Abstract
The reaction of triisopropyl phosphite with phosphine-based indenylidene pre-catalysts affords “1st generation” cis-complexes. These have been used in olefin metathesis reactions. The cis-Ru species exhibit noticeable differences with the trans-Ru parent complexes in terms of structure, thermal stability and reactivity. Experimental data underline the importance of synergistic effects between phosphites and L-type ligands.
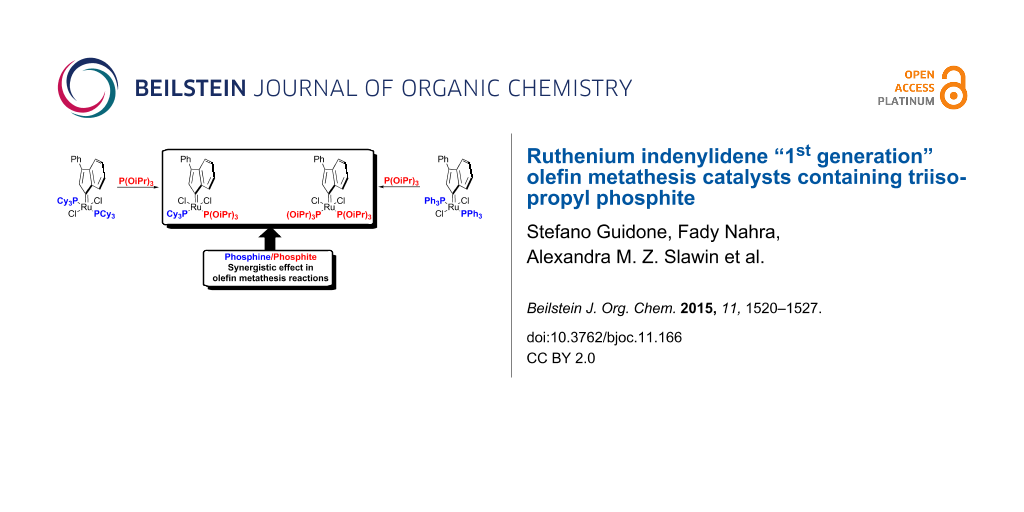
Graphical Abstract
Introduction
The olefin metathesis reaction is a powerful tool for C–C bond formation in the synthesis of highly valuable organic compounds [1-4]. Protocols involving W-, Mo- and Ru-based pre-catalysts can shorten or provide alternative synthetic pathways for the synthesis of natural products displaying complex chemical structures [5-9]. Ru-based pre-catalysts are known to be more air-, moisture- and functional-group tolerant compared to early transition metal complexes [10-13]. In general, the commonly used Ru(II)-based pre-catalysts have five ligands in the metal coordination sphere and adopt a distorted square pyramidal geometry (Figure 1).
Figure 1: Examples of ruthenium complexes used in olefin metathesis reactions.
Figure 1: Examples of ruthenium complexes used in olefin metathesis reactions.
The basic components of this structure include two L-type ligands mutually trans (e.g., phosphines and N-heterocyclic carbene) and two halides. The apex of the pyramid is occupied by an alkylidene moiety, such as a benzylidene or an indenylidene. Mixed NHC/phosphine complexes (G-II and Ind-II) known as “2nd generation” pre-catalysts generally display higher catalytic activity than “1st generation” complexes (G-I and Ind-I) containing two phosphines [14-23]. The most common phosphine, so called “throw-away ligand”, is tricyclohexyl phosphine [10-23]. In other words, such phosphorus donor ligands dissociate from the metal center to afford the 14e− active species [10-13,24]. In order to reduce the cost of the Ru-based pre-catalyst, our group has investigated the use of phosphites as an economical alternative to phosphines. The reaction of triisopropyl phosphite with the pyridine-containing indenylidene complex [RuCl2(Ind)(SIMes)(py)] (SIMes = N,N’-bis[2,4,6-(trimethyl)phenyl]imidazolidin-2-ylidene) afforded a Ru pre-catalyst displaying an unusual cis-geometry [25]. cis-Caz-1, which is more thermodynamically stable than its trans-isomer represents a breakthrough in catalyst-design for metathesis reactions of challenging hindered substrates (Figure 1) [25-34]. The latent behavior exhibited by cis-Caz-1 can be of interest in fields such as polymer chemistry, where its thermally-switchable properties can be used to inhibit polymerization during the storage of monomer-catalyst mixtures, and/or to initiate polymerization on demand through use of a stimulus [35,36]. The use of this catalyst in the ring-closing metathesis (RCM) reaction gave excellent conversions of challenging substrates, even at low catalyst loadings. The high activity and robustness of cis-Caz-1 is derived from synergistic effects between the σ-donor ligand NHC and the π-acidic triisopropyl phosphite [25,37]. Subsequently, the benzylidene analogue G-II-P(OiPr)3 was also reported. The latter displayed a typical trans-configuration, seen in other Ru pre-catalysts, and gave a similar catalytic activity to that of the phosphine-containing parent G-II [26].
Because of the recent interest in “1st generation” complexes [9,38-40], previous findings concerning “2nd generation” complexes [25-33] and the desire to further reduce catalyst cost, the aim of this contribution is to replace the phosphine ligands in Ind-I with the less expensive triisopropyl phosphite and to study the structural and catalytic properties of these new species.
Results and Discussion
Synthesis of [RuCl2(Ind)(PCy3){P(OiPr)3}] (1)
Attempts towards the synthesis of a mixed phosphine/phosphite complex involved the reaction of Ind-I with a stoichiometric amount of triisopropyl phosphite. Complex 1 was isolated in analytically pure form in 85% yield, after recrystallization, using a simple ligand exchange reaction (Scheme 1).
Scheme 1: Synthesis of the mixed phosphine/phosphite complex 1.
Scheme 1: Synthesis of the mixed phosphine/phosphite complex 1.
Similarly to the mixed NHC/phosphite species Caz-1 [25], the cis-geometry is the most thermodynamically stable conformation for the phosphine/phosphite complex 1. The corresponding trans-isomer was not isolated due to the fast isomerization occurring under the reaction conditions, although traces of transient species were detected by 31P-{1H} NMR spectroscopy (see Supporting Information File 1, section 4). The 1H NMR spectrum of 1 in CD2Cl2 showed the typical indenylidene proton system (characteristic doublet at low field, δH = 8.80 ppm). Coalescence of the aliphatic protons assigned to the cyclohexyl and the phosphite moieties was also observed at room temperature. These signals were resolved at a lower temperature (193 K). The 13C-{1H} NMR spectrum showed a doublet of doublets for the carbene carbon at δC = 290.3 ppm with two 2JCP of 12.5 and 24.5 Hz (cf., cis-Caz-1; 24.7 Hz) [25]. In the 31P-{1H} NMR spectrum, two doublets at 120.1 and 47.4 ppm with 2JPP of 37.0 Hz were observed, consistent with a cis-disposition of the phosphorus donor ligands. This geometry was confirmed by X-ray diffraction analysis on a single crystal (Figure 2).
![[1860-5397-11-166-2]](/bjoc/content/figures/1860-5397-11-166-2.png?scale=2.0&max-width=1024&background=FFFFFF)
Figure 2: Molecular structure of mixed phosphine/phosphite complex 1. Hydrogen atoms are omitted for clarity.
Figure 2: Molecular structure of mixed phosphine/phosphite complex 1. Hydrogen atoms are omitted for clarity.
Synthesis of [RuCl2(Ind){P(OiPr)3}2] (2)
The synthesis of the bis-phosphite species 2 was first attempted by the reaction of Ind-I with 2.5 equivalents of P(OiPr)3. Full conversion of the starting material was observed affording complex 2 (Scheme 2). Unfortunately, all attempts to purify 2 failed due to the presence of PCy3 decomposition products. The PPh3 adduct Ind-I0 was then employed as alternative starting material for the ligand substitution reaction with the phosphite (Scheme 2) (see Supporting Information File 1, section 4). During the recrystallization from dichloromethane/pentane, compound 3 was detected as a decomposition product [41].
Scheme 2: Synthesis of the bis-phosphite complex 2.
Scheme 2: Synthesis of the bis-phosphite complex 2.
Due to the high solubility of this species and difficulties encountered in the purification process, product 2 was isolated with traces of compound 3 still present. In the 1H NMR spectrum of 2 in CD2Cl2 (with 3 present), the characteristic doublet at δH = 8.53 ppm for the indenylidene system was observed. The 13C-{1H} NMR spectrum contains a doublet of doublets for the carbene carbon at δC = 291.1 ppm with two similar 2JCP of 22.0 Hz (cf. cis-Caz-1, 24.7 Hz) [25]. In the 31P-{1H} NMR spectrum, two singlets at δP = 123.0 ppm and 10.9 ppm corresponding to 2 and 3, respectively, were detected. Fortunately, we were able to cleanly isolate 3, which allowed its full characterization and assignment. Crystals suitable for X-ray diffraction studies were grown for both species. These studies confirmed the relative cis-disposition of the phosphite ligands in 2 and the structure of 3 (Figure 3).
![[1860-5397-11-166-3]](/bjoc/content/figures/1860-5397-11-166-3.png?scale=2.0&max-width=1024&background=FFFFFF)
Figure 3: Molecular structure of 2 and the ylide 3. Hydrogen atoms and solvent molecules are omitted for clarity. Selected bond distances (Å) and angles (°) (ESD) for compound 3: P(1)–C(1), 1.713(5); P(1)–C(28), 1.795(5); P(1)–C(22), 1.805(5); P(1)–C(16), 1.811(5); C(1)–P(1)–C(28), 106.2(2); C(1)–P(1)-C(22), 113.1(3); C(28)–P(1)–C(22), 109.0(2); C(1)–P(1)–C(16), 113.3(2); C(28)–P(1)–C(16), 109.2(2); C(22)–P(1)–C(16), 105.9(2).
Figure 3: Molecular structure of 2 and the ylide 3. Hydrogen atoms and solvent molecules are omitted for clar...
Complexes 1 and 2 display a rare distorted cis-square pyramidal geometry as observed in the case of cis-Caz-1 [25]. The cis-geometry differentiates these species from other “1st generation” complexes that display the more common trans-geometry [14-17]. Comparing the details of the three structures in Table 1 (entries 1 to 3), the Ru–CNHC bond distances are found shorter than Ru–Pphosphite, and both of them are shorter than Ru–Pphosphine (Ru(1)–P(2) complex 1) [34].
Table 1: Selected bond distances (Å) and angles (°) for 1, 2 and cis-Caz-1.
| Entry | Parameter | 1 | 2 | cis-Caz-1[25] |
|---|---|---|---|---|
| 1 | Ru(1)–C(1) | 1.873(14) | 1.869(3) | 1.881(8) |
| 2 | Ru(1)–P(1) | 2.239(4) | 2.2300(9) | 2.249(2) |
| 3 | Ru(1)–P(2) | 2.387(4) | 2.2663(8) | – |
| 4 | Ru(1)–C(NHC) | – | – | 2.067(7) |
| 5 | Ru(1)–Cl(1) | 2.398(4) | 2.3999(9) | 2.4036(18) |
| 6 | Ru(1)–Cl(2) | 2.369(3) | 2.3789(8) | 2.3974(19) |
| 7 | P(1)–Ru(1)–P(2) | 98.74(13) | 97.34(3) | – |
| 8 | C(NHC)–Ru(1)–P(1) | – | – | 100.06(19) |
| 9 | C(1)–Ru(1)–P(1) | 90.2(5) | 90.94(10) | 90.5(2) |
| 10 | C(1)–Ru(1)–P(2) | 94.3(4) | 86.41(9) | – |
| 11 | C(1)–Ru(1)–C(NHC) | – | – | 98.7(3) |
From data listed in Table 1, the Ru–P bond appears stronger in the case of the Ru–phosphite than the Ru–phosphine scenario, suggesting the latter as the leaving ligand in catalysis (see Supporting Information File 1, section 5).
Catalytic activity in ring-closing metathesis (RCM)
The reactivity of the mixed phosphine/phosphite complex 1 was first evaluated in the RCM of the easily cyclized diethyl diallylmalonate (4) (see Supporting Information File 1, section 2). The need for thermal activation for this pre-catalyst was clearly revealed by the low catalytic activity at 30–50 °C and the high conversion observed at 80 °C in toluene (0.1 mol % of 1, 94% conv.). Contrary to 1, the phosphine-based Ind-I initiates at 30 °C exhibiting good catalytic activity and undergoes fast decomposition at higher temperature with moderate conversion (see Supporting Information File 1, section 2). This trend was further studied by profiling reactions under catalytic conditions (Figure 4).
Figure 4: Reaction profiles of mixed phosphine/phosphite 1 and phosphine-based Ind-I in the RCM of 4 (lines are visual aids and not curve fits).
Figure 4: Reaction profiles of mixed phosphine/phosphite 1 and phosphine-based Ind-I in the RCM of 4 (lines a...
An induction period was observed for 1 at the early stage of the catalysis, a behavior similar to cis-Caz-1 [25], followed by a fast reaction with full conversion of the substrate at 80 °C in less than 50 min. These features prompted us to hypothesize an isomerization step from the cis-pre-catalyst 1 to the corresponding trans isomer as reported for cis-Caz-1 (see Supporting Information File 1, section 2) [25]. Under the same conditions, instant pre-catalyst initiation and fast decomposition of the active species were observed for the phosphine-based pre-catalyst Ind-I (86% conversion after 30 min). When the experiment was performed at 30 °C, Ind-I exhibited slower conversion of the substrate, reaching complete conversion after 4 h [22]. The reaction profiles show the importance of synergistic effects in the case of the mixed phosphine/phosphite system. Complex 1 is a thermally-switchable, latent pre-catalyst displaying higher thermal stability compared to the phosphine-based Ind-I.
Consequently, a brief study of the scope of the reaction was investigated employing “1st generation” complexes 1 and Ind-I (Table 2).
Table 2: Scope of the reaction employing 1 and Ind-I.a
| Entry | Substrate | Product | Pre-catalyst (mol %) | T (°C) | Conv. (%)b |
|---|---|---|---|---|---|
|
1
2 3 4 |
|
|
1 (0.1)
1 (1) Ind-I (0.1) Ind-I (0.1) |
80
80 80 30 |
<1
4 94 94 |
|
5
6 7 |
|
|
1 (1)
Ind-I (1) Ind-I (1) |
80
80 30 |
79 (71)
33 53 |
|
8
9 10 11 12 13 |
|
|
1 (1)
1 (2) Ind-I (1) Ind-I (1) Ind-I (2) Ind-I (2) |
80
80 80 30 80 30 |
41
41 22 21 30 77 |
|
14
15 |
|
|
1 (1)
Ind-I (1) |
80
30 |
78
98 |
aReaction conditions: substrate (0.25 mmol), pre-catalyst (0.1 to 2 mol %), toluene (0.5 mL), 19 h. bConversions were determined by GC analysis. Isolated yields in parentheses.
The diene 6 was poorly converted by mixed PCy3/P(OR)3 complex 1, whereas 94% conversion was obtained with Ind-I (Table 2, entries 1–4). In the case of tri-substituted diene 8, a more challenging substrate compared to 6, pre-catalyst 1 gave 79% conversion while Ind-I converted 33% of the substrate at 80 °C and 53% at 30 °C (Table 2, entries 5–7). The tosylamide derivative 10 was converted into product 11 by 1 (1 mol %) with 41% conversion (Table 2, entry 8). When the loading was increased to 2 mol %, no improvement in the conversion was detected (Table 2, entry 9). A higher catalytic activity was observed for Ind-I with 77% conversion when using 2 mol % pre-catalyst at 30 °C (Table 2, entry 13). Complex 1 was active in the ring-closing enyne metathesis (RCEYM) with 78% conversion of substrate 12 obtained with 1 mol % catalyst loading (Table 2, entry 14). A higher conversion of compound 12 was detected with Ind-I (98%, Table 2, entry 15).
Conclusion
The influence of triisopropyl phosphite in Ru-based indenylidene “1st generation” complexes has been presented. The mixed phosphine/phosphite complex 1 and the bis-phosphite complex 2 adopt distorted square pyramidal geometries with the P-donor ligands mutually cis as the most thermodynamically stable conformation. The isolation of the corresponding trans-isomers was not possible due to a fast isomerization process occurring during the synthesis of the complexes. Pre-catalyst 1 was found to be active in olefin metathesis reaction showing similarities with cis-Caz-1 in terms of reactivity. Both pre-catalysts need thermal activation; they display an induction period in the reaction profiling and exhibit higher thermal stability compared to their phosphine-based analogues. In terms of catalytic efficiency, Ind-I was found more active than 1 unless higher thermal stability is needed. Indeed, in the case of the malonate derivative 8, pre-catalyst 1 afforded the tri-substituted ring-closed product in 71% isolated yield. The similar structural and catalytic properties observed in the mixed phosphine/phosphite complex 1 and the mixed NHC/phosphite cis-Caz-1 suggest the importance of synergistic effects involving phosphites, an inexpensive alternative to phosphines for Ru-based pre-catalysts, and the L-type ligands, a concept that can be used to incite further improvements in catalyst design.
Experimental
Synthesis and characterization of [RuCl2(Ind)(PCy3){P(OiPr)3}] (1): Under an inert atmosphere of argon, triisopropyl phosphite (364 μL, 1.53 mmol) was added to a solution of Ind-I (1.414 g, 1.53 mmol) in dichloromethane (20 mL). The mixture was stirred for 24 h at room temperature and the solvent was then removed in vacuo. The crude product was recrystallized twice from dichloromethane/pentane. The solid was collected by filtration and washed with pentane (3 × 10, 2 × 15 mL). The product was obtained as a brownish red solid (1.116 g, 85%). During NMR experiments, peaks originating from the decomposition of the PCy3 were observed. 1H NMR of the mixture: (400 MHz, CD2Cl2) δ 1.08–1.33 (m, 6H, PCy3), 1.11 (d, 3JHH = 6.3 Hz, 9H, CH-CH3), 1.30 (d, 3JHH = 6.3 Hz, 9H, CH-CH3), 1.40–1.55 (m, 9H, PCy3), 1.60–1.85 (m, 15H, PCy3), 2.50 (m, 3H, CH PCy3), 4.55 (m, 3H, CH-CH3), 6.79 (s, 1H, H2), 7.27 (d, 3JHH = 7.1 Hz, 1H, H4), 7.43 (dd, 3JHH = 6.7 Hz, 3JHH = 6.3 Hz, 1H, H5), 7.44 (dd, 3JHH = 7.4 Hz, 3JHH = 6.3 Hz, 2H, H10), 7.50 (dd, 3JHH = 7.4 Hz, 3JHH = 7.7 Hz, 1H, H11), 7.53 (dd, 3JHH = 7.4 Hz, 3JHH = 7.4 Hz, 1H, H6), 7.76 (d, 3JHH = 7.3 Hz, 2H, H9), 8.80 (d, 3JHH = 7.3 Hz, 1H, H7) ppm; 13C-{1H} NMR of the mixture (100.6 MHz, CD2Cl2) δ 24.3 (s, CH-CH3), 24.5 (d, 3JCP = 4 Hz, CH-CH3), 26.9 (s, CH2 PCy3), 28.1 (d, 2JCP = 11 Hz, CH2 PCy3), 28.4 (d, 2JCP = 10 Hz, CH2 PCy3), 30.1 (s, CH2 PCy3), 30.6 (s, CH2 PCy3), 35.3 (s, CH PCy3), 71.4 (s, CH-CH3), 118.4 (s, C4), 127.0 (s, C9), 129.6 (s, C10), 129.7 (s, C11), 130.2 (s, C7), 130.3 (s, C6), 130. 7 (s, C5), 135.1 (s, C8), 136.5 (s, C3), 140.6 (s, C3a), 141.3 (dd, 3JCP = 5.0 Hz, 3JCP = 14.0 Hz, C2) 147.8 (s, C7a), 290.3 (dd, 3JCP = 12.5 Hz, 2JCP = 24.5 Hz, C1) ppm; 31P-{1H} NMR of the mixture (162 MHz, CD2Cl2) δ 120.1 (d, 2JPP = 37.0 Hz, PCy3), 47.4 (d, 2JPP = 37.0 Hz, P(OiPr)3) ppm; anal. calcd for C42H64Cl2O3P2Ru: C, 59.29; H, 7.58; found: C, 59.45; H, 7.66. CCDC-889638 contains the supplementary crystallographic data for 1. These data can be obtained free of charge from the Cambridge Crystallographic Data Centre via http://www.ccdc.cam.ac.uk/data_request/cif.
Synthesis and characterization of [RuCl2(Ind){P(OiPr)3}2] (2): Under an inert atmosphere of argon, triisopropyl phosphite (65 μL, 0.28 mmol) was added to a solution of Ind-I0 (0.100 g, 0.113 mmol) in dichloromethane (1.2 mL). The mixture was stirred for 24 h at room temperature and the solvent was removed in vacuo. The crude product was recrystallized from dichloromethane/pentane. The solid was collected by filtration and washed with pentane (3 × 3 mL). The product was obtained as a brownish green solid in a mixture with the phosphonium ylide 3 (0.032 g, 35%). 1H NMR of the mixture (400 MHz, CD2Cl2) δ 1.13 (d, 3JHH = 6.3 Hz, 18H, CH-CH3), 1.31 (d, 3JHH = 6.1 Hz, 18H, CH-CH3), 4.53 (m, 3JHH = 6.3 Hz, 6H, CH-CH3), 7.18 (s, 1H, H2), 7.26 (d, 3JHH = 6.9 Hz, 1H, H4), 7.40 (dd, 3JHH = 7.1 Hz, 3JHH = 7.1 Hz, 1H, H5), 7.43 (dd, 3JHH = 7.4 Hz, 3JHH = 7.4 Hz, 1H, H6), 7.46 (dd, 3JHH = 7.4 Hz, 3JHH = 7.4 Hz, 2H, H10), 7.53 (dd, 3JHH = 7.4 Hz, 3JHH = 7.4 Hz, 1H, H11), 7.77 (d, 3JHH = 7.3 Hz, 2H, H9), 8.53 (d, 3JHH = 7.0 Hz, 1H, H7) ppm; 13C-{1H} NMR of the mixture (100.6 MHz, CD2Cl2) δ 24.0 (s, CH-CH3), 24.4 (s, CH-CH3), 71.5 (m, CH-CH3), 119.0 (s, C4), 127.1 (s, C9), 129.6 (s, C11), 130.0 (s, C10), 130.2 (s, C6), 130.3 (s, C7), 131. 2 (s, C5), 134.8 (s, C8), 136.8 (s, C3), 140.3 (s, C3a), 140.7 (dd, 3JCP = 8.5 Hz, C2) 150.2 (s, C7a), 291.1 (dd, 2JCP = 22.0 Hz, 2JCP = 22.0 Hz, C1) ppm; 31P-{1H} NMR (162 MHz, CD2Cl2) δ 123.0 (s, P(OiPr)3) ppm; CCDC-889639 contains the supplementary crystallographic data for 2. These data can be obtained free of charge from the Cambridge Crystallographic Data Centre via http://www.ccdc.cam.ac.uk/data_request/cif.
Characterization of Ph3P(Ind) (3): Under an inert atmosphere of argon, compound 3 was obtained as a side product from the reaction of Ind-I0 (0.303 mg, 0.34 mmol, 1 equiv) with triisopropyl phosphite (179 μL, 0.75 mmol, 2.2 equiv) for 24 h. During recrystallization in a dichloromethane/pentane mixture, compound 3 was isolated as a yellow solid (63.4 mg, 41%). 1H NMR (400 MHz; CD2Cl2) δ 6.73 (d, 2JHP = 4.9 Hz, 1H, H2), 6.77 (dd, 3JHH = 7.5 Hz, 3JHH = 7.5 Hz, 1H, H6), 6.89 (d, 3JHH = 7.9 Hz, 1H, H7), 6.97 (dd, 3JHH = 7.5 Hz, 3JHH = 7.5 Hz, 1H, H5), 7.08 (dd, 3JHH = 7.4 Hz, 3JHH = 7.4 Hz, 1H, H11), 7.32 (dd, 3JHH = 7.6 Hz, 3JHH = 7.6 Hz, 2H, H10), 7.52–7.60 (m, 6H, C6H5), 7.61 (d, 3JHH = 7.9 Hz, 2H, H9), 7.66–7.75 (m, 9H, C6H5), 7.96 (d, 3JHH = 8.1 Hz, 1H, H4) ppm; 13C-{1H} NMR (100.6 MHz; CD2Cl2) δ 68.5 (d, 1JCP = 121.6 Hz, C1), 118.2 (s, C5), 118.5 (s, C6), 118.7 (s, C7), 119.4 (s, C4), 121.2 (d, 3JCP = 15.8 Hz, C3), 123.8 (s, C11), 125.6 (d, 1JCP = 90.3 Hz, i-C6H5), 127.1 (s, C9), 127.8 (d, 2JCP = 16.5 Hz, C2), 128.7 (s, C10), 129.5 (d, 3JCP = 12.3 Hz, m-C6H5), 133.3 (d, 4JCP = 2.7 Hz, p-C6H5), 134.2 (d, 2JCP = 10.3 Hz, o-C6H5), 135.0 (d, 3JCP = 13.9 Hz, C3a), 137.1 (d, 2JCP = 13.2 Hz, C7a), 140.5 (s, C8) ppm; 31P-{1H} NMR (162 MHz; CD2Cl2) δ 10.9 (s, Ph3P(Ind)) ppm; HMRS (APCI): m/z calcd for [C33H25P + H] 453.18; found 453.1754. CCDC-889640 contains the supplementary crystallographic data for 3. These data can be obtained free of charge from the Cambridge Crystallographic Data Centre via http://www.ccdc.cam.ac.uk/data_request/cif.
General procedure for catalysis: Substrates 6 [42], 8 [43], 10 [42], 12 [22], were synthesized following previous reports in the literature. Compound 4 was obtained from a commercial source and its purity confirmed prior to use. 1H NMR data of product 9 were compared to previously reported analyses [22].
A 5 mL screwcap-vial fitted with a septum equipped with a magnetic stirring bar was charged with the olefin (0.25 mmol) then purged with nitrogen, closed and introduced in a glovebox. The solvent and pre-catalyst (stock solution for <1 mol %, or weighed in the vial) were added to the reaction mixture (total amount of solvent 0.5 mL). Once out of the glovebox, the reaction mixture was heated at the desired temperature and stirred for 14 or 19 hours. The reaction mixture was then analyzed by GC and/or purified by flash chromatography.
General procedure for kinetic experiments. A Schlenk flask was charged with the olefin (0.5 mmol), then closed, placed under vacuum and introduced in a glovebox. The solvent was added (5 mL) and then the pre-catalyst was weighed and charged into the Schlenk flask. Out of the glovebox, the reaction was performed at the desired temperature. Samples were taken under nitrogen flow and quenched with ethyl vinyl ether. Data were obtained by GC analysis.
Supporting Information
Crystallographic data for complexes 1–3 in CIF format can be obtained free of charge from the Cambridge Crystallographic Data Centre via http://www.ccdc.cam.ac.uk/data_request/cif (CCDC/889638-889640).
| Supporting Information File 1: Crystallographic data for compounds 1–3, NMR spectra of all the complexes, spectroscopic data. | ||
| Format: PDF | Size: 1.9 MB | Download |
| Supporting Information File 2: CIF file for complex 2. | ||
| Format: CIF | Size: 27.1 KB | Download |
| Supporting Information File 3: CIF file for complex 3. | ||
| Format: CIF | Size: 26.3 KB | Download |
| Supporting Information File 4: CIF file for complex 4. | ||
| Format: CIF | Size: 33.4 KB | Download |
References
-
Hoveyda, A. H.; Zhugralin, A. R. Nature 2007, 450, 243–251. doi:10.1038/nature06351
Return to citation in text: [1] -
Chauvin, Y. Angew. Chem., Int. Ed. 2006, 45, 3740–3747. doi:10.1002/anie.200601234
Return to citation in text: [1] -
Schrock, R. R. Angew. Chem., Int. Ed. 2006, 45, 3748–3759. doi:10.1002/anie.200600085
Return to citation in text: [1] -
Grubbs, R. H. Angew. Chem., Int. Ed. 2006, 45, 3760–3765. doi:10.1002/anie.200600680
Return to citation in text: [1] -
Fürstner, A. Angew. Chem., Int. Ed. 2000, 39, 3012–3043. doi:10.1002/1521-3773(20000901)39:17<3012::AID-ANIE3012>3.0.CO;2-G
Return to citation in text: [1] -
Buchmeiser, M. R. Chem. Rev. 2000, 100, 1565–1604. doi:10.1021/cr990248a
Return to citation in text: [1] -
Connon, S. J.; Blechert, S. Angew. Chem., Int. Ed. 2003, 42, 1900–1923. doi:10.1002/anie.200200556
Return to citation in text: [1] -
Grubbs, R. H.; Miller, S. J.; Fu, G. C. Acc. Chem. Res. 1995, 28, 446–452. doi:10.1021/ar00059a002
Return to citation in text: [1] -
Nicolaou, K. C.; Bulger, P. G.; Sarlah, D. Angew. Chem., Int. Ed. 2005, 44, 4490–4527. doi:10.1002/anie.200500369
Return to citation in text: [1] [2] -
Trnka, T. M.; Grubbs, R. H. Acc. Chem. Res. 2001, 34, 18–29. doi:10.1021/ar000114f
Return to citation in text: [1] [2] [3] -
Schrock, R. R.; Hoveyda, A. H. Angew. Chem., Int. Ed. 2003, 42, 4592–4633. doi:10.1002/anie.200300576
Return to citation in text: [1] [2] [3] -
Grubbs, R. H., Ed. Handbook of Metathesis: Catalyst Development; Wiley-VCH: Weinheim, Germany, 2003. doi:10.1002/9783527619481
Return to citation in text: [1] [2] [3] -
Grela, K., Ed. Olefin Metathesis – Theory and Practice; Wiley-VCH: Weinheim, Germany, 2014.
Return to citation in text: [1] [2] [3] -
Nguyen, S. T.; Johnson, L. K.; Grubbs, R. H.; Ziller, J. W. J. Am. Chem. Soc. 1992, 114, 3974–3975. doi:10.1021/ja00036a053
Return to citation in text: [1] [2] [3] -
Nguyen, S. T.; Grubbs, R. H.; Ziller, J. W. J. Am. Chem. Soc. 1993, 115, 9858–9859. doi:10.1021/ja00074a086
Return to citation in text: [1] [2] [3] -
Schwab, P.; Grubbs, R. H.; Ziller, J. W. J. Am. Chem. Soc. 1996, 118, 100–110. doi:10.1021/ja952676d
Return to citation in text: [1] [2] [3] -
Boeda, F.; Clavier, H.; Nolan, S. P. Chem. Commun. 2008, 2726–2740. doi:10.1039/b718287b
Return to citation in text: [1] [2] [3] -
Weskamp, T.; Kohl, F. J.; Herrmann, W. A. J. Organomet. Chem. 1999, 582, 362–365. doi:10.1016/S0022-328X(99)00163-1
Return to citation in text: [1] [2] -
Huang, J.; Schanz, H.-J.; Stevens, E. D.; Nolan, S. P. Organometallics 1999, 18, 5375–5380. doi:10.1021/om990788y
Return to citation in text: [1] [2] -
Scholl, M.; Ding, S.; Lee, C. W.; Grubbs, R. H. Org. Lett. 1999, 1, 953–956. doi:10.1021/ol990909q
Return to citation in text: [1] [2] -
Sanford, M. S.; Love, J. A.; Grubbs, R. H. J. Am. Chem. Soc. 2001, 123, 6543–6554. doi:10.1021/ja010624k
Return to citation in text: [1] [2] -
Clavier, H.; Nolan, S. P. Chem. – Eur. J. 2007, 13, 8029–8036. doi:10.1002/chem.200700256
Return to citation in text: [1] [2] [3] [4] [5] -
Monsaert, S.; Drozdzak, R.; Dragutan, V.; Dragutan, I.; Verpoort, F. Eur. J. Inorg. Chem. 2008, 432–440. doi:10.1002/ejic.200700879
Return to citation in text: [1] [2] -
Nelson, D. J.; Manzini, S.; Urbina-Blanco, C. A.; Nolan, S. P. Chem. Commun. 2014, 50, 10355–10375. doi:10.1039/C4CC02515F
Return to citation in text: [1] -
Bantreil, X.; Schmid, T. E.; Randall, R. A. M.; Slawin, A. M. Z.; Cazin, C. S. J. Chem. Commun. 2010, 46, 7115–7117. doi:10.1039/c0cc02448a
Return to citation in text: [1] [2] [3] [4] [5] [6] [7] [8] [9] [10] [11] -
Schmid, T. E.; Bantreil, X.; Citadelle, C. A.; Slawin, A. M. Z.; Cazin, C. S. J. Chem. Commun. 2011, 47, 7060–7062. doi:10.1039/c1cc10825e
Return to citation in text: [1] [2] [3] -
Songis, O.; Slawin, A. M. Z.; Cazin, C. S. J. Chem. Commun. 2012, 48, 1266–1268. doi:10.1039/C2CC15903A
Return to citation in text: [1] [2] -
Bantreil, X.; Poater, A.; Urbina-Blanco, C. A.; Bidal, Y. D.; Falivene, L.; Randall, R. A. M.; Cavallo, L.; Slawin, A. M. Z.; Cazin, C. S. J. Organometallics 2012, 31, 7415–7426. doi:10.1021/om300703p
Return to citation in text: [1] [2] -
Urbina-Blanco, C. A.; Bantreil, X.; Wappel, J.; Schmid, T. E.; Slawin, A. M. Z.; Slugovc, C.; Cazin, C. S. J. Organometallics 2013, 32, 6240–6247. doi:10.1021/om4004362
Return to citation in text: [1] [2] -
Falivene, L.; Poater, A.; Cazin, C. S. J.; Slugovc, C.; Cavallo, L. Dalton Trans. 2013, 42, 7312–7317. doi:10.1039/C2DT32277C
Return to citation in text: [1] [2] -
Guidone, S.; Songis, O.; Nahra, F.; Cazin, C. S. J. ACS Catal. 2015, 5, 2697–2701. doi:10.1021/acscatal.5b00197
Return to citation in text: [1] [2] -
Guidone, S.; Songis, O.; Falivene, L.; Nahra, F.; Slawin, A. M. Z.; Jacobsen, H.; Cavallo, L.; Cazin, C. S. J. ACS Catal. 2015, 5, 3932–3939. doi:10.1021/acscatal.5b00219
Return to citation in text: [1] [2] -
Bantreil, X.; Cazin, C. S. J. Monatsh. Chem. 2015, 146, 1043–1052. doi:10.1007/s00706-015-1487-7
Return to citation in text: [1] [2] -
Cazin, C. S. J., Ed. N-Heterocyclic Carbenes in Transition Metal Catalysis and Organocatalysis; Catalysis by Metal Complexes, Vol. 32; Springer: New York, NY, U.S.A., 2011. doi:10.1007/978-90-481-2866-2
Return to citation in text: [1] [2] -
Ung, T.; Hejl, A.; Grubbs, R. H.; Schrodi, Y. Organometallics 2004, 23, 5399–5401. doi:10.1021/om0493210
Return to citation in text: [1] -
Slugovc, C.; Burtscher, D.; Stelzer, F.; Mereiter, K. Organometallics 2005, 24, 2255–2258. doi:10.1021/om050141f
Return to citation in text: [1] -
Ho, C.-Y.; Jamison, T. F. Angew. Chem., Int. Ed. 2007, 46, 782–785. doi:10.1002/anie.200603907
Return to citation in text: [1] -
Wallace, D. J.; Reamer, R. A. J. Org. Chem. 2014, 79, 5644–5651. doi:10.1021/jo500815q
Return to citation in text: [1] -
Chatterjee, S.; Guchhait, S.; Goswami, R. K. J. Org. Chem. 2014, 79, 7689–7695. doi:10.1021/jo501184t
Return to citation in text: [1] -
Keck, C. G.; Kendall, J. L.; Caster, K. C. Adv. Synth. Catal. 2007, 349, 165–174. doi:10.1002/adsc.200600455
Return to citation in text: [1] -
Hong, S. H.; Day, M. W.; Grubbs, R. H. J. Am. Chem. Soc. 2004, 126, 7414–7415. doi:10.1021/ja0488380
Phosphorus ylide bearing the alkylidene moiety has already been proposed.
Return to citation in text: [1] -
Terada, Y.; Arisawa, M.; Nishida, A. Angew. Chem., Int. Ed. 2004, 43, 4063–4067. doi:10.1002/anie.200454157
Return to citation in text: [1] [2] -
Kirkland, T. A.; Grubbs, R. H. J. Org. Chem. 1997, 62, 7310–7318. doi:10.1021/jo970877p
Return to citation in text: [1]
| 22. | Clavier, H.; Nolan, S. P. Chem. – Eur. J. 2007, 13, 8029–8036. doi:10.1002/chem.200700256 |
| 22. | Clavier, H.; Nolan, S. P. Chem. – Eur. J. 2007, 13, 8029–8036. doi:10.1002/chem.200700256 |
| 1. | Hoveyda, A. H.; Zhugralin, A. R. Nature 2007, 450, 243–251. doi:10.1038/nature06351 |
| 2. | Chauvin, Y. Angew. Chem., Int. Ed. 2006, 45, 3740–3747. doi:10.1002/anie.200601234 |
| 3. | Schrock, R. R. Angew. Chem., Int. Ed. 2006, 45, 3748–3759. doi:10.1002/anie.200600085 |
| 4. | Grubbs, R. H. Angew. Chem., Int. Ed. 2006, 45, 3760–3765. doi:10.1002/anie.200600680 |
| 10. | Trnka, T. M.; Grubbs, R. H. Acc. Chem. Res. 2001, 34, 18–29. doi:10.1021/ar000114f |
| 11. | Schrock, R. R.; Hoveyda, A. H. Angew. Chem., Int. Ed. 2003, 42, 4592–4633. doi:10.1002/anie.200300576 |
| 12. | Grubbs, R. H., Ed. Handbook of Metathesis: Catalyst Development; Wiley-VCH: Weinheim, Germany, 2003. doi:10.1002/9783527619481 |
| 13. | Grela, K., Ed. Olefin Metathesis – Theory and Practice; Wiley-VCH: Weinheim, Germany, 2014. |
| 14. | Nguyen, S. T.; Johnson, L. K.; Grubbs, R. H.; Ziller, J. W. J. Am. Chem. Soc. 1992, 114, 3974–3975. doi:10.1021/ja00036a053 |
| 15. | Nguyen, S. T.; Grubbs, R. H.; Ziller, J. W. J. Am. Chem. Soc. 1993, 115, 9858–9859. doi:10.1021/ja00074a086 |
| 16. | Schwab, P.; Grubbs, R. H.; Ziller, J. W. J. Am. Chem. Soc. 1996, 118, 100–110. doi:10.1021/ja952676d |
| 17. | Boeda, F.; Clavier, H.; Nolan, S. P. Chem. Commun. 2008, 2726–2740. doi:10.1039/b718287b |
| 18. | Weskamp, T.; Kohl, F. J.; Herrmann, W. A. J. Organomet. Chem. 1999, 582, 362–365. doi:10.1016/S0022-328X(99)00163-1 |
| 19. | Huang, J.; Schanz, H.-J.; Stevens, E. D.; Nolan, S. P. Organometallics 1999, 18, 5375–5380. doi:10.1021/om990788y |
| 20. | Scholl, M.; Ding, S.; Lee, C. W.; Grubbs, R. H. Org. Lett. 1999, 1, 953–956. doi:10.1021/ol990909q |
| 21. | Sanford, M. S.; Love, J. A.; Grubbs, R. H. J. Am. Chem. Soc. 2001, 123, 6543–6554. doi:10.1021/ja010624k |
| 22. | Clavier, H.; Nolan, S. P. Chem. – Eur. J. 2007, 13, 8029–8036. doi:10.1002/chem.200700256 |
| 23. | Monsaert, S.; Drozdzak, R.; Dragutan, V.; Dragutan, I.; Verpoort, F. Eur. J. Inorg. Chem. 2008, 432–440. doi:10.1002/ejic.200700879 |
| 25. | Bantreil, X.; Schmid, T. E.; Randall, R. A. M.; Slawin, A. M. Z.; Cazin, C. S. J. Chem. Commun. 2010, 46, 7115–7117. doi:10.1039/c0cc02448a |
| 14. | Nguyen, S. T.; Johnson, L. K.; Grubbs, R. H.; Ziller, J. W. J. Am. Chem. Soc. 1992, 114, 3974–3975. doi:10.1021/ja00036a053 |
| 15. | Nguyen, S. T.; Grubbs, R. H.; Ziller, J. W. J. Am. Chem. Soc. 1993, 115, 9858–9859. doi:10.1021/ja00074a086 |
| 16. | Schwab, P.; Grubbs, R. H.; Ziller, J. W. J. Am. Chem. Soc. 1996, 118, 100–110. doi:10.1021/ja952676d |
| 17. | Boeda, F.; Clavier, H.; Nolan, S. P. Chem. Commun. 2008, 2726–2740. doi:10.1039/b718287b |
| 18. | Weskamp, T.; Kohl, F. J.; Herrmann, W. A. J. Organomet. Chem. 1999, 582, 362–365. doi:10.1016/S0022-328X(99)00163-1 |
| 19. | Huang, J.; Schanz, H.-J.; Stevens, E. D.; Nolan, S. P. Organometallics 1999, 18, 5375–5380. doi:10.1021/om990788y |
| 20. | Scholl, M.; Ding, S.; Lee, C. W.; Grubbs, R. H. Org. Lett. 1999, 1, 953–956. doi:10.1021/ol990909q |
| 21. | Sanford, M. S.; Love, J. A.; Grubbs, R. H. J. Am. Chem. Soc. 2001, 123, 6543–6554. doi:10.1021/ja010624k |
| 22. | Clavier, H.; Nolan, S. P. Chem. – Eur. J. 2007, 13, 8029–8036. doi:10.1002/chem.200700256 |
| 23. | Monsaert, S.; Drozdzak, R.; Dragutan, V.; Dragutan, I.; Verpoort, F. Eur. J. Inorg. Chem. 2008, 432–440. doi:10.1002/ejic.200700879 |
| 41. |
Hong, S. H.; Day, M. W.; Grubbs, R. H. J. Am. Chem. Soc. 2004, 126, 7414–7415. doi:10.1021/ja0488380
Phosphorus ylide bearing the alkylidene moiety has already been proposed. |
| 10. | Trnka, T. M.; Grubbs, R. H. Acc. Chem. Res. 2001, 34, 18–29. doi:10.1021/ar000114f |
| 11. | Schrock, R. R.; Hoveyda, A. H. Angew. Chem., Int. Ed. 2003, 42, 4592–4633. doi:10.1002/anie.200300576 |
| 12. | Grubbs, R. H., Ed. Handbook of Metathesis: Catalyst Development; Wiley-VCH: Weinheim, Germany, 2003. doi:10.1002/9783527619481 |
| 13. | Grela, K., Ed. Olefin Metathesis – Theory and Practice; Wiley-VCH: Weinheim, Germany, 2014. |
| 25. | Bantreil, X.; Schmid, T. E.; Randall, R. A. M.; Slawin, A. M. Z.; Cazin, C. S. J. Chem. Commun. 2010, 46, 7115–7117. doi:10.1039/c0cc02448a |
| 26. | Schmid, T. E.; Bantreil, X.; Citadelle, C. A.; Slawin, A. M. Z.; Cazin, C. S. J. Chem. Commun. 2011, 47, 7060–7062. doi:10.1039/c1cc10825e |
| 27. | Songis, O.; Slawin, A. M. Z.; Cazin, C. S. J. Chem. Commun. 2012, 48, 1266–1268. doi:10.1039/C2CC15903A |
| 28. | Bantreil, X.; Poater, A.; Urbina-Blanco, C. A.; Bidal, Y. D.; Falivene, L.; Randall, R. A. M.; Cavallo, L.; Slawin, A. M. Z.; Cazin, C. S. J. Organometallics 2012, 31, 7415–7426. doi:10.1021/om300703p |
| 29. | Urbina-Blanco, C. A.; Bantreil, X.; Wappel, J.; Schmid, T. E.; Slawin, A. M. Z.; Slugovc, C.; Cazin, C. S. J. Organometallics 2013, 32, 6240–6247. doi:10.1021/om4004362 |
| 30. | Falivene, L.; Poater, A.; Cazin, C. S. J.; Slugovc, C.; Cavallo, L. Dalton Trans. 2013, 42, 7312–7317. doi:10.1039/C2DT32277C |
| 31. | Guidone, S.; Songis, O.; Nahra, F.; Cazin, C. S. J. ACS Catal. 2015, 5, 2697–2701. doi:10.1021/acscatal.5b00197 |
| 32. | Guidone, S.; Songis, O.; Falivene, L.; Nahra, F.; Slawin, A. M. Z.; Jacobsen, H.; Cavallo, L.; Cazin, C. S. J. ACS Catal. 2015, 5, 3932–3939. doi:10.1021/acscatal.5b00219 |
| 33. | Bantreil, X.; Cazin, C. S. J. Monatsh. Chem. 2015, 146, 1043–1052. doi:10.1007/s00706-015-1487-7 |
| 5. | Fürstner, A. Angew. Chem., Int. Ed. 2000, 39, 3012–3043. doi:10.1002/1521-3773(20000901)39:17<3012::AID-ANIE3012>3.0.CO;2-G |
| 6. | Buchmeiser, M. R. Chem. Rev. 2000, 100, 1565–1604. doi:10.1021/cr990248a |
| 7. | Connon, S. J.; Blechert, S. Angew. Chem., Int. Ed. 2003, 42, 1900–1923. doi:10.1002/anie.200200556 |
| 8. | Grubbs, R. H.; Miller, S. J.; Fu, G. C. Acc. Chem. Res. 1995, 28, 446–452. doi:10.1021/ar00059a002 |
| 9. | Nicolaou, K. C.; Bulger, P. G.; Sarlah, D. Angew. Chem., Int. Ed. 2005, 44, 4490–4527. doi:10.1002/anie.200500369 |
| 25. | Bantreil, X.; Schmid, T. E.; Randall, R. A. M.; Slawin, A. M. Z.; Cazin, C. S. J. Chem. Commun. 2010, 46, 7115–7117. doi:10.1039/c0cc02448a |
| 35. | Ung, T.; Hejl, A.; Grubbs, R. H.; Schrodi, Y. Organometallics 2004, 23, 5399–5401. doi:10.1021/om0493210 |
| 36. | Slugovc, C.; Burtscher, D.; Stelzer, F.; Mereiter, K. Organometallics 2005, 24, 2255–2258. doi:10.1021/om050141f |
| 26. | Schmid, T. E.; Bantreil, X.; Citadelle, C. A.; Slawin, A. M. Z.; Cazin, C. S. J. Chem. Commun. 2011, 47, 7060–7062. doi:10.1039/c1cc10825e |
| 25. | Bantreil, X.; Schmid, T. E.; Randall, R. A. M.; Slawin, A. M. Z.; Cazin, C. S. J. Chem. Commun. 2010, 46, 7115–7117. doi:10.1039/c0cc02448a |
| 26. | Schmid, T. E.; Bantreil, X.; Citadelle, C. A.; Slawin, A. M. Z.; Cazin, C. S. J. Chem. Commun. 2011, 47, 7060–7062. doi:10.1039/c1cc10825e |
| 27. | Songis, O.; Slawin, A. M. Z.; Cazin, C. S. J. Chem. Commun. 2012, 48, 1266–1268. doi:10.1039/C2CC15903A |
| 28. | Bantreil, X.; Poater, A.; Urbina-Blanco, C. A.; Bidal, Y. D.; Falivene, L.; Randall, R. A. M.; Cavallo, L.; Slawin, A. M. Z.; Cazin, C. S. J. Organometallics 2012, 31, 7415–7426. doi:10.1021/om300703p |
| 29. | Urbina-Blanco, C. A.; Bantreil, X.; Wappel, J.; Schmid, T. E.; Slawin, A. M. Z.; Slugovc, C.; Cazin, C. S. J. Organometallics 2013, 32, 6240–6247. doi:10.1021/om4004362 |
| 30. | Falivene, L.; Poater, A.; Cazin, C. S. J.; Slugovc, C.; Cavallo, L. Dalton Trans. 2013, 42, 7312–7317. doi:10.1039/C2DT32277C |
| 31. | Guidone, S.; Songis, O.; Nahra, F.; Cazin, C. S. J. ACS Catal. 2015, 5, 2697–2701. doi:10.1021/acscatal.5b00197 |
| 32. | Guidone, S.; Songis, O.; Falivene, L.; Nahra, F.; Slawin, A. M. Z.; Jacobsen, H.; Cavallo, L.; Cazin, C. S. J. ACS Catal. 2015, 5, 3932–3939. doi:10.1021/acscatal.5b00219 |
| 33. | Bantreil, X.; Cazin, C. S. J. Monatsh. Chem. 2015, 146, 1043–1052. doi:10.1007/s00706-015-1487-7 |
| 34. | Cazin, C. S. J., Ed. N-Heterocyclic Carbenes in Transition Metal Catalysis and Organocatalysis; Catalysis by Metal Complexes, Vol. 32; Springer: New York, NY, U.S.A., 2011. doi:10.1007/978-90-481-2866-2 |
| 9. | Nicolaou, K. C.; Bulger, P. G.; Sarlah, D. Angew. Chem., Int. Ed. 2005, 44, 4490–4527. doi:10.1002/anie.200500369 |
| 38. | Wallace, D. J.; Reamer, R. A. J. Org. Chem. 2014, 79, 5644–5651. doi:10.1021/jo500815q |
| 39. | Chatterjee, S.; Guchhait, S.; Goswami, R. K. J. Org. Chem. 2014, 79, 7689–7695. doi:10.1021/jo501184t |
| 40. | Keck, C. G.; Kendall, J. L.; Caster, K. C. Adv. Synth. Catal. 2007, 349, 165–174. doi:10.1002/adsc.200600455 |
| 25. | Bantreil, X.; Schmid, T. E.; Randall, R. A. M.; Slawin, A. M. Z.; Cazin, C. S. J. Chem. Commun. 2010, 46, 7115–7117. doi:10.1039/c0cc02448a |
| 10. | Trnka, T. M.; Grubbs, R. H. Acc. Chem. Res. 2001, 34, 18–29. doi:10.1021/ar000114f |
| 11. | Schrock, R. R.; Hoveyda, A. H. Angew. Chem., Int. Ed. 2003, 42, 4592–4633. doi:10.1002/anie.200300576 |
| 12. | Grubbs, R. H., Ed. Handbook of Metathesis: Catalyst Development; Wiley-VCH: Weinheim, Germany, 2003. doi:10.1002/9783527619481 |
| 13. | Grela, K., Ed. Olefin Metathesis – Theory and Practice; Wiley-VCH: Weinheim, Germany, 2014. |
| 24. | Nelson, D. J.; Manzini, S.; Urbina-Blanco, C. A.; Nolan, S. P. Chem. Commun. 2014, 50, 10355–10375. doi:10.1039/C4CC02515F |
| 25. | Bantreil, X.; Schmid, T. E.; Randall, R. A. M.; Slawin, A. M. Z.; Cazin, C. S. J. Chem. Commun. 2010, 46, 7115–7117. doi:10.1039/c0cc02448a |
| 37. | Ho, C.-Y.; Jamison, T. F. Angew. Chem., Int. Ed. 2007, 46, 782–785. doi:10.1002/anie.200603907 |
| 14. | Nguyen, S. T.; Johnson, L. K.; Grubbs, R. H.; Ziller, J. W. J. Am. Chem. Soc. 1992, 114, 3974–3975. doi:10.1021/ja00036a053 |
| 15. | Nguyen, S. T.; Grubbs, R. H.; Ziller, J. W. J. Am. Chem. Soc. 1993, 115, 9858–9859. doi:10.1021/ja00074a086 |
| 16. | Schwab, P.; Grubbs, R. H.; Ziller, J. W. J. Am. Chem. Soc. 1996, 118, 100–110. doi:10.1021/ja952676d |
| 17. | Boeda, F.; Clavier, H.; Nolan, S. P. Chem. Commun. 2008, 2726–2740. doi:10.1039/b718287b |
| 25. | Bantreil, X.; Schmid, T. E.; Randall, R. A. M.; Slawin, A. M. Z.; Cazin, C. S. J. Chem. Commun. 2010, 46, 7115–7117. doi:10.1039/c0cc02448a |
| 25. | Bantreil, X.; Schmid, T. E.; Randall, R. A. M.; Slawin, A. M. Z.; Cazin, C. S. J. Chem. Commun. 2010, 46, 7115–7117. doi:10.1039/c0cc02448a |
| 43. | Kirkland, T. A.; Grubbs, R. H. J. Org. Chem. 1997, 62, 7310–7318. doi:10.1021/jo970877p |
| 42. | Terada, Y.; Arisawa, M.; Nishida, A. Angew. Chem., Int. Ed. 2004, 43, 4063–4067. doi:10.1002/anie.200454157 |
| 22. | Clavier, H.; Nolan, S. P. Chem. – Eur. J. 2007, 13, 8029–8036. doi:10.1002/chem.200700256 |
| 42. | Terada, Y.; Arisawa, M.; Nishida, A. Angew. Chem., Int. Ed. 2004, 43, 4063–4067. doi:10.1002/anie.200454157 |
| 25. | Bantreil, X.; Schmid, T. E.; Randall, R. A. M.; Slawin, A. M. Z.; Cazin, C. S. J. Chem. Commun. 2010, 46, 7115–7117. doi:10.1039/c0cc02448a |
| 25. | Bantreil, X.; Schmid, T. E.; Randall, R. A. M.; Slawin, A. M. Z.; Cazin, C. S. J. Chem. Commun. 2010, 46, 7115–7117. doi:10.1039/c0cc02448a |
| 34. | Cazin, C. S. J., Ed. N-Heterocyclic Carbenes in Transition Metal Catalysis and Organocatalysis; Catalysis by Metal Complexes, Vol. 32; Springer: New York, NY, U.S.A., 2011. doi:10.1007/978-90-481-2866-2 |
| 25. | Bantreil, X.; Schmid, T. E.; Randall, R. A. M.; Slawin, A. M. Z.; Cazin, C. S. J. Chem. Commun. 2010, 46, 7115–7117. doi:10.1039/c0cc02448a |
© 2015 Guidone et al; licensee Beilstein-Institut.
This is an Open Access article under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
The license is subject to the Beilstein Journal of Organic Chemistry terms and conditions: (http://www.beilstein-journals.org/bjoc)