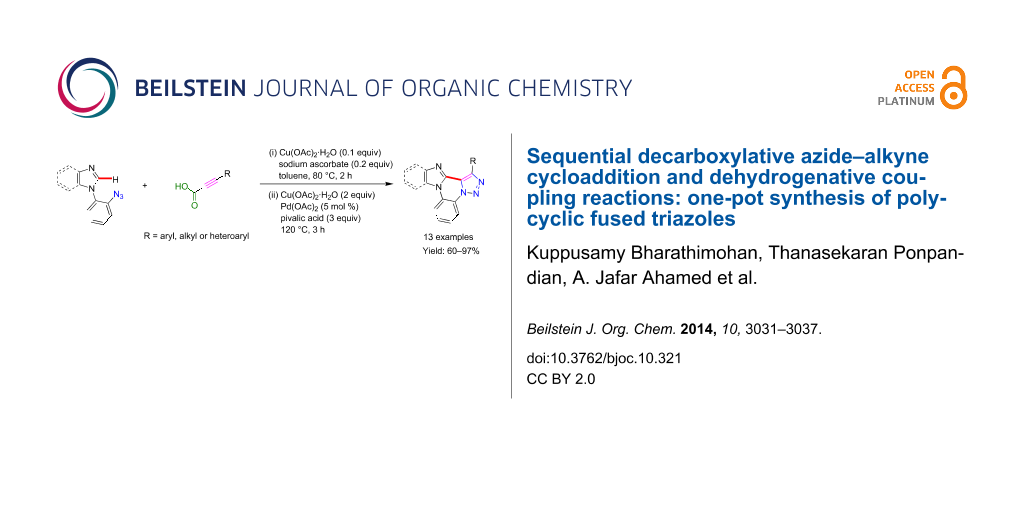
Abstract
Herein, we describe a one-pot protocol for the synthesis of a novel series of polycyclic triazole derivatives. Transition metal-catalyzed decarboxylative CuAAC and dehydrogenative cross coupling reactions are combined in a single flask and achieved good yields of the respective triazoles (up to 97% yield). This methodology is more convenient to produce the complex polycyclic molecules in a simple way.
Graphical Abstract
Introduction
The copper-catalyzed Huisgen [3 + 2] cycloaddition (or copper-catalyzed azide–alkyne cycloaddition, CuAAC) between an organic azide and a terminal alkyne is a well-established strategy for the construction of 1,4-disubstituted 1,2,3-triazoles [1-4]. In a recent development, this decarboxylative coupling reaction was well documented for the generation of C–C bonds [5]. This method has several advantages over the classical C–C bond formation method including the stability and preparation of the starting material and the non-hazardous byproducts. In 2011, Kolarovič et al. [6] first reported the copper-catalyzed decarboxylative [3 + 2] cycloaddition reaction of 2-alkynoic acid with organic azides. This kind of decarboxylative CuAAC reaction has not been further investigated. Transition metal-mediated C–H bond activation has become a hot topic in recent years [7-11]. Formally, it requires insertion of a transition metal (usually Pd, Ru, Rh or Ir) across a strong C–H bond (90–105 kcal/mol) to form a new, weaker C–M bond (50–80 kcal/mol), followed by generation of a new C–C bond. Generally, transition metal-catalyzed sp2 C–H activation is facilitated by directing groups [10-13] or heteroatoms in the heterocyclic compounds [14-18]. This methodology has been applied in the synthesis of polycyclic frameworks as well as in the preparation of biologically important compounds [19-23]. Further development of this reaction has led to double C–H activation which has been used for the construction of biaryl compounds [24-33]. The double C–H activation (dehydrogenative cross coupling) reaction can be classified into two categories: intermolecular and intramolecular. There are several reports in literature describing intermolecular sp2 C–H/C–H coupling reactions [24-33], whereas only limited reports are available for intramolecular sp2 C–H/C–H coupling reactions [34-38]. Compounds containing a fused triazole skeleton show remarkable biologically activities [39] and new strategies to prepare this class of molecules are highly warranted. Several methodologies were developed for the synthesis of fused triazoles [40]. Ackermann referred to an intramolecular dehydrogenative coupling of 1,4-disubstituted triazoles to achieve tri- and tetracyclic triazoles [34]. Recently, Lautens et al. [41] described a one-pot synthesis of fused triazoles through CuAAC reaction followed by C–H functionalization (Scheme 1).
Scheme 1: Synthesis of polycyclic fused triazoles.
Scheme 1: Synthesis of polycyclic fused triazoles.
Specifically, they demonstrated a C–H functionalization of an indole nucleus with 5-iodo-1,2,3-triazoles. In the present study, we replaced the 5-iodo-1,2,3-triazoles with 5H-1,2,3-triazoles with intramolecular sp2 C–H/C–H cross coupling reaction. To the best of our knowledge, until now there have been no reports describing the combination of decarboxylative CuAAC reaction and C–H activation in an one-pot fashion. This strategy describes the preparation of fused triazoles by one-pot reaction of 2-alkynoic acid and azide derivatives.
Results and Discussion
According to the report of Kolarovič et al., the decarboxylative CuAAC reaction occurs efficiently with a CuSO4/NaAsc/DMSO catalytic system [6]. The palladium-catalyzed oxidative dehydrogenative coupling reaction may be effected by various oxidants [42,43] such as Ag2O, AgOAc, Ag2CO3, Na2S2O8, Cu(OPiv)2, Cu(OAc)2, benzoquinone and O2 among others. In the present study, we have chosen a Cu2+ salt because it can be used as an oxidant and as a pre-catalyst for the C–H functionalization and the decarboxylative CuAAC reaction, respectively. 1-(2-Azidophenyl)-1H-benzo[d]imidazole (1a) and phenylpropiolic acid (2a) were selected as model substrates to optimize the reaction conditions. Initially, the decarboxylative CuAAC reactions were carried out with 10 mol % of CuSO4∙5H2O and 20 mol % of NaAsc in DMSO at 80 °C. After 2 h, TLC showed the completion of the cycloaddition reaction and mass spectrometric analysis, [M + 1] peak at 338.1, of the reaction mixture confirmed the formation of 3a. The reaction mixture was divided into three equal portions and transferred to separate round bottom flasks and the cross coupling was carried out with 5 mol % of three different Pd2+ catalysts and 2 equivalents of CuSO4∙5H2O (Cu2+ used for decarboxylative CuAAC) at 120 °C for 12 h. It failed to undergo the oxidative dehydrogenative coupling reaction and the triazole derivative 3a was isolated in 79–82% yield (Table 1, entries 1–1b). A similar reaction sequence was performed with different copper salts such as CuCl2∙H2O, Cu(OAc)2∙H2O and Cu(NO3)2∙3H2O instead of CuSO4∙5H2O (Table 1, entries 2–4b). Among the Cu2+ salts tested, Cu(OAc)2∙H2O was found to be better than others and yielded 10% of 4a (Table 1, entries 3–4b). In the literature, we found that additives, such as Brønsted acids, enhance the acidity of the C–H bond in several C–H activation reactions [44-48]. Thus, the reaction was carried out with additives such as pivalic acid, acetic acid or trifluoroacetic acid in the above catalytic system (Table 1, entries 5–5b). When pivalic acid was used, the product formation was improved to 35% (Table 1, entry 5a) whereas acetic acid and trifluoroacetic acid conditions yielded 15% and 19% of 4a, respectively. None of these modifications provided the desired product in good yield. Therefore, finally we studied the effect of solvents on these reactions. Several polar and non-polar solvents such as dioxane, toluene, 1,2-dichloroethane, DMF, and NMP were tested in this sequential reaction (Table 1, entries 6–10). Toluene was found to be superior to other solvents tested, affording a good yield (87%) of fused triazole 4a (Table 1, entry 7). No product formation was observed if the reaction was carried out in the absence of Pd(OAc)2 (Table 1, entry 11) and without pivalic acid the yield of 4a was only 22% (Table 1, entry 12). All these results demonstrated that the additive and solvent played a crucial role in the dehydrogenative coupling reaction.
Table 1: Optimization of reaction conditions for the preparation of 4a.
|
|
|||||||
| Entry | Cu2+ | Solvent | Pd2+ | Additive | Time [h] | Yield(%)a | |
|---|---|---|---|---|---|---|---|
| 3a | 4a | ||||||
| 1 | CuSO4∙5H2O | DMSO | Pd(OAc)2 | – | 12 | 80 | – |
| 1a | PdCl2 | – | 82 | – | |||
| 1b | Pd(PPh3)2Cl2 | – | 79 | – | |||
| 2 | CuCl2∙H2O | DMSO | Pd(OAc)2 | – | 12 | 81 | – |
| 2a | PdCl2 | – | 85 | – | |||
| 2b | Pd(PPh3)2Cl2 | – | 80 | – | |||
| 3 | Cu(OAc)2∙H2O | DMSO | Pd(OAc)2 | – | 12 | 76 | 10 |
| 3a | PdCl2 | – | 77 | trace | |||
| 3b | Pd(PPh3)2Cl2 | – | 78 | – | |||
| 4 | Cu(NO3)2∙3H2O | DMSO | Pd(OAc)2 | – | 12 | 50 | – |
| 4a | PdCl2 | – | 58 | – | |||
| 4b | Pd(PPh3)2Cl2 | – | 52 | – | |||
| 5 | Cu(OAc)2∙H2O | DMSO | Pd(OAc)2 | pivalic acid | 12 | 58 | 35 |
| 5a | AcOH | 78 | 15 | ||||
| 5b | TFA | 76 | 19 | ||||
| 6 | Cu(OAc)2∙H2O | dioxane | Pd(OAc)2 | pivalic acid | 12 | 38 | 39 |
| 7 | Cu(OAc)2∙H2O | toluene | Pd(OAc)2 | pivalic acid | 3 | – | 87 |
| 8b | Cu(OAc)2∙H2O | 1,2-DCE | Pd(OAc)2 | pivalic acid | 12 | 46 | trace |
| 9 | Cu(OAc)2∙H2O | DMF | Pd(OAc)2 | pivalic acid | 12 | 70 | 21 |
| 10 | Cu(OAc)2∙H2O | NMP | Pd(OAc)2 | pivalic acid | 12 | 66 | 24 |
| 11 | Cu(OAc)2∙H2O | toluene | – | pivalic acid | 12 | 97 | – |
| 12 | Cu(OAc)2∙H2O | toluene | Pd(OAc)2 | – | 12 | 75 | 22 |
aIsolated yield. bReaction was performed at 100 °C.
The sequential reaction was performed with phenylacetylene instead of phenylpropiolic acid and the product 4a was isolated in 79% yield (Scheme 2). This result clearly shows that the use of 2-alkynoic acid is more advantageous for this reaction.
Scheme 2: Synthesis of fused triazole 4a using phenylacetylene.
Scheme 2: Synthesis of fused triazole 4a using phenylacetylene.
The 1-(2-azidophenyl)-1H-imidazole derivatives 1b and 1c also participates effectively in the optimized reaction conditions. The azide derivatives 1a, 1b and 1c were prepared from 1-fluoro-2-nitrobenzene (Scheme 3) according to literature procedure [49]. Using the optimized reaction conditions, the reactivity of different 2-alkynoic acids was investigated with 1a and 1b and the results are shown in Scheme 4.
Scheme 4: Synthesis of fused polycyclic triazole analogs 4.
Scheme 4: Synthesis of fused polycyclic triazole analogs 4.
The electron-donating substituents (OMe)-bearing phenyl ring in 2b resulted in a good yield of triazole analogs 4b and 4i in contrast 2c with electron withdrawing methoxycarbonyl substitution provided moderate yields of 4c and 4h (62% and 60%). Notably, the carboxylate group in 4c and 4h offers a versatile synthetic functionality for further derivatization reactions. The thiophene-derived alkynoic acid, 2d, provided the corresponding triazole analogs 4d and 4j in 79% and 73%, respectively. Likewise, the alkynoic acid derived from short and long linear alkyl chains also provided good yields (75–88%) of polycyclic fused triazoles 4e, 4f, 4k, 4l and 4m (Scheme 4).
The structures were fully characterized by NMR, IR and mass spectroscopic techniques. Furthermore, the structure of 4f has been confirmed by single crystal X-ray crystallographic study (Figure 1).
![[1860-5397-10-321-1]](/bjoc/content/figures/1860-5397-10-321-1.png?scale=2.0&max-width=1024&background=FFFFFF)
Figure 1: ORTEP diagram of 4f (CCDC 979471).
Figure 1: ORTEP diagram of 4f (CCDC 979471).
The proposed reaction mechanism for the formation of 4 is described in Scheme 5. Initially alkynoic acid 2 undergoes decarboxylation to form the copper acetylide (A) in the presence of the Cu+ catalyst which is generated by the reduction of Cu2+ with sodium ascorbate. The obtained copper acetylide undergoes regioselective [3 + 2] cycloaddition with azide derivative 1 to yield the copper salt of 3 and a transmetalation reaction gave the intermediate B. We assumed that the pivalate group replaces the acetate group in B and may produce C. The pivalate group in C facilitates the palladium insertion to the C–H bond to give D and subsequent reductive elimination reaction yields the polycyclic triazoles 4.
Scheme 5: Proposed mechanism for the formation of 4.
Scheme 5: Proposed mechanism for the formation of 4.
Conclusion
In summary, we have successfully developed an efficient and convenient one-pot protocol for the synthesis of novel benzimidazole and imidazole-fused 1,2,3-triazoloquinoxaline derivatives. The key finding of this work is the bifunctional behavior of Cu(OAc)2∙H2O in the reaction sequence.
Experimental
General procedure for the synthesis of fused triazoloquinoxaline derivatives 4
Substituted phenylpropiolic acids (2) were prepared by the literature procedure [50]. To a mixture of 1-(2-azidophenyl)-1H-benzo[d]imidazole (1a) or 1-(2-azidophenyl)-1H-imidazole (1b) (0.85 mmol), 2-alkynoic acid (2) (1.02 mmol) and Cu(OAc)2∙H2O (0.085 mmol, 10 mol %) in toluene (8 mL) was added to sodium ascorbate (0.17 mmol, 20 mol %) at room temperature. The mixture was stirred at 80 °C for 2 h. Cu(OAc)2∙H2O (1.7 mmol), Pd(OAc)2 (0.043 mmol, 5 mol %) and pivalic acid (2.55 mmol) were added into the above reaction mixture and then refluxed at 120 °C for 3 h. The reaction mixture was cooled to room temperature and diluted with ethyl acetate (200 mL). The mixture was filtered through a pad of celite and the filtrate was washed with water, dried over anhydrous Na2SO4 and concentrated under vacuum. The residue was purified by column chromatography using hexane/ethyl acetate as eluent to obtain the desired product 4 (60–97%).
Supporting Information
| Supporting Information File 1: X-ray crystallographic data of 4f, characterization, 1H and 13C NMR data of compounds 3a and 4a–m. | ||
| Format: PDF | Size: 2.2 MB | Download |
References
-
Rostovtsev, V. V.; Green, L. G.; Fokin, V. V.; Sharpless, K. B. Angew. Chem., Int. Ed. 2002, 41, 2596–2599. doi:10.1002/1521-3773(20020715)41:14<2596::AID-ANIE2596>3.0.CO;2-4
Return to citation in text: [1] -
Himo, F.; Lovell, T.; Hilgraf, R.; Rostovtsev, V. V.; Noodleman, L.; Sharpless, K. B.; Fokin, V. V. J. Am. Chem. Soc. 2005, 127, 210–216. doi:10.1021/ja0471525
Return to citation in text: [1] -
Shao, C.; Wang, X.; Xu, J.; Zhao, J.; Zhang, Q.; Hu, Y. J. Org. Chem. 2010, 75, 7002–7005. doi:10.1021/jo101495k
Return to citation in text: [1] -
Shin, J.-A.; Lim, Y.-G.; Lee, K.-H. J. Org. Chem. 2012, 77, 4117–4122. doi:10.1021/jo3000095
Return to citation in text: [1] -
Rodriquez, N.; Goossen, L. J. Chem. Soc. Rev. 2011, 40, 5030–5048. doi:10.1039/C1CS15093F
Return to citation in text: [1] -
Kolarovič, A.; Schnürch, M.; Mihovilovic, M. D. J. Org. Chem. 2011, 76, 2613–2618. doi:10.1021/jo1024927
Return to citation in text: [1] [2] -
Dyker, G. Angew. Chem., Int. Ed. 1999, 38, 1698–1712. doi:10.1002/(SICI)1521-3773(19990614)38:12<1698::AID-ANIE1698>3.0.CO;2-6
Return to citation in text: [1] -
Kakiuchi, F.; Chatani, N. Adv. Synth. Catal. 2003, 345, 1077–1101. doi:10.1002/adsc.200303094
Return to citation in text: [1] -
Dick, A. R.; Sanford, M. S. Tetrahedron 2006, 62, 2439–2463. doi:10.1016/j.tet.2005.11.027
Return to citation in text: [1] -
Alberico, D.; Scott, M. E.; Lautens, M. Chem. Rev. 2007, 107, 174–238. doi:10.1021/cr0509760
Return to citation in text: [1] [2] -
Ackermann, L.; Vicente, R.; Kapdi, A. R. Angew. Chem., Int. Ed. 2009, 48, 9792–9826. doi:10.1002/anie.200902996
Return to citation in text: [1] [2] -
Daugulis, O.; Do, H.-Q.; Shabashov, D. Acc. Chem. Res. 2009, 42, 1074–1086. doi:10.1021/ar9000058
Return to citation in text: [1] -
Chen, X.; Engle, K. M.; Wang, D.-H.; Yu, J.-Q. Angew. Chem., Int. Ed. 2009, 48, 5094–5115. doi:10.1002/anie.200806273
Return to citation in text: [1] -
Carrer, A.; Brion, J.-D.; Messaoudi, S.; Alami, M. Org. Lett. 2013, 15, 5606–5609. doi:10.1021/ol4028946
Return to citation in text: [1] -
Li, Q.; Zhang, S.-Y.; He, G.; Ai, Z.; Nack, W. A.; Chen, G. Org. Lett. 2014, 16, 1764–1767. doi:10.1021/ol500464x
Return to citation in text: [1] -
Hull, K. L.; Sanford, M. S. J. Am. Chem. Soc. 2009, 131, 9651–9653. doi:10.1021/ja901952h
Return to citation in text: [1] -
Ackermann, L.; Lygin, A. V. Org. Lett. 2011, 13, 3332–3335. doi:10.1021/ol2010648
Return to citation in text: [1] -
Zaitzev, V. G.; Shabashov, D.; Daugulis, O. J. Am. Chem. Soc. 2005, 127, 13154–13155. doi:10.1021/ja054549f
Return to citation in text: [1] -
Dangel, B. D.; Godula, K.; Youn, S. W.; Sezen, B.; Sames, D. J. Am. Chem. Soc. 2002, 124, 11856–11857. doi:10.1021/ja027311p
Return to citation in text: [1] -
Hinman, A.; Du Bois, J. J. Am. Chem. Soc. 2003, 125, 11510–11511. doi:10.1021/ja0368305
Return to citation in text: [1] -
O’Malley, S. J.; Tan, K. L.; Watzke, A.; Bergman, R. G.; Ellman, J. A. J. Am. Chem. Soc. 2005, 127, 13496–13497. doi:10.1021/ja052680h
Return to citation in text: [1] -
Yamaguchi, J.; Yamaguchi, A. D.; Itami, K. Angew. Chem., Int. Ed. 2012, 51, 8960–9009. doi:10.1002/anie.201201666
Return to citation in text: [1] -
Ramkumar, N.; Nagarajan, R. J. Org. Chem. 2013, 78, 2802–2807. doi:10.1021/jo302821v
Return to citation in text: [1] -
Stuart, D. R.; Villemure, E.; Fagnou, K. J. Am. Chem. Soc. 2007, 129, 12072–12073. doi:10.1021/ja0745862
Return to citation in text: [1] [2] -
Liang, Z.; Zhao, J.; Zhang, Y. J. Org. Chem. 2010, 75, 170–177. doi:10.1021/jo902265s
Return to citation in text: [1] [2] -
Fan, S.; Chen, Z.; Zhang, X. Org. Lett. 2012, 14, 4950–4953. doi:10.1021/ol3023165
Return to citation in text: [1] [2] -
Willis, N. J.; Smith, J. M. RSC Adv. 2014, 4, 11059–11063. doi:10.1039/C3RA44411B
Return to citation in text: [1] [2] -
Truong, T.; Alvarado, J.; Tran, L. D.; Daugulis, O. Org. Lett. 2010, 12, 1200–1203. doi:10.1021/ol902970z
Return to citation in text: [1] [2] -
Mao, Z.; Wang, Z.; Xu, Z.; Huang, F.; Yu, Z.; Wang, R. Org. Lett. 2012, 14, 3854–3857. doi:10.1021/ol301517y
Return to citation in text: [1] [2] -
Xi, P.; Yang, F.; Qin, S.; Zhao, D.; Lan, J.; Gao, G.; Hu, C.; You, J. J. Am. Chem. Soc. 2010, 132, 1822–1824. doi:10.1021/ja909807f
Return to citation in text: [1] [2] -
Nishino, M.; Hirano, K.; Satoh, T.; Miura, M. Angew. Chem., Int. Ed. 2012, 51, 6993–6997. doi:10.1002/anie.201201491
Return to citation in text: [1] [2] -
Grimster, N. P.; Gauntlett, C.; Godfrey, C. R. A.; Gaunt, M. J. Angew. Chem., Int. Ed. 2005, 44, 3125–3129. doi:10.1002/anie.200500468
Return to citation in text: [1] [2] -
Jiang, H.; Feng, Z.; Wang, A.; Liu, X.; Chen, Z. Eur. J. Org. Chem. 2010, 1227–1230. doi:10.1002/ejoc.200901282
Return to citation in text: [1] [2] -
Ackermann, L.; Jeyachandran, R.; Potukuchi, H. K.; Novak, P.; Büttner, L. Org. Lett. 2010, 12, 2056–2059. doi:10.1021/ol1005517
Return to citation in text: [1] [2] -
Meng, G.; Niu, H.-Y.; Qu, G.-R.; Fossey, J. S.; Li, J.-P.; Guo, H.-M. Chem. Commun. 2012, 48, 9601–9603. doi:10.1039/C2CC34158A
Return to citation in text: [1] -
Dwight, T. A.; Rue, N. R.; Charyk, D.; Josselyn, R.; DeBoef, B. Org. Lett. 2007, 9, 3137–3139. doi:10.1021/ol071308z
Return to citation in text: [1] -
Pintori, D. G.; Greaney, M. F. J. Am. Chem. Soc. 2011, 133, 1209–1211. doi:10.1021/ja1090854
Return to citation in text: [1] -
Reddy, V. P.; Iwasaki, T.; Kambe, N. Org. Biomol. Chem. 2013, 11, 2249–2253. doi:10.1039/c3ob27396b
Return to citation in text: [1] -
Agalave, S. G.; Maujan, S. R.; Pore, V. S. Chem. – Asian J. 2011, 6, 2696–2718. doi:10.1002/asia.201100432
Return to citation in text: [1] -
Shafran, E. A.; Bakulev, V. A.; Rozin, Yu. A.; Shafran, Yu. M. Chem. Heterocycl. Compd. 2008, 44, 1040–1069. doi:10.1007/s10593-008-0155-9
Return to citation in text: [1] -
Panteleev, J.; Geyer, K.; Aguilar-Aguilar, A.; Wang, L.; Lautens, M. Org. Lett. 2010, 12, 5092–5095. doi:10.1021/ol102342y
Return to citation in text: [1] -
Jiao, L.-Y.; Oestreich, M. Chem. – Eur. J. 2013, 19, 10845–10848. doi:10.1002/chem.201302140
Return to citation in text: [1] -
Wu, Y.; Wang, J.; Mao, F.; Kwong, F. Y. Chem. – Asian J. 2014, 9, 26–47. doi:10.1002/asia.201300990
Return to citation in text: [1] -
Lafrance, M.; Fagnou, K. J. Am. Chem. Soc. 2006, 128, 16496–16497. doi:10.1021/ja067144j
Return to citation in text: [1] -
Ackermann, L.; Vicente, R.; Althammer, A. Org. Lett. 2008, 10, 2299–2302. doi:10.1021/ol800773x
Return to citation in text: [1] -
Ackermann, L.; Novák, P. Org. Lett. 2009, 11, 4966–4969. doi:10.1021/ol902115f
Return to citation in text: [1] -
Lapointe, D.; Fagnou, K. Chem. Lett. 2010, 39, 1118–1126. doi:10.1246/cl.2010.1118
Return to citation in text: [1] -
Rousseaux, S.; Gorelsky, S. I.; Chung, B. K.; Fagnou, K. J. Am. Chem. Soc. 2010, 132, 10692–10705. doi:10.1021/ja103081n
Return to citation in text: [1] -
Blake, A. J.; Clark, B. A. J.; McNab, H.; Sommerville, C. C. J. Chem. Soc., Perkin Trans. 1 1997, 1605–1608. doi:10.1039/A700457E
Return to citation in text: [1] -
Ponpandian, T.; Muthusubramanian, S. Tetrahedron Lett. 2012, 53, 4248–4252. doi:10.1016/j.tetlet.2012.06.023
Return to citation in text: [1]
| 50. | Ponpandian, T.; Muthusubramanian, S. Tetrahedron Lett. 2012, 53, 4248–4252. doi:10.1016/j.tetlet.2012.06.023 |
| 44. | Lafrance, M.; Fagnou, K. J. Am. Chem. Soc. 2006, 128, 16496–16497. doi:10.1021/ja067144j |
| 45. | Ackermann, L.; Vicente, R.; Althammer, A. Org. Lett. 2008, 10, 2299–2302. doi:10.1021/ol800773x |
| 46. | Ackermann, L.; Novák, P. Org. Lett. 2009, 11, 4966–4969. doi:10.1021/ol902115f |
| 47. | Lapointe, D.; Fagnou, K. Chem. Lett. 2010, 39, 1118–1126. doi:10.1246/cl.2010.1118 |
| 48. | Rousseaux, S.; Gorelsky, S. I.; Chung, B. K.; Fagnou, K. J. Am. Chem. Soc. 2010, 132, 10692–10705. doi:10.1021/ja103081n |
| 49. | Blake, A. J.; Clark, B. A. J.; McNab, H.; Sommerville, C. C. J. Chem. Soc., Perkin Trans. 1 1997, 1605–1608. doi:10.1039/A700457E |
| 1. | Rostovtsev, V. V.; Green, L. G.; Fokin, V. V.; Sharpless, K. B. Angew. Chem., Int. Ed. 2002, 41, 2596–2599. doi:10.1002/1521-3773(20020715)41:14<2596::AID-ANIE2596>3.0.CO;2-4 |
| 2. | Himo, F.; Lovell, T.; Hilgraf, R.; Rostovtsev, V. V.; Noodleman, L.; Sharpless, K. B.; Fokin, V. V. J. Am. Chem. Soc. 2005, 127, 210–216. doi:10.1021/ja0471525 |
| 3. | Shao, C.; Wang, X.; Xu, J.; Zhao, J.; Zhang, Q.; Hu, Y. J. Org. Chem. 2010, 75, 7002–7005. doi:10.1021/jo101495k |
| 4. | Shin, J.-A.; Lim, Y.-G.; Lee, K.-H. J. Org. Chem. 2012, 77, 4117–4122. doi:10.1021/jo3000095 |
| 10. | Alberico, D.; Scott, M. E.; Lautens, M. Chem. Rev. 2007, 107, 174–238. doi:10.1021/cr0509760 |
| 11. | Ackermann, L.; Vicente, R.; Kapdi, A. R. Angew. Chem., Int. Ed. 2009, 48, 9792–9826. doi:10.1002/anie.200902996 |
| 12. | Daugulis, O.; Do, H.-Q.; Shabashov, D. Acc. Chem. Res. 2009, 42, 1074–1086. doi:10.1021/ar9000058 |
| 13. | Chen, X.; Engle, K. M.; Wang, D.-H.; Yu, J.-Q. Angew. Chem., Int. Ed. 2009, 48, 5094–5115. doi:10.1002/anie.200806273 |
| 6. | Kolarovič, A.; Schnürch, M.; Mihovilovic, M. D. J. Org. Chem. 2011, 76, 2613–2618. doi:10.1021/jo1024927 |
| 7. | Dyker, G. Angew. Chem., Int. Ed. 1999, 38, 1698–1712. doi:10.1002/(SICI)1521-3773(19990614)38:12<1698::AID-ANIE1698>3.0.CO;2-6 |
| 8. | Kakiuchi, F.; Chatani, N. Adv. Synth. Catal. 2003, 345, 1077–1101. doi:10.1002/adsc.200303094 |
| 9. | Dick, A. R.; Sanford, M. S. Tetrahedron 2006, 62, 2439–2463. doi:10.1016/j.tet.2005.11.027 |
| 10. | Alberico, D.; Scott, M. E.; Lautens, M. Chem. Rev. 2007, 107, 174–238. doi:10.1021/cr0509760 |
| 11. | Ackermann, L.; Vicente, R.; Kapdi, A. R. Angew. Chem., Int. Ed. 2009, 48, 9792–9826. doi:10.1002/anie.200902996 |
| 42. | Jiao, L.-Y.; Oestreich, M. Chem. – Eur. J. 2013, 19, 10845–10848. doi:10.1002/chem.201302140 |
| 43. | Wu, Y.; Wang, J.; Mao, F.; Kwong, F. Y. Chem. – Asian J. 2014, 9, 26–47. doi:10.1002/asia.201300990 |
| 6. | Kolarovič, A.; Schnürch, M.; Mihovilovic, M. D. J. Org. Chem. 2011, 76, 2613–2618. doi:10.1021/jo1024927 |
| 34. | Ackermann, L.; Jeyachandran, R.; Potukuchi, H. K.; Novak, P.; Büttner, L. Org. Lett. 2010, 12, 2056–2059. doi:10.1021/ol1005517 |
| 5. | Rodriquez, N.; Goossen, L. J. Chem. Soc. Rev. 2011, 40, 5030–5048. doi:10.1039/C1CS15093F |
| 41. | Panteleev, J.; Geyer, K.; Aguilar-Aguilar, A.; Wang, L.; Lautens, M. Org. Lett. 2010, 12, 5092–5095. doi:10.1021/ol102342y |
| 24. | Stuart, D. R.; Villemure, E.; Fagnou, K. J. Am. Chem. Soc. 2007, 129, 12072–12073. doi:10.1021/ja0745862 |
| 25. | Liang, Z.; Zhao, J.; Zhang, Y. J. Org. Chem. 2010, 75, 170–177. doi:10.1021/jo902265s |
| 26. | Fan, S.; Chen, Z.; Zhang, X. Org. Lett. 2012, 14, 4950–4953. doi:10.1021/ol3023165 |
| 27. | Willis, N. J.; Smith, J. M. RSC Adv. 2014, 4, 11059–11063. doi:10.1039/C3RA44411B |
| 28. | Truong, T.; Alvarado, J.; Tran, L. D.; Daugulis, O. Org. Lett. 2010, 12, 1200–1203. doi:10.1021/ol902970z |
| 29. | Mao, Z.; Wang, Z.; Xu, Z.; Huang, F.; Yu, Z.; Wang, R. Org. Lett. 2012, 14, 3854–3857. doi:10.1021/ol301517y |
| 30. | Xi, P.; Yang, F.; Qin, S.; Zhao, D.; Lan, J.; Gao, G.; Hu, C.; You, J. J. Am. Chem. Soc. 2010, 132, 1822–1824. doi:10.1021/ja909807f |
| 31. | Nishino, M.; Hirano, K.; Satoh, T.; Miura, M. Angew. Chem., Int. Ed. 2012, 51, 6993–6997. doi:10.1002/anie.201201491 |
| 32. | Grimster, N. P.; Gauntlett, C.; Godfrey, C. R. A.; Gaunt, M. J. Angew. Chem., Int. Ed. 2005, 44, 3125–3129. doi:10.1002/anie.200500468 |
| 33. | Jiang, H.; Feng, Z.; Wang, A.; Liu, X.; Chen, Z. Eur. J. Org. Chem. 2010, 1227–1230. doi:10.1002/ejoc.200901282 |
| 39. | Agalave, S. G.; Maujan, S. R.; Pore, V. S. Chem. – Asian J. 2011, 6, 2696–2718. doi:10.1002/asia.201100432 |
| 24. | Stuart, D. R.; Villemure, E.; Fagnou, K. J. Am. Chem. Soc. 2007, 129, 12072–12073. doi:10.1021/ja0745862 |
| 25. | Liang, Z.; Zhao, J.; Zhang, Y. J. Org. Chem. 2010, 75, 170–177. doi:10.1021/jo902265s |
| 26. | Fan, S.; Chen, Z.; Zhang, X. Org. Lett. 2012, 14, 4950–4953. doi:10.1021/ol3023165 |
| 27. | Willis, N. J.; Smith, J. M. RSC Adv. 2014, 4, 11059–11063. doi:10.1039/C3RA44411B |
| 28. | Truong, T.; Alvarado, J.; Tran, L. D.; Daugulis, O. Org. Lett. 2010, 12, 1200–1203. doi:10.1021/ol902970z |
| 29. | Mao, Z.; Wang, Z.; Xu, Z.; Huang, F.; Yu, Z.; Wang, R. Org. Lett. 2012, 14, 3854–3857. doi:10.1021/ol301517y |
| 30. | Xi, P.; Yang, F.; Qin, S.; Zhao, D.; Lan, J.; Gao, G.; Hu, C.; You, J. J. Am. Chem. Soc. 2010, 132, 1822–1824. doi:10.1021/ja909807f |
| 31. | Nishino, M.; Hirano, K.; Satoh, T.; Miura, M. Angew. Chem., Int. Ed. 2012, 51, 6993–6997. doi:10.1002/anie.201201491 |
| 32. | Grimster, N. P.; Gauntlett, C.; Godfrey, C. R. A.; Gaunt, M. J. Angew. Chem., Int. Ed. 2005, 44, 3125–3129. doi:10.1002/anie.200500468 |
| 33. | Jiang, H.; Feng, Z.; Wang, A.; Liu, X.; Chen, Z. Eur. J. Org. Chem. 2010, 1227–1230. doi:10.1002/ejoc.200901282 |
| 40. | Shafran, E. A.; Bakulev, V. A.; Rozin, Yu. A.; Shafran, Yu. M. Chem. Heterocycl. Compd. 2008, 44, 1040–1069. doi:10.1007/s10593-008-0155-9 |
| 19. | Dangel, B. D.; Godula, K.; Youn, S. W.; Sezen, B.; Sames, D. J. Am. Chem. Soc. 2002, 124, 11856–11857. doi:10.1021/ja027311p |
| 20. | Hinman, A.; Du Bois, J. J. Am. Chem. Soc. 2003, 125, 11510–11511. doi:10.1021/ja0368305 |
| 21. | O’Malley, S. J.; Tan, K. L.; Watzke, A.; Bergman, R. G.; Ellman, J. A. J. Am. Chem. Soc. 2005, 127, 13496–13497. doi:10.1021/ja052680h |
| 22. | Yamaguchi, J.; Yamaguchi, A. D.; Itami, K. Angew. Chem., Int. Ed. 2012, 51, 8960–9009. doi:10.1002/anie.201201666 |
| 23. | Ramkumar, N.; Nagarajan, R. J. Org. Chem. 2013, 78, 2802–2807. doi:10.1021/jo302821v |
| 14. | Carrer, A.; Brion, J.-D.; Messaoudi, S.; Alami, M. Org. Lett. 2013, 15, 5606–5609. doi:10.1021/ol4028946 |
| 15. | Li, Q.; Zhang, S.-Y.; He, G.; Ai, Z.; Nack, W. A.; Chen, G. Org. Lett. 2014, 16, 1764–1767. doi:10.1021/ol500464x |
| 16. | Hull, K. L.; Sanford, M. S. J. Am. Chem. Soc. 2009, 131, 9651–9653. doi:10.1021/ja901952h |
| 17. | Ackermann, L.; Lygin, A. V. Org. Lett. 2011, 13, 3332–3335. doi:10.1021/ol2010648 |
| 18. | Zaitzev, V. G.; Shabashov, D.; Daugulis, O. J. Am. Chem. Soc. 2005, 127, 13154–13155. doi:10.1021/ja054549f |
| 34. | Ackermann, L.; Jeyachandran, R.; Potukuchi, H. K.; Novak, P.; Büttner, L. Org. Lett. 2010, 12, 2056–2059. doi:10.1021/ol1005517 |
| 35. | Meng, G.; Niu, H.-Y.; Qu, G.-R.; Fossey, J. S.; Li, J.-P.; Guo, H.-M. Chem. Commun. 2012, 48, 9601–9603. doi:10.1039/C2CC34158A |
| 36. | Dwight, T. A.; Rue, N. R.; Charyk, D.; Josselyn, R.; DeBoef, B. Org. Lett. 2007, 9, 3137–3139. doi:10.1021/ol071308z |
| 37. | Pintori, D. G.; Greaney, M. F. J. Am. Chem. Soc. 2011, 133, 1209–1211. doi:10.1021/ja1090854 |
| 38. | Reddy, V. P.; Iwasaki, T.; Kambe, N. Org. Biomol. Chem. 2013, 11, 2249–2253. doi:10.1039/c3ob27396b |
© 2014 Bharathimohan et al; licensee Beilstein-Institut.
This is an Open Access article under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
The license is subject to the Beilstein Journal of Organic Chemistry terms and conditions: (http://www.beilstein-journals.org/bjoc)