Abstract
A very simple and convenient reaction for 1,3-diketone preparation from carboxylic acids and aromatic ketones in TFAA/TfOH system is described. When the β-phenylpropionic acids were used as starting materials, they initially gave 1-indanones and then underwent further acylation with the formation of 2-(β-phenylpropionyl)-1-indanones as the main reaction products. In addition, the application of the proposed protocol allowed for the synthesis of selected polysubstituted pyrazoles in a one-pot procedure directly from acids and ketones.
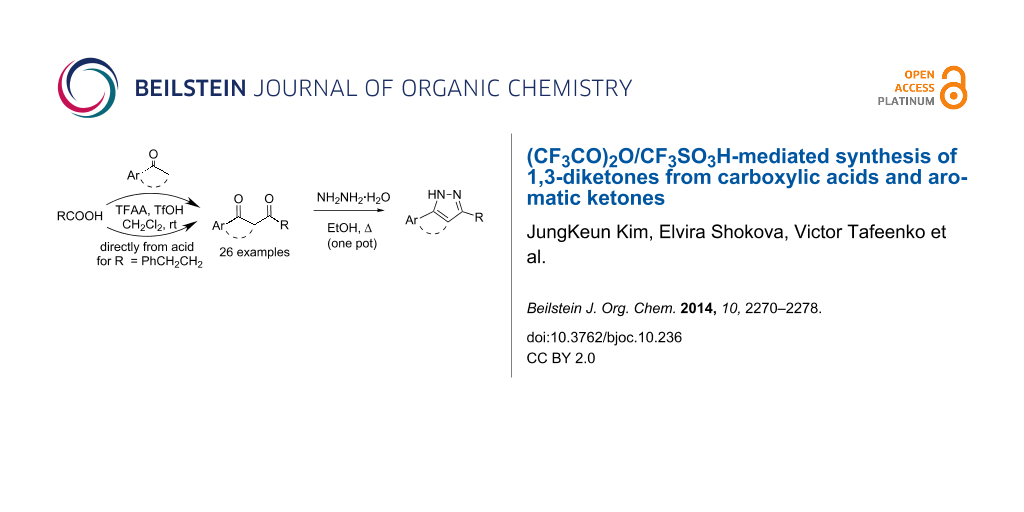
Graphical Abstract
Introduction
1,3-Diketones represent one of the most important class of organic compounds, since they are applied as key structural blocks in organic syntheses, exhibit different kinds of biological activities, and display a broad range of ionophoric properties [1-3]. The method most frequently used for 1,3-diketone synthesis is the Claisen condensation, which comprises the C-acylation of the α-position of ketones in the form of their metal enolates, enamines or silyl ethers, with or without a catalyst. To appear as an acylating agent one of the following compounds could be required: acyl halides and acid esters, including formates and oxalates, and acid anhydrides, dialkyl carbonates, methoxymagnesium methyl carbonate, N-acylimidazoles, acyl cyanides, and acylbenzotriazoles [4]. Though recently many modifications of this method have been proposed [5-11], it is noticeable that none of these techniques implements a direct synthesis of β-diketones from acids and ketones with an immediate activation of both carbonyl and methylene components in the course of the reaction.
Herein, we would like to describe a direct and operationally simple TFAA/TfOH-mediated synthesis of 1,3-diketones from unmodified carboxylic acids and ketones.
Results and Discussion
Initially, an unusual transformation of the β-phenylpropionic acids was observed by us in a TFAA/TfOH/CH2Cl2 system (Table 1), which gave the impulse for this research. Surprisingly, it turned out that β-phenylpropionic acid (1a) in a TFAA/CH2Cl2 medium in the presence of TfOH (0.25 equiv, Table 1, entry 1) gave 2-(β-phenylpropionyl)-1-indanone (3а) in 51% yield as the major product, even though we expected 1-indanone (2а, <2%). When 0.5 equiv of TfOH was applied, 3а and 2a were obtained in 75 and 16% yield, respectively (Table 1, entry 2). Evidently, in this reaction, 1-indanone (2а), which was initially formed as the result of an intramolecular cyclization of 1a, underwent a further acylation with the formation of 1,3-diketone 3a. In contrast, γ-phenylbutanoic acid (1c) was quantitatively transformed only to the tetralone 2с (Table 1, entries 10 and 11).
Table 1: TFAA/TfOH-mediated self-acylation of ω-phenylalkanoic acids 1a–ca.
|
|
|||||
| Entry | 1 | TfOH (equiv) | Time (h) | Yield, %b | |
|---|---|---|---|---|---|
| 2 | 3 | ||||
| 1 | 1a | 0.25 | 2 | 2a, <2 | 3a, 55, (51)c |
| 2 | 1a | 0.5 | 2 | 2a, 19, (16) | 3a, 79, (75) |
| 3 | 1a | 1.0 | 1 | 2a, 81 | 3a, 16 |
| 4 | 1a | 1.5 | 0.5 | 2a, 96, (94) | 3a, <1 |
| 5 | 1b | 0.25 | 2 | 2b, 0 | 3b, 27 |
| 6 | 1b | 0.5 | 2 | 2b, 0 | 3b, 67, (59) |
| 7 | 1b | 1.0 | 2 | 2b, 9, (8) | 3b, 78, (70) |
| 8 | 1b | 1.5 | 2 | 2b, 28 | 3b, 58 |
| 9 | 1b | 3.0 | 2 | 2b, 67, (62) | 3b, <1 |
| 10 | 1c | 0.25 | 1.5 | 2с, 98, (96) | |
| 11 | 1c | 0.5 | 1 | 2с, 96 | |
a Reactions were performed by the addition of TfOH to a solution of 1a–c (1.0 mmol) and TFAA (6 mmol) in 1.0 mL of dry CH2Cl2 at rt; byield determined by1H NMR; cyield of individual product, purified by using silica gel column chromatography, is given in parentheses.
The acid-catalyzed cyclization of 3-arylpropanoic and 4-arylbutanoic acids to 1-indanones and 1-tetralones is well-known [12-18], but it appears that the further acylation and β-diketone formation has not been reported yet. While the use of acyl trifluoroacetates, generated in situ from a carboxylic acid and TFAA, for the aromatic acylation catalyzed by acid (H3PO4 [19-22] or TfOH [23-25]) has been reported, the 1,3-diketone formation has not been observed yet.
We concluded that in the work of reference [25], an apparently larger quantity of the super acidic TfOH was employed (4 equiv vs 0.25–1.5 equiv in our work), which possibly slowed down the reaction of ketone acylation. This is corroborated in the case of phenylpropionic acid 1а. Here, the yield of the diketone 3a decreased with an increase of the quantity of TfOH, whereas the yield of 1-indanone (2a) increased and reached 94% with 1.5 equiv of TfOH (Table 1, entry 4).
In the case of β-(4-bromophenyl)propionic acid (1b) with 0.5 equiv of TfOH, the reaction chemoselectively proceeds to give the only diketone 3b (59%, Table 1, entry 6). The maximal yield of the diketone 3b is obtained with 1 equiv of TfOH (70%, Table 1, entry 7), and a further increase of the TfOH quantity to 3 equiv results in the indanone 2b as the only product (62%, Table 1, entry 9). Evidently, the intra- and intermolecular acylation of β-phenylpropionic acids in this reaction is dependent on the used TfOH quantity and the nature of the substituent in the phenyl moiety.
On the basis of the above results, we supposed that the acylation of ketones with carboxylic acids in a TFAA/TfOH/CH2Cl2 system could be applied as an effective method for the synthesis of β-diketone. It turned out that the acylation of different alkyl aryl ketones 2a–k (indanones, tetralone, acetophenones, 2-acetylthiophene and methyl benzyl ketone) with alkanoic acids RCOOH 1d–h (where R = 1-adamantylmethyl, neopentyl, isopropyl, methyl, phenyl) gave the corresponding β-diketones 3c–t in 37–86% yields (Table 2).
Table 2: TFAA/TfOH-mediated acylation of aromatic ketones with alkanoic acidsа.
|
|
|||||
| Entry | Acid 1, R | Ketone | 1,3-Diketone | Yield,% | |
|---|---|---|---|---|---|
| 1 | 1-AdCH2 | 1d |
2a |
3c |
79 |
| 2 | t-BuCH2 | 1e | 2a |
3d |
74 |
| 3 | iPr | 1f | 2a |
3e |
50 |
| 4 | Me | 1g | 2a |
3f |
77b |
| 5 | Ph | 1h | 2a |
3g |
66c |
| 6 | 1-AdCH2 | 1d |
2c |
3h |
(65)d |
| 7 | t-BuCH2 | 1e | 2c |
3i |
57 |
| 8 | Me | 1g | 2c |
3j |
53b |
| 9 | t-BuCH2 | 1e |
2d |
3k |
86 |
| 10 | 1-AdCH2 | 1d |
2e |
3l |
47 |
| 11 | t-BuCH2 | 1e | 2e |
3m |
69b |
| 12 | t-BuCH2 | 1e |
2f |
3n |
61 |
| 13 | Me | 1g | 2f |
3o |
41b,c |
| 14 | 1-AdCH2 | 1d |
2g |
3p |
48 |
| 15 | 1-AdCH2 | 1d |
2h |
3q |
37 |
| 16 | 1-AdCH2 | 1d |
2i |
3r |
49 |
| 17 | 1-AdCH2 | 1d |
2j |
3s |
43 |
| 18 | 1-AdCH2 | 1d |
2k |
3t |
64 |
aReaction conditions: ketone (1 mmol), acid (1 mmol), TFAA (6 mmol), TfOH (0.5 mmol) in 1 mL CH2Cl2, 2–4 h, rt; b2 mmol of acid was used; c1.5 mmol of TfOH was used; dyield determined by 1H NMR.
In most cases reactions were carried out at the molar ratios of acid:ketone:TFAA:TfOH = 1:1:6:0.5. For the reaction of 1-indanone (2а) and 1-adamantylacetic acid (1d) it was shown that in the absence of TfOH the yield of diketone 3c significantly dropped (<2%). With 0.25–1.5 equiv of TfOH the yield of the diketone 3c reached its maximal value (~80%) and decreased with a greater excess (3 equiv) of TfOH (57%). Apparently, the reduction of the TFAA excess (from 6 to 3 equiv) slightly lowered the yield of the diketone 3c to 68%, although the quantity of TFAA was not optimized for these reactions. An excess of the acid 1e (2 equiv vs 1 equiv) in the acylation of acetophenone (2e) only modestly increase the yield of diketone 3d from 64 to 69% (Table 2, entry 11), and usually we added equimolecular quantities of an acid and a ketone. An excess of acetic acid in acylation reactions (Table 2, entries 4, 8 and 13) was employed considering that this acid was partially self-acylated under the reaction conditions. A probable mechanism of the reaction of ketone acylation by acids in TFAA/TfOH media may be that after the acyl trifluoroacetates are generated in situ, triflic acid becomes involved in the enolization of ketones and increases the acylating ability of acyl trifluoroacetates. However, with an increase of the TfOH quantity (>1.5 equiv) a retardation of the ketone acylation is observed, presumably as a result of its protonation.
Since the γ-phenylbutanoic acid (1c) was quantitatively converted to 1-tetralone (2c) under the acylation conditions, the diketone 3h (Table 2, entry 6) was also obtained by a two-stage one-pot approach, i.e., the intramolecular cyclization of the acid 1c (to form the tetralone 2c) and the following acylation with 1-adamantylacetic acid (1d) (Scheme 1).
Scheme 1: One-pot synthesis of diketone 3h from acids 1d and 1c.
Scheme 1: One-pot synthesis of diketone 3h from acids 1d and 1c.
The acylation of the methyl benzyl ketone (2k) by 1-adamantylacetic acid (1d) proceeded with regioselectivity at the α-СН2-group and gave the diketone 3t in good yield (Table 2, entry 18), which is apparently associated with the enolization ability of 2k in the benzyl fragment of the molecule.
To enhance the application of the considered reaction, we investigated the acylation of the ketones by several functionally substituted carboxylic acids. Whereas glycine did not react with the ketones, β-alanine (1i) reacting with 1-indanone, 1-tetralone and acetophenone formed the corresponding trifluoroacylated β-aminodiketones 3u–w (reaction 1 in Scheme 2). A fuller acylation for 2a and 2c was achieved when 1.5 equiv of the acid 1i and 1 equiv of TfOH were used, whereas the yield of diketone 3w still remained low under these conditions. The hydrolysis of trifluoroacetates 3u, 3v under reflux in dilute HCl was followed by the intramolecular cyclization and led to the unknown heterocycles 4а,b.
Unambiguous evidence for the structure of heterocycle 4a was obtained by X-ray diffraction analysis [26]. The crystal structure of 4a is mediated by hydrogen bonds with a cation, a chloride anion and a solvating water molecule as participants (Figure 1).
By acylation of 1-indanone (2a) with (diphenylphosphoryl)acetic acid (1j), the self-acylation of the acid 1j already occurred at room temperature, which complicated the separation of the desired dicarbonyl compound. When the acid 1j was heated under the conditions of TFAA/TfOH-mediated self-acylation of alkanoic acids recently reported by us [27] and decarboxylation was subsequently carried out, 1,3-diphenylphosphorylated acetone 5 could be obtained (reaction 2 in Scheme 2).
The two-stage one-pot reaction of γ-phenylbutanoic acid (1c) and 3-hydroxy-1-adamantylacetic acid (1k) gave as a result the trifluoroacetylated hydroxydiketone 3x, which could be hydrolyzed to the corresponding alcohol 3y (reaction 3 in Scheme 2). The acylation of 1-indanone (2а) with 2,2’-(adamantane-1,3-diyl)diacetic acid (1l) gave the tetraketone 3z.
Finally, we used our method in a two-stage one-pot syntheses of pyrazoles, which find extensive use in the pharmaceutical industry [8,28]. Diketones 3a–c,f, essential for synthesis of the pyrazoles 6a–d, were obtained by one of the three routes (Scheme 3). The one-pot intra- and intermolecular acylation of β-phenylpropionic acids 1а,b gave 3а,b. The acylation of the 1-indanone (2а) by acetic acid (1g) yielded 3f. The selective intramolecular cyclization of acid 1а was used to obtain the intermediate ketone 2а, which was further acylated by the acid 1d to finally give 3c. Upon the formation of the diketones, the reaction mixtures were evaporated in vacuum, the residues were dissolved in ethanol, and the following reaction with hydrazine hydrate gave the pyrazoles 6a–d in 62–76% yield.
Scheme 3: One-pot synthesis of pyrazoles 6.
Scheme 3: One-pot synthesis of pyrazoles 6.
The products were identified by 1H and 13C NMR spectroscopy, microanalysis and by spectral comparison with the known compounds. The molecular structures of compounds 3с, 3u, 4a, 6b were confirmed by X-ray diffraction [26]. The obtained diketones were mainly present in the enol form in CDCl3 solution. The enol structures of the β-diketones 3 were supported by 1H/13C NMR spectra, which showed a set of singlets at δ 5.9–6.2 corresponding to the α-olefinic protons for 3l–3r, 3s and 3w, signals at δ 106.5–116.1 and at δ 96.1–102.7 were assigned to the α-olefinic carbon for 3a–3k, 3u, 3v, 3x–3z and 3l–3r, 3s, 3w, respectively. The minor keto tautomers (2–30%) were characterized by 1H multiplets or singlets between δ 3.6 and 4.2 (4.70 for 3t) and 13C NMR signals between δ 59.0 and 61.5.
Conclusion
In summary, we proposed a simple and effective synthetic approach to 1,3-diketones based on the TFAA/TfOH-mediated acylation of ketones with carboxylic acids. Advantages of this approach are the ready availability of the starting materials, the simple operational procedure, and the possibility to realize the one-pot immediate syntheses of 1,3-diketones and pyrazoles directly from acids and ketones. Moreover, 1,3-diketones and pyrazoles can be obtained from unmodified β-phenylpropionic acids. Given the importance of 1,3-dicarbonyl compounds in general, we expect that this reaction has a wide application in the realm of synthetic chemistry.
Experimental
General procedure for the synthesis of diketones: A solution of carboxylic acid (1 mmol), ketone (1 mmol, if required) and TFAA (0.85 mL, 6 mmol) in dichloromethane (1 mL) was stirred for 15 min at rt. The required quantity of triflic acid (usually 44 μL, 0.5 mmol) was then added, and the resulting solution was stirred at rt for 1–4 h (24 h for 3v and 3w) under the conditions indicated in Scheme 1, Scheme 2, Table 1, and Table 2 (TLC monitoring). The reaction mixture was evaporated under reduced pressure, and after quenching with water, the residue was redissolved in dichloromethane (10 mL), washed with 5% NaHCO3 (2 × 3 mL), water (2 × 3 mL), and dried over MgSO4. The solvent was removed in vacuum, and the crude reaction mixture was purified by silica gel chromatography (n-hexane/CH2Cl2/MeOH).
As illustrative examples, compounds 3a and 3c are prepared as follows.
2-(3-Phenylpropionyl)-1-indanone (3a): Obtained from β-phenylpropionic acid (1a, 150 mg, 1 mmol), TFAA (0.85 mL, 6 mmol) and TfOH (44 μL, 0.5 mmol) in 75% (100 mg) yield as a red solid. Mp 65–66 °C (Lit. [29]: mp 68–69 °C); 1H NMR (400 MHz, CDCl3) keto-enol (20:80); enol tautomer: δ 7.80 (d, J = 7.6 MHz, HAr, 1H), 7.55–7.33 (m, HAr, 3H), 7.32–7.15 (m, HAr, 5H), 3.40 (s, CH2Ind, 2H), 3.04 (t, J = 7.7 Hz, CH2, 2H), 2.73 (t, J = 7.7 Hz, CH2, 2H); 13С NMR (100 MHz, CDCl3) δ 191.1 (CO), 179.7 (C=C(OH)), 147.4 (CAr), 140.6 (CAr), 138.1 (CAr), 132.7 (CAr), 128.5 (CHAr), 128.3 (CHAr), 127.2 (CHAr), 126.3 (CHAr), 125.6 (CHAr), 123.0 (CHAr), 110.5 (C=C(OH)), 36.8 (CH2), 31.6 (CH2), 29.9 (CH2).
2-[2-(1-Adamantyl)acetyl]-1-indanone (3c): Obtained from 1-adamantylacetic acid (1d, 194 mg, 1 mmol), 1-indanone (2a, 132 mg, 1 mmol), TFAA (0.85 mL, 6 mmol) and TfOH (44 μL, 0.5 mmol) in 79% (240 mg) yield as a red solid. Mp 154 °C; 1H NMR (400 MHz, CDCl3) keto-enol (2:98), enol tautomer: δ 7.81 (d, J = 7.6 Hz, HAr, 1H), 7.52 (t, J = 7.4 Hz, HAr, 1H), 7.46 (d, J = 7.5 Hz, HAr, 1H), 7.38 (t, J = 7.4 Hz, HAr, 1H), 3.58 (s, CH2Ind, 2H), 2.18 (s, CH2Ad, 2H), 1.98 (bs, CHAd, 3H,), 1.75–1.59 (m, CH2Ad, 12H); 13С NMR (100 MHz, CDCl3) δ 193.6 (CO), 177.1 (C=C(OH)), 148.0 (CAr), 138.5 (CAr), 132.9 (CHAr), 127.2 (CHAr), 125.6 (CHAr), 123.2 (CHAr), 111.7 (C=C(OH)), 48.6 (CH2Ad), 43.0 (CH2Ad), 36.7 (CH2Ad), 35.1 (CAd), 30.7 (CH2), 28.7 (CHAd); Anal. calcd for C21H24O2: C, 81.78; H, 7.84; found: C, 82.23; H 7.67.
Typical procedure for the synthesis of heterocycles 4a,b: A solution of diketone 3u or 3v (1 mmol) in an ethanol (20 mL)/water (4 mL)/HClconc (4 mL) mixture was heated under reflux for 6 h. After the reaction was completed (TLC control) the solvent was evaporated, the product was washed with diethyl ether and hexane and dried.
1-Hydroxy-4-aza-2,3-dihydrofluorene hydrochloride (4a): Obtained from diketone 3u (299 mg, 1 mmol) in 95% (210 mg) yield as a brown solid. Mp 110–112 °С; 1H NMR (400 MHz, methanol-d4) δ 7.97 (d, J = 7.8 Hz, HAr, 1H), 7.69 (t, J = 7.5 Hz, HAr, 1H), 7.59 (d, J = 7.7 Hz, HAr, 1H), 7.56 (t, J = 7.5 Hz, HAr, 1H), 3.99 (t, J = 8.5 Hz, CH2, 2H), 3.79 (s, CH2, 2H), 2.91 (t, J = 8.5 Hz, CH2, 2H); 13С NMR (100 MHz, methanol-d4) δ 173.7 (C), 173.2 (C), 149.5 (C), 135.1 (CH), 132.0 (C), 127.4 (CH), 126.1 (CH), 123.8 (CH), 105.9 (C), 41.1 (CH2), 30.7 (CH2), 27.3 (CH2); Anal. calcd for C12H11NO·HCl: C, 65.02; H, 5.46; N, 6.32; found: C, 64.72; H, 5.61; N, 6.24.
1,3-Bis(diphenylphosphoryl)acetone (5): A solution of diphenylphosphorylacetic acid (260 mg, 1 mmol) in a mixture of TFAA (1.95 mL, 13.8 mmol) and TfOH (0.05 mL, 0.57 mmol) was kept at 60–65 °C for 1.5 h. The solvent was evaporated under reduced pressure, the obtained residue was stirred in a water (4 mL)/EtOH (4 mL) solution under reflux for 2 h, cooled and concentrated. The residue was adjusted to pH 7.5 with 1 N NaHCO3, the solid formed was filtered, washed with water, and dried. The product was purified by column chromatography (eluent: CH2Cl2/MeOH 50:1). Yield: 44% (101 mg), white solid; mp 173–175 °С (Lit. [30]: mp 175–176 °C); 1H NMR (400 MHz, CDCl3) δ 7.80–7.68 (m, HAr, 8H), 7.57–7.41 (m, HAr, 12H), 3.97 (d, J = 14.3 Hz, CH2, 4H); 13С NMR (100 MHz, CDCl3) δ 195.6 (CO), 132.2 (CHAr), 131.2 (CAr), 130.9 (d, J = 10.6 Hz, CHAr,), 128.7 (CHAr, J = 12.7 Hz), 48.2 (d, J = 55.7, CH2, Hz); 31P NMR (CDCl3) δ 27.1.
The synthesis of pyrazoles 6. As an illustrative example, compound 6a is prepared as follows. A solution of β-phenylpropionic acid (1a, 150 mg, 1 mmol) and TFAA (0.85 mL, 6 mmol) in 1 mL CH2Cl2 was stirred for 15 min at rt. Then TfOH (44 μL, 0.5 mmol) was added, and the reaction mixture was kept for 2 h. On completion of the reaction, the solvent was removed under reduced pressure. The crude 3a was dissolved in 5 mL ethanol and heated under reflux with hydrazine hydrate (0.1 mL, 2 mmol). After 2 h the solvent was evaporated, and the remaining oil was dissolved in CH2Cl2, washed with 5% NaHCO3, water, and dried over MgSO4. The product was purified by means of column chromatography (SiO2 60, eluent: CH2Cl2/MeOH 50:1). Yield: 69% (90 mg), brown solid; mp 108–110 °С; 1H NMR (400 MHz, CDCl3) δ 7.71 (d, J = 7.2 Hz, HAr, 1H), 7.44 (d, J = 7.2 Hz, HAr, 1H), 7.40–7.17 (m, HAr, 4H), 7.14 (d, J = 6.8 Hz, HAr, 2H), 3.42 (s, CH2, 2H), 3.04 (m, CH2, 4H); 13С NMR (100 MHz, CDCl3) δ 148.7 (C), 140.8 (C), 137.9 (C), 134.7 (C), 128.5 (CH), 128.4 (CH), 126.4 (2CH), 126.3 (CH), 125.8 (CH), 122.7 (CH), 121.6 (C), 119.9 (CH), 34.8 (CH2), 28.4 (CH2), 27.5 (CH2); Anal. calcd for C18H16N2: C, 83.05; H, 6.19; N, 10.76; found: C, 82.67; H, 6.35; N, 10.37.
Supporting Information
| Supporting Information File 1: Detailed experimental procedures and characterization of compounds 3c–z, 4b and 6b–d, figures of the molecular structures of compounds 3c, 3u, 4a and 6b, and copies of 1H, 13C and 31P NMR spectra for all new compounds. | ||
| Format: PDF | Size: 1.7 MB | Download |
| Supporting Information File 2: Crystallographic information for compound 3c. | ||
| Format: CIF | Size: 12.7 KB | Download |
| Supporting Information File 3: Crystallographic information for compound 3u. | ||
| Format: CIF | Size: 20.5 KB | Download |
| Supporting Information File 4: Crystallographic information for compound 4a. | ||
| Format: CIF | Size: 11.8 KB | Download |
| Supporting Information File 5: Crystallographic information for compound 6b. | ||
| Format: CIF | Size: 12.9 KB | Download |
References
-
Kel’in, A. V. Curr. Org. Chem. 2003, 7, 1691–1711. doi:10.2174/1385272033486233
Return to citation in text: [1] -
Kel’in, A. V.; Maioli, A. Curr. Org. Chem. 2003, 7, 1855–1886. doi:10.2174/1385272033486134
Return to citation in text: [1] -
Vigato, P. A.; Peruzzo, V.; Tamburini, S. Coord. Chem. Rev. 2009, 253, 1099–1201. doi:10.1016/j.ccr.2008.07.013
Return to citation in text: [1] -
Katritzky, A. R.; Pastor, A. J. Org. Chem. 2000, 65, 3679–3682. doi:10.1021/jo991878f
Return to citation in text: [1] -
Katritzky, A. R.; Wang, Z.; Wang, M.; Wilkerson, C. R.; Hall, C. D.; Akhmedov, N. G. J. Org. Chem. 2004, 69, 6617–6622. doi:10.1021/jo049274l
Return to citation in text: [1] -
Shen, Z.; Li, B.; Wang, L.; Zhang, Y. Tetrahedron Lett. 2005, 46, 8785–8788. doi:10.1016/j.tetlet.2005.10.036
Return to citation in text: [1] -
Katritzky, A. R.; Meher, N. K.; Singh, S. K. J. Org. Chem. 2005, 70, 7792–7794. doi:10.1021/jo051087f
Return to citation in text: [1] -
Heler, S. T.; Natarajan, S. R. Org. Lett. 2006, 8, 2675–2678. doi:10.1021/ol060570p
Return to citation in text: [1] [2] -
Iida, A.; Osada, J.; Nagase, R.; Misaki, T.; Tanabe, Y. Org. Lett. 2007, 9, 1859–1862. doi:10.1021/ol070191b
Return to citation in text: [1] -
Lim, D.; Fang, F.; Zhou, G.; Coltart, D. M. Org. Lett. 2007, 9, 4139–4142. doi:10.1021/ol701599v
Return to citation in text: [1] -
Zhou, G.; Lim, D.; Coltart, D. M. Org. Lett. 2008, 10, 3809–3812. doi:10.1021/ol801498u
Return to citation in text: [1] -
Sharma, A. K.; Subramani, A. V.; Gorman, C. B. Tetrahedron 2007, 63, 389–395. doi:10.1016/j.tet.2006.10.065
Return to citation in text: [1] -
Rendy, R.; Zhang, Y.; McElrea, A.; Gomez, A.; Klumpp, D. A. J. Org. Chem. 2004, 69, 2340–2347. doi:10.1021/jo030327t
Return to citation in text: [1] -
Prakash, G. K. S.; Yan, P.; Török, B.; Olah, G. A. Catal. Lett. 2003, 87, 109–112. doi:10.1023/A:1023482904174
Return to citation in text: [1] -
Yamato, T.; Hideshima, C.; Prakash, G. K. S.; Olah, G. A. J. Org. Chem. 1991, 56, 3955–3957. doi:10.1021/jo00012a033
Return to citation in text: [1] -
Dallemagne, P.; Rault, S.; Pilo, J. C.; Foloppe, M. P.; Robba, M. Tetrahedron Lett. 1991, 32, 6327–6328. doi:10.1016/0040-4039(91)80160-8
Return to citation in text: [1] -
Hulin, B.; Koreeda, M. J. Org. Chem. 1984, 49, 207–209. doi:10.1021/jo00175a055
Return to citation in text: [1] -
Premasagar, V.; Palaniswamy, V. A.; Eisenbraun, E. J. J. Org. Chem. 1981, 46, 2974–2976. doi:10.1021/jo00327a028
Return to citation in text: [1] -
Luke, G. P.; Seekamp, C. K.; Wang, Z.-Q.; Chenard, B. L. J. Org. Chem. 2008, 73, 6397–6400. doi:10.1021/jo800973e
Return to citation in text: [1] -
Pal, S.; Khan, M. A.; Bindu, P.; Dubey, P. K. Beilstein J. Org. Chem. 2007, 3, No. 35. doi:10.1186/1860-5397-3-35
Return to citation in text: [1] -
Gray, A. D.; Smyth, T. P. J. Org. Chem. 2001, 66, 7113–7117. doi:10.1021/jo0158074
Return to citation in text: [1] -
Galli, C. Synthesis 1979, 303–304. doi:10.1055/s-1979-28661
Return to citation in text: [1] -
Plażuk, D.; Zakrzewski, J.; Salmain, M. Org. Biomol. Chem. 2011, 9, 408–417. doi:10.1039/c0ob00319k
Return to citation in text: [1] -
Plażuk, D.; Zakrzewski, J. J. Organomet. Chem. 2009, 694, 1802–1806. doi:10.1016/j.jorganchem.2009.01.007
Return to citation in text: [1] -
Plażuk, D.; Zakrzewski, J. Synth. Commun. 2004, 34, 99–107. doi:10.1081/SCC-120027243
Return to citation in text: [1] [2] -
CCDC 942538 (3c), CCDC 942537 (3u), CCDC 942536 (4a), CCDC 950017 (6b) contain the supplementary crystallographic data for this paper. These data can be obtained, free of charge, from the Cambridge Crystallographic Data Centre via http://www.ccdc.cam.ac.uk/data_request/cif.
Return to citation in text: [1] [2] -
Kovalev, V.; Shokova, E.; Shmailov, A.; Vatsouro, I.; Tafeenko, V. Eur. J. Org. Chem. 2010, 3754–3761. doi:10.1002/ejoc.201000312
Return to citation in text: [1] -
Kost, A. N.; Grandberg, I. I. Adv. Heterocycl. Chem. 1966, 6, 347–429. doi:10.1016/S0065-2725(08)60579-6
Return to citation in text: [1] -
Burton, H.; Munday, D. A. J. Chem. Soc. 1957, 1718–1726. doi:10.1039/JR9570001718
Return to citation in text: [1] -
Fattakhov, S. A.; Tarasova, R. I.; Voskresenskaya, O. V.; Gazizov, M. B. Russ. J. Gen. Chem. 2010, 80, 2065–2066. doi:10.1134/S1070363210100324
Return to citation in text: [1]
| 1. | Kel’in, A. V. Curr. Org. Chem. 2003, 7, 1691–1711. doi:10.2174/1385272033486233 |
| 2. | Kel’in, A. V.; Maioli, A. Curr. Org. Chem. 2003, 7, 1855–1886. doi:10.2174/1385272033486134 |
| 3. | Vigato, P. A.; Peruzzo, V.; Tamburini, S. Coord. Chem. Rev. 2009, 253, 1099–1201. doi:10.1016/j.ccr.2008.07.013 |
| 19. | Luke, G. P.; Seekamp, C. K.; Wang, Z.-Q.; Chenard, B. L. J. Org. Chem. 2008, 73, 6397–6400. doi:10.1021/jo800973e |
| 20. | Pal, S.; Khan, M. A.; Bindu, P.; Dubey, P. K. Beilstein J. Org. Chem. 2007, 3, No. 35. doi:10.1186/1860-5397-3-35 |
| 21. | Gray, A. D.; Smyth, T. P. J. Org. Chem. 2001, 66, 7113–7117. doi:10.1021/jo0158074 |
| 22. | Galli, C. Synthesis 1979, 303–304. doi:10.1055/s-1979-28661 |
| 12. | Sharma, A. K.; Subramani, A. V.; Gorman, C. B. Tetrahedron 2007, 63, 389–395. doi:10.1016/j.tet.2006.10.065 |
| 13. | Rendy, R.; Zhang, Y.; McElrea, A.; Gomez, A.; Klumpp, D. A. J. Org. Chem. 2004, 69, 2340–2347. doi:10.1021/jo030327t |
| 14. | Prakash, G. K. S.; Yan, P.; Török, B.; Olah, G. A. Catal. Lett. 2003, 87, 109–112. doi:10.1023/A:1023482904174 |
| 15. | Yamato, T.; Hideshima, C.; Prakash, G. K. S.; Olah, G. A. J. Org. Chem. 1991, 56, 3955–3957. doi:10.1021/jo00012a033 |
| 16. | Dallemagne, P.; Rault, S.; Pilo, J. C.; Foloppe, M. P.; Robba, M. Tetrahedron Lett. 1991, 32, 6327–6328. doi:10.1016/0040-4039(91)80160-8 |
| 17. | Hulin, B.; Koreeda, M. J. Org. Chem. 1984, 49, 207–209. doi:10.1021/jo00175a055 |
| 18. | Premasagar, V.; Palaniswamy, V. A.; Eisenbraun, E. J. J. Org. Chem. 1981, 46, 2974–2976. doi:10.1021/jo00327a028 |
| 5. | Katritzky, A. R.; Wang, Z.; Wang, M.; Wilkerson, C. R.; Hall, C. D.; Akhmedov, N. G. J. Org. Chem. 2004, 69, 6617–6622. doi:10.1021/jo049274l |
| 6. | Shen, Z.; Li, B.; Wang, L.; Zhang, Y. Tetrahedron Lett. 2005, 46, 8785–8788. doi:10.1016/j.tetlet.2005.10.036 |
| 7. | Katritzky, A. R.; Meher, N. K.; Singh, S. K. J. Org. Chem. 2005, 70, 7792–7794. doi:10.1021/jo051087f |
| 8. | Heler, S. T.; Natarajan, S. R. Org. Lett. 2006, 8, 2675–2678. doi:10.1021/ol060570p |
| 9. | Iida, A.; Osada, J.; Nagase, R.; Misaki, T.; Tanabe, Y. Org. Lett. 2007, 9, 1859–1862. doi:10.1021/ol070191b |
| 10. | Lim, D.; Fang, F.; Zhou, G.; Coltart, D. M. Org. Lett. 2007, 9, 4139–4142. doi:10.1021/ol701599v |
| 11. | Zhou, G.; Lim, D.; Coltart, D. M. Org. Lett. 2008, 10, 3809–3812. doi:10.1021/ol801498u |
| 30. | Fattakhov, S. A.; Tarasova, R. I.; Voskresenskaya, O. V.; Gazizov, M. B. Russ. J. Gen. Chem. 2010, 80, 2065–2066. doi:10.1134/S1070363210100324 |
| 4. | Katritzky, A. R.; Pastor, A. J. Org. Chem. 2000, 65, 3679–3682. doi:10.1021/jo991878f |
| 27. | Kovalev, V.; Shokova, E.; Shmailov, A.; Vatsouro, I.; Tafeenko, V. Eur. J. Org. Chem. 2010, 3754–3761. doi:10.1002/ejoc.201000312 |
| 26. | CCDC 942538 (3c), CCDC 942537 (3u), CCDC 942536 (4a), CCDC 950017 (6b) contain the supplementary crystallographic data for this paper. These data can be obtained, free of charge, from the Cambridge Crystallographic Data Centre via http://www.ccdc.cam.ac.uk/data_request/cif. |
| 26. | CCDC 942538 (3c), CCDC 942537 (3u), CCDC 942536 (4a), CCDC 950017 (6b) contain the supplementary crystallographic data for this paper. These data can be obtained, free of charge, from the Cambridge Crystallographic Data Centre via http://www.ccdc.cam.ac.uk/data_request/cif. |
| 29. | Burton, H.; Munday, D. A. J. Chem. Soc. 1957, 1718–1726. doi:10.1039/JR9570001718 |
| 25. | Plażuk, D.; Zakrzewski, J. Synth. Commun. 2004, 34, 99–107. doi:10.1081/SCC-120027243 |
| 23. | Plażuk, D.; Zakrzewski, J.; Salmain, M. Org. Biomol. Chem. 2011, 9, 408–417. doi:10.1039/c0ob00319k |
| 24. | Plażuk, D.; Zakrzewski, J. J. Organomet. Chem. 2009, 694, 1802–1806. doi:10.1016/j.jorganchem.2009.01.007 |
| 25. | Plażuk, D.; Zakrzewski, J. Synth. Commun. 2004, 34, 99–107. doi:10.1081/SCC-120027243 |
| 8. | Heler, S. T.; Natarajan, S. R. Org. Lett. 2006, 8, 2675–2678. doi:10.1021/ol060570p |
| 28. | Kost, A. N.; Grandberg, I. I. Adv. Heterocycl. Chem. 1966, 6, 347–429. doi:10.1016/S0065-2725(08)60579-6 |
© 2014 Kim et al; licensee Beilstein-Institut.
This is an Open Access article under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
The license is subject to the Beilstein Journal of Organic Chemistry terms and conditions: (http://www.beilstein-journals.org/bjoc)




![[1860-5397-10-236-1]](/bjoc/content/figures/1860-5397-10-236-1.png?scale=2.0&max-width=1024&background=FFFFFF)