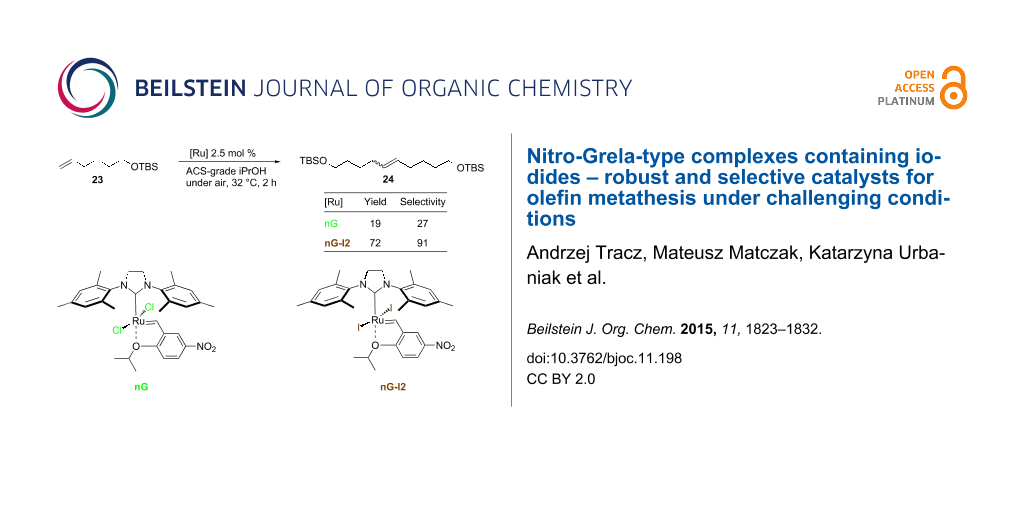
Abstract
Iodide-containing nitro-Grela-type catalysts have been synthesized and applied to ring closing metathesis (RCM) and cross metathesis (CM) reactions. These new catalysts have exhibited improved efficiency in the transformation of sterically, non-demanding alkenes. Additional steric hindrance in the vicinity of ruthenium related to the presence of iodides ensures enhanced catalyst stability. The benefits are most apparent under challenging conditions, such as very low reaction concentrations, protic solvents or with the occurrence of impurities.
Graphical Abstract
Introduction
Olefin metathesis (OM) is a mild and versatile catalytic method which allows the formation of carbon–carbon double bonds [1]. Understanding the key events in ruthenium-catalyzed olefin metathesis [2] and developing efficient and selective catalysts [3] provides opportunities for industrial applications of this technology. In many cases, however, the achievement of high turn over numbers (TONs) requires tedious purification of starting materials and solvents. New catalysts with increased efficiency and selectivity, especially under challenging conditions, are therefore of high interest. Currently, the second generation Hoveyda-type catalysts, such as HII [4], A [5], B [6], and C [7] are considered to be the most versatile tool for OM (Figure 1).
Figure 1: The diversity of Hoveyda-type complexes (Mes – 2,4,6-trimethylphenyl, DIPP – 2,6-diisopropylphenyl).
Figure 1: The diversity of Hoveyda-type complexes (Mes – 2,4,6-trimethylphenyl, DIPP – 2,6-diisopropylphenyl)....
Modifications of ligands permanently bound to the ruthenium center appear to be the most efficient methods for altering the catalyst properties. Great improvement of catalyst efficiency in the transformation of sterically non-demanding alkenes have been achieved by the replacement of the classical SIMes ligand with the bulkier SIPr ligand (Scheme 1) [8,9]. Metathesis catalysts with even larger NHC ligands have also been reported, but their syntheses require additional steps because the necessary anilines – the starting materials for the preparation of NHCs precursors – are not commercially available [10,11]. Up until now, there had been no disclosures of increased catalyst efficiency caused by the exchange of chlorides with larger anionic ligands. Grubbs et al. showed that the exchange of chlorides for bromides or iodides in the second generation Grubbs’ catalysts facilitated the initiation, but reduced the propagation rate and eventually provided no overall improvement [12]. More recently Slugovc et al. synthesized bromo- and iodo- analogues of HII, but no improvement was noted [13-15]. Moreover, the presence of iodide ligands reduced initiation rates for Hoveyda second generation complex bearing iodides (HII-I2) in ring-closing metathesis (RCM). Similarly, Schrodi and colleagues did not find any advantages for halide exchanged Hoveyda-type complexes in cross metathesis of methyl oleate with ethylene [16]. Complexes containing iodide lead to products of asymmetric OM with better enantio- and diastereoselectivity, but this came at the price of lower activity [17]. In the past few years the replacement of chloride ligands created the first Z-selective catalysts [18-21]. Their efficiency, however, is noticeably lower than that observed for classical complexes. The second generation indenylidene catalysts with phosphite ligand (frequently reported as “Cazin-type catalysts”) bearing mixed chloride–fluoride or difluoride anionic ligands were also reported very recently [22]. The former catalyst exhibited thermal stability and efficiency comparable with the original complex having two chlorides, while the difluoride catalyst showed low catalytic activity. Finally, alternative anionic ligands have been used in order to heterogenize catalysts, which resulted in the formation of materials with reduced activity and efficiency [23,24].
Scheme 1: Modifications of the 2nd generation alkylidene complexes.
Scheme 1: Modifications of the 2nd generation alkylidene complexes.
It is well recognized that the benzylidene ligand structure strongly influences initiation rates for Hoveyda-type catalysts [25]. As a consequence of the “boomerang effect”, which was recently strongly supported by Fogg et al. [26], the benzylidene ligand also most likely affects propagation rates.
In our search for active, more robust and selective catalysts, we synthesized iodide-containing nitro-Grela type catalysts. A synergistic effect of the ligands was sought: the nitro-substituted benzylidene ligand was expected to ensure fast initiation, while the bulky iodides were anticipated to provide additional stabilization of the active species.
Results and Discussion
The new iodide-containing catalysts, nG-I2 and nG-SIPr-I2, were prepared with a 93% yield from commercially available complexes, nG and nG-SIPr, and with the use of potassium iodide as the iodide anions source (Scheme 2). In the synthesis of both catalysts, the isolated material contained 99% of the expected diiodo catalyst and 1% of the “mixed halogen” complex, which was identified by field desorption mass spectrometry (FD–MS) and quantified by 1H NMR.
Scheme 2: Synthesis of iodide-containing nitro-Grela type catalysts.
Scheme 2: Synthesis of iodide-containing nitro-Grela type catalysts.
In order to determine the differences in the initiation rate between the new and parent complexes, we ran the RCM of diethyl diallylmalonate (DEDAM) in toluene (C0DEDAM 0.2 M) at a relatively low temperature (18 °C) with only 0.15 mol % of the catalyst (Figure 2). The nG-I2 catalyst initiated slightly more slowly than the parent nG, but was more stable and after 1 h gave greater than a 10% better conversion of the substrate as indicated in Figure 2. The catalytic performance of nG-I2 was almost identical to that observed for nG-SIPr, suggesting that the exchange of chloride with iodide can – at least for some substrates – provide similar catalyst stabilization as the introduction of a bulky NHC ligand. In the case of the most sterically crowded nG-SIPr-I2, initiation was delayed, but a very fast reaction propagation was observed. This catalyst was the most stable and efficient among all tested complexes.
![[1860-5397-11-198-2]](/bjoc/content/figures/1860-5397-11-198-2.png?scale=2.0&max-width=1024&background=FFFFFF)
Figure 2: Reaction profiles for RCM of DEDAM; toluene, 0.2 M, 18 °C, [Ru] 0.15 mol %; conversion determined by GC.
Figure 2: Reaction profiles for RCM of DEDAM; toluene, 0.2 M, 18 °C, [Ru] 0.15 mol %; conversion determined b...
To gain more information about the scope of application of the obtained catalysts, we carried out a set of standard RCM and CM transformations (Table 1 and Table 2). The reactions were performed in dry, degassed toluene, at 70 °C with varied catalyst loadings to demonstrate differences in their efficiencies.
The efficiency pattern observed in RCM of DEDAM was confirmed in the synthesis of five- to seven-membered, disubstituted heterocycles (Table 1, entries 1–3). Both nG-I2 and nG-SIPr-I2 proved to be sensitive to the steric bulk in close proximity to the double bond. Thus, RCM with substrate 7 having one double bond terminally substituted with the phenyl ring as well as the formation of the trisubstituted heterocycle 9 proceeded better with chloride-containing catalysts. When proline derivative 10 was used, the diiodo catalysts performed better than nG but slightly worse than nG-SIPr.
Table 1: Results of RCM reactions.a
| Entry | Substrate | Product | Catalyst (mol %) | GC Conversion [%] |
|---|---|---|---|---|
| 1 |
|
|
nG (0.0025) | 32 |
| nG-I2 (0.0025) | 72 | |||
| nG-SIPr (0.0025) | 85 | |||
| nG-SIPr-I2 (0.0025) | 95 | |||
| 2 |
|
|
nG (0.003) | 67 |
| nG-I2 (0.003) | 91 | |||
| nG-SIPr (0.003) | 90 | |||
| nG-SIPr-I2 (0.003) | 97 | |||
| 3 |
|
|
nG (0.0075) | 57 |
| nG-I2 (0.0075) | 87 | |||
| nG-SIPr (0.0075) | 86 | |||
| nG-SIPr-I2 (0.0075) | 94 | |||
| 4 |
|
|
nG (0.015) | 89 |
| nG-I2 (0.015) | 82 | |||
| nG-SIPr (0.015) | 95 | |||
| nG-SIPr-I2 (0.015) | 47 | |||
| 5 |
|
|
nG (0.05) | 93 |
| nG-I2 (0.05) | 79 | |||
| nG-SIPr (0.05) | 99 | |||
| nG-SIPr-I2 (0.05) | 75 | |||
| 6 |
|
|
nG (0.04) | 80 |
| nG-I2 (0.04) | 94 | |||
| nG-SIPr (0.04) | 99 | |||
| nG-SIPr-I2 (0.04) | 94 | |||
aToluene, 0.2 M, 70 °C, 2 h.
As outlined in Table 2, all tested catalysts were similarly effective in CM of methyl undecenoate 12 with cis-1,4-diacetoxy-2-butene (13), but parent dichloro complexes provided smaller quantities of dimerization product of 12. In CM of 12 with electron deficient methyl acrylate 15, diiodo derivatives were significantly less efficient and provided much more dimer of 12. Apparently nG-I2 and nG-SIPr-I2 can perform noticeably better than parent dichloro complexes only in metathesis of sterically non-demanding substrates. With this knowledge, we decided to test their applicability under conditions which require high stability of the active species. Macrocyclization of dienes having low effective molarity provides access to a number of valuable musk-like compounds [27,28]. This type of transformation must be carried out at a very low concentration (usually <10 mM) in order to avoid formation of oligomeric/polymeric byproducts. Moreover, high temperature is required to complete the reaction in an acceptably short time. Therefore, a very stable and efficient catalyst is required to perform macrocyclization at reasonable loadings. The additional challenge related to high dilutions is the efficient removal of ethylene, which can be especially difficult on a large scale. Accordingly, the optimal catalyst for macrocyclization should form stable active species (usually ruthenium methylidenes), but it should also exhibit high preference of productive metathesis over unproductive metathesis.
Table 2: Results of CM reactions.a
| Entry | Substrates | Product | Catalyst (mol %) | GC Yield (selectivity) [%] | E/Z |
|---|---|---|---|---|---|
| 1b |
|
|
nG (0.4) | 84 (99) | 6/1 |
| nG-I2 (0.4) | 88 (96) | 4.8/1 | |||
| nG-SIPr (0.4) | 88 (98) | 5/1 | |||
| nG-SIPr-I2 (0.4) | 84 (90) | 3/1 | |||
| 2c |
|
|
nG (0.5) | 98 (>99) | 19/1 |
| nG-I2 (0.5) | 74 (88) | 9/1 | |||
| nG-SIPr (0.5) | 98 (>99) | 9/1 | |||
| nG-SIPr-I2 (0.5) | 30 (44) | 9/1 | |||
aToluene, 0.2 M, 70 °C, 2 h; b3 equiv of 13; c3 equiv of 15.
Experiments with ethylene
To gain more information about the behavior of tested catalysts in the presence of ethylene, we performed two experiments. In the first test, 100 ppm of each catalyst was stirred for 45 minutes at 25 °C in an ethylene atmosphere [29,30]. During that period, ruthenium methylidenes were generated and involved in the unproductive metathesis of ethylene (Figure 3). Subsequently, the atmosphere was changed to argon and the substrate 1 (C01 0.05 M) was added. To our surprise, ethylene pre-treatment had the strongest negative effect on the most sterically crowded nG-SIPr-I2, which in our initial tests showed the highest efficiency in RCM of 1. In contrast, nG-I2 turned out to be the least sensitive to ethylene. Both dichloro complexes showed similar levels of stability. These results suggest that most stable ruthenium methylidenes were generated from nG-I2.
![[1860-5397-11-198-3]](/bjoc/content/figures/1860-5397-11-198-3.png?scale=2.0&max-width=1024&background=FFFFFF)
Figure 3: RCM of 1 (toluene, 0.05 M, 25 °C, [Ru] 0.01 mol %); blue diamonds – original (pre)catalysts; red squares – complexes pretreated with ethylene for 45 minutes.
Figure 3: RCM of 1 (toluene, 0.05 M, 25 °C, [Ru] 0.01 mol %); blue diamonds – original (pre)catalysts; red sq...
Next, the RCM of 1 was carried out under ethylene atmosphere which increases the probability of unproductive events (Figure 4). In this setup, the efficiency of catalysts decreased in the following order: nG-I2 = nG-SIPr-I2 > nG-SIPr > nG. Good conversion obtained with nG-SIPr-I2 indicated high preference of this catalyst toward productive RCM over non-productive metathesis. This observation partially explains the high efficiency of this catalyst obtained in RCM of 1 under conventional conditions. On the other hand, fast initiation of nG-SIPr-I2 under ethylene suggests that in the first catalytic turn-over, the small molecule of ethylene is coordinated to the ruthenium generating highly active methylidene species.
![[1860-5397-11-198-4]](/bjoc/content/figures/1860-5397-11-198-4.png?scale=2.0&max-width=1024&background=FFFFFF)
Figure 4: RCM of 1 (toluene, 0.05 M, 25 °C, [Ru] 0.01 mol %): top – productive RCM and possible non-productive events; bottom – reaction profiles of tested catalysts (blue diamonds – reaction under Ar, red squares – reaction under ethylene).
Figure 4: RCM of 1 (toluene, 0.05 M, 25 °C, [Ru] 0.01 mol %): top – productive RCM and possible non-productiv...
Macrocyclization reactions
As model substrates for macrocyclization we choose esters 17 and 18 which are metathesized to the 16- and 14- membered lactones. The RCM was run in toluene, at 70 °C and at 5 mM concentration; the catalysts were added in 10 portions with 7 minutes intervals. The 16-membered lactone 19 was synthesized with the catalyst loading of 0.3 mol % (Table 3, entries 1–4). The highest yield (91%) along with good selectivity (93%) was obtained with nG-I2 while only a 54% yield and rather poor selectivity (70%) was observed for nG. Low selectivity of the reaction promoted by nG was the result of the formation of 13% of GC-observable byproducts (originated from double bond isomerization and ring contraction) as well as 10% of oligomeric/polymeric byproducts. The nG-SIPr-I2 was more efficient than nG-SIPr, but the difference was not as striking in this pair (85% and 69% of yield, respectively). The same efficiency profile was observed in the synthesis of 14-membered 20, which was carried out with the catalyst loading of 0.2 mol % (Table 3, entries 9–12). In this transformation each catalyst formed significant amounts of oligomeric/polymeric byproducts. Interestingly, we noticed a strong dependence of the catalyst efficiency on the argon flow over the reaction mixture which indicates the high importance of the ethylene removal in this type of RCM. The high stability of ruthenium methylidenes generated from nG-I2 proved to be of great importance when macrocyclizations were run without active removal of ethylene (no flow of argon over the reaction mixture). In these conditions, which simulate the difficult removal of ethylene on large scale processes, nG-I2 delivered expected products with fair yields (77% and 57% of 19 and 20, respectively) while other catalysts demonstrated less than a 10% yield.
Table 3: Results of the macrocyclization reactions.
|
|
|||||
| Entry | n | Conditions | Catalyst (mol %) |
GC Conversion
(selectivity) [%] |
GC Yield [%]
(E/Z) |
|---|---|---|---|---|---|
| 1 | 3 | active removal of ethylene | nG (0.3) | 77 (70) | 54 (2.9/1) |
| 2 | nG-I2 (0.3) | 98 (93) | 91 (3.1/1) | ||
| 3 | nG-SIPr (0.3) | 77 (90) | 69 (2.5/1) | ||
| 4 | nG-SIPr-I2 (0.3) | 90 (94) | 85 (2.3/1) | ||
| 5 | 3 | no active removal of ethylene | nG (0.3) | 5 (80) | 4 |
| 6 | nG-I2 (0.3) | 87 (89) | 77 (2.7/1) | ||
| 7 | nG-SIPr (0.3) | 8 (88) | 7 | ||
| 8 | nG-SIPr-I2 (0.3) | 7 (100) | 7 | ||
| 9 | 1 | active removal of ethylene | nG (0.2) | 72 (62) | 45 (8/1) |
| 10 | nG-I2 (0.2) | 99 (82) | 81 (8/1) | ||
| 11 | nG-SIPr (0.2) | 97 (68) | 66 (9/1) | ||
| 12 | nG-SIPr-I2 (0.2) | 98 (73) | 72 (6/1) | ||
| 13 | 1 | no active removal of ethylene | nG (0.2) | 8 (62) | 5 |
| 14 | nG-I2 (0.2) | 68 (84) | 57 (5/1) | ||
| 15 | nG-SIPr (0.2) | 5 (60) | 3 | ||
| 16 | nG-SIPr-I2 (0.2) | 6 (50) | 3 | ||
Metathesis in ACS-grade and “green” solvents
Our continuous interest in the development of more sustainable, environmentally and user-friendly olefin metathesis has recently inspired us to test a range of commercially available, classical ruthenium initiators in ACS grade solvents under air [31]. For this study we choose substrate 1, which is highly prone to non-metathesis reactions, namely isomerization and cycloisomerization. The result we found is that esters constitute exeptionally good solvents for RCM and CM. Conversely, application of ACS grade alcohols, ethers and toluene in many cases dramatically reduced catalyst efficiency and selectivity. It was particularly noticeable in isopropanol, in which only Hoveyda–Grubbs type complexes bearing a SIPr ligand provided expected products with 80–88% yields (0.25 mol % of catalyst, 40 or 70 °C). The catalysts containing a less sterically crowded SIMEs ligand delivered 2 with poor yield, usually accompanied by significant amounts of byproducts 21 and 22. This demonstrates that large substituents in N-heterocyclic ligands (NHC) not only increased efficiency of Hoveyda-type catalysts, but also to some extent prevented formation of ruthenium species active in non-metathetical transformations.
We decided to check whether additional steric restraints around the ruthenium center caused by iodides [32] can stabilize catalysts during OM in ACS grade solvents under air. RCM of 1 carried out in toluene was accomplished by nG with only 54% yield and 89% selectivity (Table 4). This reduced efficiency and selectivity observed in ACS grade toluene is most probably related to the small amounts of basic amines present in this solvent [33,34]. As anticipated, nG-SIPR performed better, giving 92% of product and 8% of isomers. We were pleased to see that nG-I2 and nG-SIPr-I2 provided over 99% of the expected product. As observed previously, nG exhibited very low activity in 2-MeTHF while nG-SIPR gave 90% of 2 which was, however, accompanied by 10% of isomers. The yield (96–97%) and the selectivity (98%) for both iodide analogues were noticeably better. The advantage of sterically crowded catalysts was even more pronounced when reactions were carried out in alcohols. In iPrOH 0.075 mol % of nG gave only 21% of 2 with 72% selectivity; nG-SIPr was much more efficient (77% of yield), but the selectivity was limited (82%). In contrast nG-I2 delivered 84% of the product with 99% selectivity, and nG-SIPr-I2 yielded 94% of 2 with 97% selectivity. Noteworthy is that nG-SIPr-I2 was the only catalyst able to efficiently promote RCM of 1 in methanol.
Table 4: RCM of 1 in ACS-grade solvents under air.a
|
|
||||
| Catalyst | GC Yield (selectivity) [%] | |||
|---|---|---|---|---|
| tolueneb | 2-MeTHFc | iPrOHd | MeOHe | |
| nG | 54 (89) | 28 (97) | 21 (72) | 14 (88) |
| nG-I2 | >99 | 97 (98) | 84 (99) | 47 (98) |
| nG-SIPr | 92 (92) | 90 (90) | 77 (82) | 46 (87) |
| nG-SIPr-I2 | 99 (99) | 96 (98) | 94 (97) | 94 (95) |
aReactions carried out in non-degassed, non-distilled ACS grade solvents under air; b[Ru] 0.1 mol %, 70 °C; c[Ru] 0.25 mol %, 40 °C; d[Ru] 0.075 mol %, 70 °C; e[Ru] 0.25 mol %, 40 °C.
To further differentiate the tested catalysts, we performed RCM of DEDAM, which required an even higher stability of the active species. In this transformation, nG failed to give substantial amounts of the product in any solvent (Table 5). Interestingly, nG-SIPr exhibited very low efficiency in 2-MeTHF, but in other solvents ensured better yields than nG-I2. Regardless, the solvent applied, nG-SIPr-I2, was the most efficient catalyst.
Table 5: RCM of DEDAM in ACS-grade solvents under air.a
|
|
||||
| Catalyst | GC Yield [%] | |||
|---|---|---|---|---|
| tolueneb | 2-MeTHFc | iPrOHd | MeOHe | |
| nG | 33 | 31 | 15 | 9 |
| nG-I2 | 77 | 98 | 76 | 31 |
| nG-SIPr | 96 | 25 | 87 | 43 |
| nG-SIPr-I2 | 98 | 100 | 99 | 64 |
aReactions carried out in non-degassed, non-distilled ACS grade solvents under air; b[Ru] 0.1 mol %, 70 °C; c[Ru] 0.25 mol %, 40 °C; d[Ru] 0.25 mol %, 70 °C; e[Ru] 0.75 mol %, 40 °C.
In our final experiment we performed self metathesis of tert-butyldimethylsilyl (TBS)-protected 5-hexen-1-ol without any additives that are known to prevent double bond isomerization [35]. As expected, SM turned out to be much more challenging than RCM reactions in terms of the catalyst efficiency and selectivity (Table 6). With 1 mol % of nG only a minor amount of 24 was observed in toluene and no catalytic activity was noted in 2-MeTHF. nG-SIPr performed better in these solvents, but iodide catalysts were twice as efficient and in addition, were noticeably more selective. In alcohols 2.5 mol % of nG or nG-SIPr delivered from 9 to 19% of 24 with dramatically low selectivity in the range of 25–47%. Application of nG-I2 or nG-SIPr-I2 resulted in the formation of 48–72% of the expected product with fair selectivity (83–91%).
Table 6: CM of TBS protected 5-hexen-1-ol in ACS-grade solvents under air.a
|
|
||||
| Catalyst | GC Yield (selectivity) [%] | |||
|---|---|---|---|---|
| tolueneb | 2-MeTHFb | iPrOHc | MeOHc | |
| nG | 13 (87) | 0 | 19 (27) | 19 (25) |
| nG-I2 | 70 (99) | 67 (97) | 72 (91) | 48 (83) |
| nG-SIPr | 38 (93) | 33 (87) | 15 (25) | 9 (47) |
| nG-SIPr-I2 | 67 (97) | 57 (95) | 65 (86) | 65 (86) |
aReactions carried out in non-degassed, non-distilled ACS grade solvents under air; b[Ru] 1 mol %; c[Ru] 2.5 mol %.
Conclusion
The iodide-containing nitro-Grela analogues exhibit improved efficiency in RCM and CM of sterically non-demanding substrates. Additional steric hindrance in the metal center proximity caused by iodides makes the 14-electron species less sensitive to small impurities, coordinative solvents (e.g., 2-MeTHF) and protic solvents. These factors lead in some cases, to dramatic improvement in the reaction(s) yield and selectivity. Increased stability of the ruthenium methylidenes generated from nG-I2 makes this catalyst especially suitable for macrocyclization of dienes with low effective molarity.
Supporting Information
| Supporting Information File 1: Experimental and spectral data for nG-I2, nG-SIPr-I2 and the test reactions. | ||
| Format: PDF | Size: 562.1 KB | Download |
References
-
Grela, K. Olefin Metathesis Theory and Practice; Wiley: Hoboken, 2014.
Return to citation in text: [1] -
Nelson, D. J.; Manzini, S.; Urbina-Blanco, C. A.; Nolan, S. P. Chem. Commun. 2014, 50, 10355–10375. doi:10.1039/C4CC02515F
Return to citation in text: [1] -
Grubbs, R. H.; Wenzel, A. G. Handbook of Metathesis, 2nd ed.; Wiley-VCH: Weinheim, 2015; Vol. 1. doi:10.1002/9783527674107
Return to citation in text: [1] -
Garber, S. B.; Kingsbury, J. S.; Gray, B. L.; Hoveyda, A. H. J. Am. Chem. Soc. 2000, 122, 8168–8179. doi:10.1021/ja001179g
Return to citation in text: [1] -
Rosebrugh, L. E.; Herbert, M. B.; Marx, V. M.; Keitz, B. K.; Grubbs, R. H. J. Am. Chem. Soc. 2013, 135, 1276–1279. doi:10.1021/ja311916m
Return to citation in text: [1] -
Marx, V. M.; Sullivan, A. H.; Melaimi, M.; Virgil, S. C.; Keitz, B. K.; Weinberger, D. S.; Bertrand, G.; Grubbs, R. H. Angew. Chem., Int. Ed. 2015, 54, 1919–1923. doi:10.1002/anie.201410797
Return to citation in text: [1] -
Skowerski, K.; Pastva, J.; Czarnocki, S. J.; Janoscova, J. Org. Process Res. Dev. 2015, 19, 872–877. doi:10.1021/acs.oprd.5b00132
Return to citation in text: [1] -
Nelson, D. J.; Queval, P.; Rouen, M.; Magrez, M.; Toupet, L.; Caijo, F.; Borré, E.; Laurent, I.; Crévisy, C.; Baslé, O.; Mauduit, M.; Percy, J. M. ACS Catal. 2013, 3, 259–264. doi:10.1021/cs400013z
Return to citation in text: [1] -
Urbina-Blanco, C. A.; Leitgeb, A.; Slugovc, C.; Bantreil, X.; Clavier, H.; Slawin, A. M. Z.; Nolan, S. P. Chem. – Eur. J. 2011, 17, 5045–5053. doi:10.1002/chem.201003082
Return to citation in text: [1] -
Gatti, M.; Vieille-Petit, L.; Luan, X.; Mariz, R.; Drinkel, E.; Linden, A.; Dorta, R. J. Am. Chem. Soc. 2009, 131, 9498–9499. doi:10.1021/ja903554v
Return to citation in text: [1] -
Manzini, S.; Urbina-Blanco, C. A.; Slawin, A. M. Z.; Nolan, S. P. Organometallics 2012, 31, 6514–6517. doi:10.1021/om300719t
Return to citation in text: [1] -
Sanford, M. S.; Love, J. A.; Grubbs, R. H. J. Am. Chem. Soc. 2001, 123, 6543–6554. doi:10.1021/ja010624k
Return to citation in text: [1] -
Wappel, J.; Urbina-Blanco, C. A.; Abbas, M.; Albering, J. H.; Saf, R.; Nolan, S. P.; Slugovc, C. Beilstein J. Org. Chem. 2010, 6, 1091–1098. doi:10.3762/bjoc.6.125
Return to citation in text: [1] -
Abbas, M.; Wappel, J.; Slugovc, C. Macromol. Symp. 2012, 311, 122–125. doi:10.1002/masy.201000095
Return to citation in text: [1] -
Abbas, M.; Slugovc, C. Tetrahedron Lett. 2011, 52, 2560–2562. doi:10.1016/j.tetlet.2011.03.038
Return to citation in text: [1] -
Schrodi, Y.; Ung, T.; Vargas, A.; Mkrtumyan, G.; Lee, C. W.; Champagne, T. M.; Pederson, R. L.; Hong, S. H. Clean: Soil, Air, Water 2008, 36, 669–673. doi:10.1002/clen.200800088
Return to citation in text: [1] -
Gillingham, D. G.; Kataoka, O.; Garber, S. B.; Hoveyda, A. H. J. Am. Chem. Soc. 2004, 126, 12288–12290. doi:10.1021/ja0458672
Return to citation in text: [1] -
Koh, M. J.; Khan, R. K. M.; Torker, S.; Hoveyda, A. H. Angew. Chem., Int. Ed. 2014, 53, 1968–1972. doi:10.1002/anie.201309430
Return to citation in text: [1] -
Endo, K.; Grubbs, R. H. J. Am. Chem. Soc. 2011, 133, 8525–8527. doi:10.1021/ja202818v
Return to citation in text: [1] -
Teo, P.; Grubbs, R. H. Organometallics 2010, 29, 6045–6050. doi:10.1021/om1007924
Return to citation in text: [1] -
Occhipinti, G.; Hansen, F. R.; Törnroos, K. W.; Jensen, V. R. J. Am. Chem. Soc. 2013, 135, 3331–3334. doi:10.1021/ja311505v
Return to citation in text: [1] -
Guidone, S.; Songis, O.; Falivene, L.; Nahra, F.; Slawin, A. M. Z.; Jacobsen, H.; Cavallo, L.; Cazin, C. S. J. ACS Catal. 2015, 5, 3932–3939. doi:10.1021/acscatal.5b00219
Return to citation in text: [1] -
Marciniec, B.; Rogalski, S.; Potrzebowski, M. J.; Pietraszuk, C. ChemCatChem 2011, 3, 904–910. doi:10.1002/cctc.201000376
Return to citation in text: [1] -
Bek, D.; Gawin, R.; Grela, K.; Balcar, H. Catal. Commun. 2012, 21, 42–45. doi:10.1016/j.catcom.2012.01.020
Return to citation in text: [1] -
Thiel, V.; Wannowius, K.-J.; Wolff, C.; Thiele, C. M.; Plenio, H. Chem. – Eur. J. 2013, 19, 16403–16414. doi:10.1002/chem.201204150
Return to citation in text: [1] -
Bates, J. M.; Lummiss, J. A. M.; Bailey, G. A.; Fogg, D. E. ACS Catal. 2014, 4, 2387–2394. doi:10.1021/cs500539m
Return to citation in text: [1] -
Hamasaki, R.; Funakoshi, S.; Misaki, T.; Tanabe, Y. Tetrahedron 2000, 56, 7423–7425. doi:10.1016/S0040-4020(00)00654-2
Return to citation in text: [1] -
Fürstner, A.; Thiel, O. R.; Ackermann, L. Org. Lett. 2001, 3, 449–451. doi:10.1021/ol0069554
Return to citation in text: [1] -
Lysenko, Z.; Maughon, B. R.; Mokhtar-Zadeh, T.; Tulchinsky, M. L. J. Organomet. Chem. 2006, 691, 5197–5203. doi:10.1016/j.jorganchem.2006.08.031
Return to citation in text: [1] -
Skowerski, K.; Pastva, J.; Czarnocki, S. J.; Janoscocva, J. Org. Process Res. Dev. 2015, 19, 872–877. doi:10.1021/acs.oprd.5b00132
Return to citation in text: [1] -
Skowerski, K.; Białecki, J.; Tracz, A.; Olszewski, T. K. Green Chem. 2014, 16, 1125–1130. doi:10.1039/C3GC41943F
Return to citation in text: [1] -
The ionic radii of chloride and iodide are 167 pm and 206 pm, respectively; the covalent radii of chlorine and iodine are 99 pm and 133 pm, respectively.
Return to citation in text: [1] -
Nicola, T.; Brenner, M.; Donsbach, K.; Kreye, P. Org. Process Res. Dev. 2005, 9, 513–515. doi:10.1021/op0580015
Return to citation in text: [1] -
Ireland, B. J.; Dobigny, B. T.; Fogg, D. E. ACS Catal. 2015, 5, 4690–4698. doi:10.1021/acscatal.5b00813
Return to citation in text: [1] -
Hong, S. H.; Sanders, D. P.; Lee, C. W.; Grubbs, R. H. J. Am. Chem. Soc. 2005, 127, 17160–17161. doi:10.1021/ja052939w
Return to citation in text: [1]
| 27. | Hamasaki, R.; Funakoshi, S.; Misaki, T.; Tanabe, Y. Tetrahedron 2000, 56, 7423–7425. doi:10.1016/S0040-4020(00)00654-2 |
| 28. | Fürstner, A.; Thiel, O. R.; Ackermann, L. Org. Lett. 2001, 3, 449–451. doi:10.1021/ol0069554 |
| 25. | Thiel, V.; Wannowius, K.-J.; Wolff, C.; Thiele, C. M.; Plenio, H. Chem. – Eur. J. 2013, 19, 16403–16414. doi:10.1002/chem.201204150 |
| 26. | Bates, J. M.; Lummiss, J. A. M.; Bailey, G. A.; Fogg, D. E. ACS Catal. 2014, 4, 2387–2394. doi:10.1021/cs500539m |
| 5. | Rosebrugh, L. E.; Herbert, M. B.; Marx, V. M.; Keitz, B. K.; Grubbs, R. H. J. Am. Chem. Soc. 2013, 135, 1276–1279. doi:10.1021/ja311916m |
| 22. | Guidone, S.; Songis, O.; Falivene, L.; Nahra, F.; Slawin, A. M. Z.; Jacobsen, H.; Cavallo, L.; Cazin, C. S. J. ACS Catal. 2015, 5, 3932–3939. doi:10.1021/acscatal.5b00219 |
| 4. | Garber, S. B.; Kingsbury, J. S.; Gray, B. L.; Hoveyda, A. H. J. Am. Chem. Soc. 2000, 122, 8168–8179. doi:10.1021/ja001179g |
| 23. | Marciniec, B.; Rogalski, S.; Potrzebowski, M. J.; Pietraszuk, C. ChemCatChem 2011, 3, 904–910. doi:10.1002/cctc.201000376 |
| 24. | Bek, D.; Gawin, R.; Grela, K.; Balcar, H. Catal. Commun. 2012, 21, 42–45. doi:10.1016/j.catcom.2012.01.020 |
| 3. | Grubbs, R. H.; Wenzel, A. G. Handbook of Metathesis, 2nd ed.; Wiley-VCH: Weinheim, 2015; Vol. 1. doi:10.1002/9783527674107 |
| 17. | Gillingham, D. G.; Kataoka, O.; Garber, S. B.; Hoveyda, A. H. J. Am. Chem. Soc. 2004, 126, 12288–12290. doi:10.1021/ja0458672 |
| 35. | Hong, S. H.; Sanders, D. P.; Lee, C. W.; Grubbs, R. H. J. Am. Chem. Soc. 2005, 127, 17160–17161. doi:10.1021/ja052939w |
| 2. | Nelson, D. J.; Manzini, S.; Urbina-Blanco, C. A.; Nolan, S. P. Chem. Commun. 2014, 50, 10355–10375. doi:10.1039/C4CC02515F |
| 18. | Koh, M. J.; Khan, R. K. M.; Torker, S.; Hoveyda, A. H. Angew. Chem., Int. Ed. 2014, 53, 1968–1972. doi:10.1002/anie.201309430 |
| 19. | Endo, K.; Grubbs, R. H. J. Am. Chem. Soc. 2011, 133, 8525–8527. doi:10.1021/ja202818v |
| 20. | Teo, P.; Grubbs, R. H. Organometallics 2010, 29, 6045–6050. doi:10.1021/om1007924 |
| 21. | Occhipinti, G.; Hansen, F. R.; Törnroos, K. W.; Jensen, V. R. J. Am. Chem. Soc. 2013, 135, 3331–3334. doi:10.1021/ja311505v |
| 10. | Gatti, M.; Vieille-Petit, L.; Luan, X.; Mariz, R.; Drinkel, E.; Linden, A.; Dorta, R. J. Am. Chem. Soc. 2009, 131, 9498–9499. doi:10.1021/ja903554v |
| 11. | Manzini, S.; Urbina-Blanco, C. A.; Slawin, A. M. Z.; Nolan, S. P. Organometallics 2012, 31, 6514–6517. doi:10.1021/om300719t |
| 13. | Wappel, J.; Urbina-Blanco, C. A.; Abbas, M.; Albering, J. H.; Saf, R.; Nolan, S. P.; Slugovc, C. Beilstein J. Org. Chem. 2010, 6, 1091–1098. doi:10.3762/bjoc.6.125 |
| 14. | Abbas, M.; Wappel, J.; Slugovc, C. Macromol. Symp. 2012, 311, 122–125. doi:10.1002/masy.201000095 |
| 15. | Abbas, M.; Slugovc, C. Tetrahedron Lett. 2011, 52, 2560–2562. doi:10.1016/j.tetlet.2011.03.038 |
| 32. | The ionic radii of chloride and iodide are 167 pm and 206 pm, respectively; the covalent radii of chlorine and iodine are 99 pm and 133 pm, respectively. |
| 8. | Nelson, D. J.; Queval, P.; Rouen, M.; Magrez, M.; Toupet, L.; Caijo, F.; Borré, E.; Laurent, I.; Crévisy, C.; Baslé, O.; Mauduit, M.; Percy, J. M. ACS Catal. 2013, 3, 259–264. doi:10.1021/cs400013z |
| 9. | Urbina-Blanco, C. A.; Leitgeb, A.; Slugovc, C.; Bantreil, X.; Clavier, H.; Slawin, A. M. Z.; Nolan, S. P. Chem. – Eur. J. 2011, 17, 5045–5053. doi:10.1002/chem.201003082 |
| 16. | Schrodi, Y.; Ung, T.; Vargas, A.; Mkrtumyan, G.; Lee, C. W.; Champagne, T. M.; Pederson, R. L.; Hong, S. H. Clean: Soil, Air, Water 2008, 36, 669–673. doi:10.1002/clen.200800088 |
| 33. | Nicola, T.; Brenner, M.; Donsbach, K.; Kreye, P. Org. Process Res. Dev. 2005, 9, 513–515. doi:10.1021/op0580015 |
| 34. | Ireland, B. J.; Dobigny, B. T.; Fogg, D. E. ACS Catal. 2015, 5, 4690–4698. doi:10.1021/acscatal.5b00813 |
| 7. | Skowerski, K.; Pastva, J.; Czarnocki, S. J.; Janoscova, J. Org. Process Res. Dev. 2015, 19, 872–877. doi:10.1021/acs.oprd.5b00132 |
| 29. | Lysenko, Z.; Maughon, B. R.; Mokhtar-Zadeh, T.; Tulchinsky, M. L. J. Organomet. Chem. 2006, 691, 5197–5203. doi:10.1016/j.jorganchem.2006.08.031 |
| 30. | Skowerski, K.; Pastva, J.; Czarnocki, S. J.; Janoscocva, J. Org. Process Res. Dev. 2015, 19, 872–877. doi:10.1021/acs.oprd.5b00132 |
| 6. | Marx, V. M.; Sullivan, A. H.; Melaimi, M.; Virgil, S. C.; Keitz, B. K.; Weinberger, D. S.; Bertrand, G.; Grubbs, R. H. Angew. Chem., Int. Ed. 2015, 54, 1919–1923. doi:10.1002/anie.201410797 |
| 12. | Sanford, M. S.; Love, J. A.; Grubbs, R. H. J. Am. Chem. Soc. 2001, 123, 6543–6554. doi:10.1021/ja010624k |
| 31. | Skowerski, K.; Białecki, J.; Tracz, A.; Olszewski, T. K. Green Chem. 2014, 16, 1125–1130. doi:10.1039/C3GC41943F |
© 2015 Tracz et al; licensee Beilstein-Institut.
This is an Open Access article under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
The license is subject to the Beilstein Journal of Organic Chemistry terms and conditions: (http://www.beilstein-journals.org/bjoc)