return to citation in text: [1] -
Pando, O.; Stark, S.; Denkert, A.; Porzel, A.; Preusentanz, R.; Wessjohann, L. J. Am. Chem. Soc. 2011, 133, 7692–7695. doi:10.1021/ja2022027
Return to citation in text: [1] [2] -
Rivera, D. G.; León, F.; Concepción, O.; Morales, F. E.; Wessjohann, L. A. Chem.–Eur. J. 2013, 19, 6417–6428. doi:10.1002/chem.201201591
Return to citation in text: [1] -
Neves Filho, R. A. W.; Stark, S.; Westermann, B.; Wessjohann, L. A. Beilstein J. Org. Chem. 2012, 8, 2085–2090. doi:10.3762/bjoc.8.234
Return to citation in text: [1] -
Barreto, A. F. S.; Vercillo, O. E.; Birkett, M. A.; Caulfied, J. C.; Wessjohann, L. A.; Andrade, C. K. Z. Org. Biomol. Chem. 2011, 9, 5024–5027. doi:10.1039/c1ob05471f
Return to citation in text: [1] [2] -
Barreto, A. F. S.; Vercillo, O. E.; Andrade, C. K. Z. J. Braz. Chem. Soc. 2011, 22, 462–467. doi:10.1590/S0103-50532011000300008
Return to citation in text: [1] -
Barreto, A. F. S. Reações Multicomponentes de Isocianetos Consecutivas Assistidas por Micro-ondas: Síntese de Ciclopeptóides e Ciclodepsipeptóides Análogos da Verticilida e Sansalvamida A. Ph.D. Thesis, Universidade de Brasília, Brazil, 2013.
Return to citation in text: [1] [2] [3] -
Barreto, A. F. S.; Vercillo, O. E.; Wessjohann, L. A.; Andrade, C. K. Z. Blucher Chem. Proc. 2011, 1, 298.
http://blucherproceedings.com.br/articles/download/2095
Return to citation in text: [1] [2] [3] -
Hamel, E.; Covell, D. G. Curr. Med. Chem.: Anti-Cancer Agents 2002, 2, 19–53. doi:10.2174/1568011023354263
Return to citation in text: [1] -
Belofsky, G. N.; Jensen, P. R.; Fenical, W. T. Tetrahedron Lett. 1999, 40, 2913–2916. doi:10.1016/S0040-4039(99)00393-7
Return to citation in text: [1] [2] [3] -
Lee, Y.; Silverman, R. B. Org. Lett. 2000, 2, 3743–3746. doi:10.1021/ol0002830
Return to citation in text: [1] -
Gu, W.; Liu, S.; Silverman, R. B. Org. Lett. 2002, 4, 4171–4174. doi:10.1021/ol0269392
Return to citation in text: [1] [2] -
Carroll, C. L.; Johnston, J. V. C.; Kekec, A.; Brown, J. D.; Parry, E.; Cajica, J.; Medina, I.; Cook, K. M.; Corral, R.; Pan, P.-S.; McAlpine, S. R. Org. Lett. 2005, 7, 3481–3484. doi:10.1021/ol051161g
Return to citation in text: [1] [2] -
Liu, S.; Gu, W.; Lo, D.; Ding, X.-Z.; Ujiki, M.; Adrian, T. E.; Soff, G. A.; Silverman, R. B. J. Med. Chem. 2005, 48, 3630–3638. doi:10.1021/jm048952t
Return to citation in text: [1] [2] -
Otrubova, K.; Styers, T. J.; Pan, P.-S.; Rodriguez, R.; McGuire, K. L.; McAlpine, S. R. Chem. Commun. 2006, 1033–1034. doi:10.1039/b517434a
Return to citation in text: [1] [2] -
Styers, T. J.; Kekec, A.; Rodriguez, R.; Brown, J. D.; Cajica, J.; Pan, P.-S.; Parry, E.; Carroll, C. L.; Medina, I.; Corral, R.; Lapera, S.; Otrubova, K.; Pan, C.-M.; McGuire, K. L.; McAlpine, S. R. Bioorg. Med. Chem. 2006, 14, 5625–5631. doi:10.1016/j.bmc.2006.04.031
Return to citation in text: [1] [2] -
Rodriguez, R.; Pan, P.-S.; Pan, C.-M.; Ravula, S.; Lapera, S.; Singh, E.; Styers, T. J.; Brown, J. D.; Cajica, J.; Parry, E.; Otrubova, K.; McAlpine, S. R. J. Org. Chem. 2007, 72, 1980–2002. doi:10.1021/jo061830j
Return to citation in text: [1] -
Pan, P.-S.; McGuire, K. L.; McAlpine, S. R. Bioorg. Med. Chem. Lett. 2007, 17, 5072–5077. doi:10.1016/j.bmcl.2007.07.025
Return to citation in text: [1] [2] -
Davis, M. R.; Styers, T. J.; Rodriguez, R. A.; Pan, P.-S.; Vasko, R. C.; McAlpine, S. R. Org. Lett. 2008, 10, 177–180. doi:10.1021/ol702403r
Return to citation in text: [1] [2] -
Seebach, D.; Mahajan, Y. R.; Senthilkumar, R.; Rueping, M.; Jaun, B. Chem. Commun. 2002, 1598–1599. doi:10.1039/b204187c
Return to citation in text: [1] -
Aravinda, S.; Shamala, N.; Das, C.; Balaram, P. Biopolymers 2002, 64, 255–267. doi:10.1002/bip.10192
Return to citation in text: [1] -
Haque, T. S.; Little, J. C.; Gellman, S. H. J. Am. Chem. Soc. 1996, 118, 6975–6985. doi:10.1021/ja960429j
Return to citation in text: [1] -
Yang, X.; Wang, M.; Fitzgerald, M. C. Bioorg. Chem. 2004, 32, 438–449. doi:10.1016/j.bioorg.2004.06.011
Return to citation in text: [1] -
Powers, E. T.; Deechongkit, S.; Kelly, J. W. Adv. Protein Chem. 2005, 72, 39–78. doi:10.1016/S0065-3233(05)72002-7
Return to citation in text: [1] -
Vasko, R. C.; Rodriguez, R. A.; Cunningham, C. N.; Ardi, V. C.; Agard, D. A.; McAlpine, S. R. ACS Med. Chem. Lett. 2010, 1, 4–8. doi:10.1021/ml900003t
Return to citation in text: [1] -
Sellers, R. P.; Alexander, L. D.; Johnson, V. A.; Lin, C.-C.; Savage, J.; Corral, R.; Moss, J.; Slugocki, T. L.; Singh, E. K.; Davis, M. R.; Ravula, S.; Spicer, J. E.; Oelrich, J. L.; Thornquist, A.; Pan, C.-M.; McAlpine, S. R. Bioorg. Med. Chem. 2010, 18, 6822–6856. doi:10.1016/j.bmc.2010.07.042
Return to citation in text: [1] -
Ardi, V. C.; Alexander, L. D.; Johnson, V. A.; McAlpine, S. R. ACS Chem. Biol. 2011, 6, 1357–1366. doi:10.1021/cb200203m
Return to citation in text: [1] -
Kunicki, J. B.; Petersen, M. N.; Alexander, L. D.; Ardi, V. C.; McConnell, J. R.; McAlpine, S. R. Bioorg. Med. Chem. Lett. 2011, 21, 4716–4719. doi:10.1016/j.bmcl.2011.06.083
Return to citation in text: [1] -
Davis, M. R.; Singh, E. K.; Wahyudi, H.; Alexander, L. D.; Kunicki, J. B.; Nazarova, L. A.; Fairweather, K. A.; Giltrap, A. M.; Jolliffe, K. A.; McAlpine, S. R. Tetrahedron 2012, 68, 1029–1051. doi:10.1016/j.tet.2011.11.089
Return to citation in text: [1] -
Berłożecki, S.; Szymanski, W.; Ostaszewski, R. Tetrahedron 2008, 64, 9780–9783. doi:10.1016/j.tet.2008.07.064
Return to citation in text: [1] -
Gulevich, A. V.; Shpilevaya, I. V.; Nenajdenko, V. G. Eur. J. Org. Chem. 2009, 3801–3808. doi:10.1002/ejoc.200900330
Return to citation in text: [1] -
Leon, F.; Rivera, D. G.; Wessjohann, L. A. J. Org. Chem. 2008, 73, 1762–1767. doi:10.1021/jo7022125
Return to citation in text: [1] -
Henze, M.; Kreye, O.; Brauch, S.; Nitsche, C.; Naumann, K.; Wessjohann, L. A.; Westermann, B. Synthesis 2010, 2997–3003. doi:10.1055/s-0030-1258182
Return to citation in text: [1] -
Vercillo, O. E.; Andrade, C. K. Z.; Wessjohann, L. A. Org. Lett. 2008, 10, 205–208. doi:10.1021/ol702521g
Return to citation in text: [1] [2] -
Neves Filho, R. A. W.; Westermann, B.; Wessjohann, L. A. Beilstein J. Org. Chem. 2011, 7, 1504–1507. doi:10.3762/bjoc.7.175
Return to citation in text: [1]
| 1. | Wessjohann, L. A.; Andrade, C. K. Z.; Vercillo, O. E.; Rivera, D. G. Targets Heterocycl. Syst. 2006, 10, 24–53. |
| 2. | Wessjohann, L. A.; Rhoden, C. R. B.; Rivera, D. G.; Vercillo, O. E. Top. Heterocycl. Chem. 2010, 23, 199–226. doi:10.1007/7081_2009_25 |
| 3. | Messeguer, J.; Masip, I.; Montolio, M.; del Rio, J. A.; Soriano, E.; Messeguer, A. Tetrahedron 2010, 66, 2444–2454. doi:10.1016/j.tet.2010.01.090 |
| 4. | Fowler, S. A.; Blackwell, H. E. Org. Biomol. Chem. 2009, 7, 1508–1524. doi:10.1039/b817980h |
| 5. | Miller, S. M.; Simon, R. J.; Ng, S.; Zuckermann, R. N.; Kerr, J. M.; Moos, W. H. Bioorg. Med. Chem. Lett. 1994, 4, 2657–2662. doi:10.1016/S0960-894X(01)80691-0 |
| 6. | Wender, P. A.; Mitchell, D. J.; Pattabiraman, K.; Pelkey, E. T.; Steinman, L.; Rothbard, J. B. Proc. Natl. Acad. Sci. U. S. A. 2000, 97, 13003–13008. doi:10.1073/pnas.97.24.13003 |
| 7. | Kwon, Y.-U.; Kodadek, T. J. Am. Chem. Soc. 2007, 129, 1508–1509. doi:10.1021/ja0668623 |
| 19. | Hamel, E.; Covell, D. G. Curr. Med. Chem.: Anti-Cancer Agents 2002, 2, 19–53. doi:10.2174/1568011023354263 |
| 15. | Barreto, A. F. S.; Vercillo, O. E.; Birkett, M. A.; Caulfied, J. C.; Wessjohann, L. A.; Andrade, C. K. Z. Org. Biomol. Chem. 2011, 9, 5024–5027. doi:10.1039/c1ob05471f |
| 17. | Barreto, A. F. S. Reações Multicomponentes de Isocianetos Consecutivas Assistidas por Micro-ondas: Síntese de Ciclopeptóides e Ciclodepsipeptóides Análogos da Verticilida e Sansalvamida A. Ph.D. Thesis, Universidade de Brasília, Brazil, 2013. |
| 18. |
Barreto, A. F. S.; Vercillo, O. E.; Wessjohann, L. A.; Andrade, C. K. Z. Blucher Chem. Proc. 2011, 1, 298.
http://blucherproceedings.com.br/articles/download/2095 |
| 15. | Barreto, A. F. S.; Vercillo, O. E.; Birkett, M. A.; Caulfied, J. C.; Wessjohann, L. A.; Andrade, C. K. Z. Org. Biomol. Chem. 2011, 9, 5024–5027. doi:10.1039/c1ob05471f |
| 16. | Barreto, A. F. S.; Vercillo, O. E.; Andrade, C. K. Z. J. Braz. Chem. Soc. 2011, 22, 462–467. doi:10.1590/S0103-50532011000300008 |
| 17. | Barreto, A. F. S. Reações Multicomponentes de Isocianetos Consecutivas Assistidas por Micro-ondas: Síntese de Ciclopeptóides e Ciclodepsipeptóides Análogos da Verticilida e Sansalvamida A. Ph.D. Thesis, Universidade de Brasília, Brazil, 2013. |
| 18. |
Barreto, A. F. S.; Vercillo, O. E.; Wessjohann, L. A.; Andrade, C. K. Z. Blucher Chem. Proc. 2011, 1, 298.
http://blucherproceedings.com.br/articles/download/2095 |
| 44. | Vercillo, O. E.; Andrade, C. K. Z.; Wessjohann, L. A. Org. Lett. 2008, 10, 205–208. doi:10.1021/ol702521g |
| 8. | Wessjohann, L. A.; Rivera, D. G.; Vercillo, O. E. Chem. Rev. 2009, 109, 796–814. doi:10.1021/cr8003407 |
| 9. | Dömling, A. Chem. Rev. 2006, 106, 17–89. doi:10.1021/cr0505728 |
| 10. | Dömling, A.; Wang, W.; Wang, K. Chem. Rev. 2012, 112, 3083–3135. doi:10.1021/cr100233r |
| 11. |
Dömling, A.; Beck, B.; Eichelberger, U.; Sakamuri, S.; Menon, S.; Chen, Q.-Z.; Lu, Y.; Wessjohann, L. A. Angew. Chem., Int. Ed. 2006, 45, 7235–7239. doi:10.1002/anie.200601259
Erratum: Angew. Chem., Int. Ed. 2007, 46, 2347–2348. doi:10.1002/anie.200790053 |
| 12. | Pando, O.; Stark, S.; Denkert, A.; Porzel, A.; Preusentanz, R.; Wessjohann, L. J. Am. Chem. Soc. 2011, 133, 7692–7695. doi:10.1021/ja2022027 |
| 13. | Rivera, D. G.; León, F.; Concepción, O.; Morales, F. E.; Wessjohann, L. A. Chem.–Eur. J. 2013, 19, 6417–6428. doi:10.1002/chem.201201591 |
| 14. | Neves Filho, R. A. W.; Stark, S.; Westermann, B.; Wessjohann, L. A. Beilstein J. Org. Chem. 2012, 8, 2085–2090. doi:10.3762/bjoc.8.234 |
| 40. | Berłożecki, S.; Szymanski, W.; Ostaszewski, R. Tetrahedron 2008, 64, 9780–9783. doi:10.1016/j.tet.2008.07.064 |
| 41. | Gulevich, A. V.; Shpilevaya, I. V.; Nenajdenko, V. G. Eur. J. Org. Chem. 2009, 3801–3808. doi:10.1002/ejoc.200900330 |
| 42. | Leon, F.; Rivera, D. G.; Wessjohann, L. A. J. Org. Chem. 2008, 73, 1762–1767. doi:10.1021/jo7022125 |
| 43. | Henze, M.; Kreye, O.; Brauch, S.; Nitsche, C.; Naumann, K.; Wessjohann, L. A.; Westermann, B. Synthesis 2010, 2997–3003. doi:10.1055/s-0030-1258182 |
| 5. | Miller, S. M.; Simon, R. J.; Ng, S.; Zuckermann, R. N.; Kerr, J. M.; Moos, W. H. Bioorg. Med. Chem. Lett. 1994, 4, 2657–2662. doi:10.1016/S0960-894X(01)80691-0 |
| 6. | Wender, P. A.; Mitchell, D. J.; Pattabiraman, K.; Pelkey, E. T.; Steinman, L.; Rothbard, J. B. Proc. Natl. Acad. Sci. U. S. A. 2000, 97, 13003–13008. doi:10.1073/pnas.97.24.13003 |
| 7. | Kwon, Y.-U.; Kodadek, T. J. Am. Chem. Soc. 2007, 129, 1508–1509. doi:10.1021/ja0668623 |
| 12. | Pando, O.; Stark, S.; Denkert, A.; Porzel, A.; Preusentanz, R.; Wessjohann, L. J. Am. Chem. Soc. 2011, 133, 7692–7695. doi:10.1021/ja2022027 |
| 17. | Barreto, A. F. S. Reações Multicomponentes de Isocianetos Consecutivas Assistidas por Micro-ondas: Síntese de Ciclopeptóides e Ciclodepsipeptóides Análogos da Verticilida e Sansalvamida A. Ph.D. Thesis, Universidade de Brasília, Brazil, 2013. |
| 18. |
Barreto, A. F. S.; Vercillo, O. E.; Wessjohann, L. A.; Andrade, C. K. Z. Blucher Chem. Proc. 2011, 1, 298.
http://blucherproceedings.com.br/articles/download/2095 |
| 44. | Vercillo, O. E.; Andrade, C. K. Z.; Wessjohann, L. A. Org. Lett. 2008, 10, 205–208. doi:10.1021/ol702521g |
| 45. | Neves Filho, R. A. W.; Westermann, B.; Wessjohann, L. A. Beilstein J. Org. Chem. 2011, 7, 1504–1507. doi:10.3762/bjoc.7.175 |
| 22. | Gu, W.; Liu, S.; Silverman, R. B. Org. Lett. 2002, 4, 4171–4174. doi:10.1021/ol0269392 |
| 28. | Pan, P.-S.; McGuire, K. L.; McAlpine, S. R. Bioorg. Med. Chem. Lett. 2007, 17, 5072–5077. doi:10.1016/j.bmcl.2007.07.025 |
| 29. | Davis, M. R.; Styers, T. J.; Rodriguez, R. A.; Pan, P.-S.; Vasko, R. C.; McAlpine, S. R. Org. Lett. 2008, 10, 177–180. doi:10.1021/ol702403r |
| 30. | Seebach, D.; Mahajan, Y. R.; Senthilkumar, R.; Rueping, M.; Jaun, B. Chem. Commun. 2002, 1598–1599. doi:10.1039/b204187c |
| 31. | Aravinda, S.; Shamala, N.; Das, C.; Balaram, P. Biopolymers 2002, 64, 255–267. doi:10.1002/bip.10192 |
| 32. | Haque, T. S.; Little, J. C.; Gellman, S. H. J. Am. Chem. Soc. 1996, 118, 6975–6985. doi:10.1021/ja960429j |
| 33. | Yang, X.; Wang, M.; Fitzgerald, M. C. Bioorg. Chem. 2004, 32, 438–449. doi:10.1016/j.bioorg.2004.06.011 |
| 34. | Powers, E. T.; Deechongkit, S.; Kelly, J. W. Adv. Protein Chem. 2005, 72, 39–78. doi:10.1016/S0065-3233(05)72002-7 |
| 20. | Belofsky, G. N.; Jensen, P. R.; Fenical, W. T. Tetrahedron Lett. 1999, 40, 2913–2916. doi:10.1016/S0040-4039(99)00393-7 |
| 23. | Carroll, C. L.; Johnston, J. V. C.; Kekec, A.; Brown, J. D.; Parry, E.; Cajica, J.; Medina, I.; Cook, K. M.; Corral, R.; Pan, P.-S.; McAlpine, S. R. Org. Lett. 2005, 7, 3481–3484. doi:10.1021/ol051161g |
| 25. | Otrubova, K.; Styers, T. J.; Pan, P.-S.; Rodriguez, R.; McGuire, K. L.; McAlpine, S. R. Chem. Commun. 2006, 1033–1034. doi:10.1039/b517434a |
| 26. | Styers, T. J.; Kekec, A.; Rodriguez, R.; Brown, J. D.; Cajica, J.; Pan, P.-S.; Parry, E.; Carroll, C. L.; Medina, I.; Corral, R.; Lapera, S.; Otrubova, K.; Pan, C.-M.; McGuire, K. L.; McAlpine, S. R. Bioorg. Med. Chem. 2006, 14, 5625–5631. doi:10.1016/j.bmc.2006.04.031 |
| 35. | Vasko, R. C.; Rodriguez, R. A.; Cunningham, C. N.; Ardi, V. C.; Agard, D. A.; McAlpine, S. R. ACS Med. Chem. Lett. 2010, 1, 4–8. doi:10.1021/ml900003t |
| 36. | Sellers, R. P.; Alexander, L. D.; Johnson, V. A.; Lin, C.-C.; Savage, J.; Corral, R.; Moss, J.; Slugocki, T. L.; Singh, E. K.; Davis, M. R.; Ravula, S.; Spicer, J. E.; Oelrich, J. L.; Thornquist, A.; Pan, C.-M.; McAlpine, S. R. Bioorg. Med. Chem. 2010, 18, 6822–6856. doi:10.1016/j.bmc.2010.07.042 |
| 37. | Ardi, V. C.; Alexander, L. D.; Johnson, V. A.; McAlpine, S. R. ACS Chem. Biol. 2011, 6, 1357–1366. doi:10.1021/cb200203m |
| 38. | Kunicki, J. B.; Petersen, M. N.; Alexander, L. D.; Ardi, V. C.; McConnell, J. R.; McAlpine, S. R. Bioorg. Med. Chem. Lett. 2011, 21, 4716–4719. doi:10.1016/j.bmcl.2011.06.083 |
| 39. | Davis, M. R.; Singh, E. K.; Wahyudi, H.; Alexander, L. D.; Kunicki, J. B.; Nazarova, L. A.; Fairweather, K. A.; Giltrap, A. M.; Jolliffe, K. A.; McAlpine, S. R. Tetrahedron 2012, 68, 1029–1051. doi:10.1016/j.tet.2011.11.089 |
| 20. | Belofsky, G. N.; Jensen, P. R.; Fenical, W. T. Tetrahedron Lett. 1999, 40, 2913–2916. doi:10.1016/S0040-4039(99)00393-7 |
| 20. | Belofsky, G. N.; Jensen, P. R.; Fenical, W. T. Tetrahedron Lett. 1999, 40, 2913–2916. doi:10.1016/S0040-4039(99)00393-7 |
| 21. | Lee, Y.; Silverman, R. B. Org. Lett. 2000, 2, 3743–3746. doi:10.1021/ol0002830 |
| 22. | Gu, W.; Liu, S.; Silverman, R. B. Org. Lett. 2002, 4, 4171–4174. doi:10.1021/ol0269392 |
| 23. | Carroll, C. L.; Johnston, J. V. C.; Kekec, A.; Brown, J. D.; Parry, E.; Cajica, J.; Medina, I.; Cook, K. M.; Corral, R.; Pan, P.-S.; McAlpine, S. R. Org. Lett. 2005, 7, 3481–3484. doi:10.1021/ol051161g |
| 24. | Liu, S.; Gu, W.; Lo, D.; Ding, X.-Z.; Ujiki, M.; Adrian, T. E.; Soff, G. A.; Silverman, R. B. J. Med. Chem. 2005, 48, 3630–3638. doi:10.1021/jm048952t |
| 25. | Otrubova, K.; Styers, T. J.; Pan, P.-S.; Rodriguez, R.; McGuire, K. L.; McAlpine, S. R. Chem. Commun. 2006, 1033–1034. doi:10.1039/b517434a |
| 26. | Styers, T. J.; Kekec, A.; Rodriguez, R.; Brown, J. D.; Cajica, J.; Pan, P.-S.; Parry, E.; Carroll, C. L.; Medina, I.; Corral, R.; Lapera, S.; Otrubova, K.; Pan, C.-M.; McGuire, K. L.; McAlpine, S. R. Bioorg. Med. Chem. 2006, 14, 5625–5631. doi:10.1016/j.bmc.2006.04.031 |
| 27. | Rodriguez, R.; Pan, P.-S.; Pan, C.-M.; Ravula, S.; Lapera, S.; Singh, E.; Styers, T. J.; Brown, J. D.; Cajica, J.; Parry, E.; Otrubova, K.; McAlpine, S. R. J. Org. Chem. 2007, 72, 1980–2002. doi:10.1021/jo061830j |
| 28. | Pan, P.-S.; McGuire, K. L.; McAlpine, S. R. Bioorg. Med. Chem. Lett. 2007, 17, 5072–5077. doi:10.1016/j.bmcl.2007.07.025 |
| 29. | Davis, M. R.; Styers, T. J.; Rodriguez, R. A.; Pan, P.-S.; Vasko, R. C.; McAlpine, S. R. Org. Lett. 2008, 10, 177–180. doi:10.1021/ol702403r |
| 24. | Liu, S.; Gu, W.; Lo, D.; Ding, X.-Z.; Ujiki, M.; Adrian, T. E.; Soff, G. A.; Silverman, R. B. J. Med. Chem. 2005, 48, 3630–3638. doi:10.1021/jm048952t |
© 2014 Barreto et al; licensee Beilstein-Institut.
This is an Open Access article under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
The license is subject to the Beilstein Journal of Organic Chemistry terms and conditions: (http://www.beilstein-journals.org/bjoc)