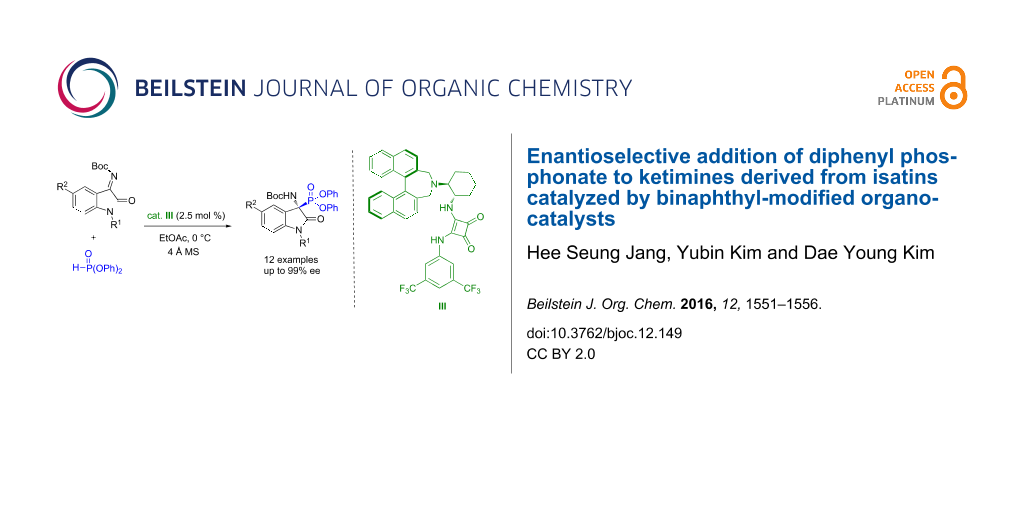
Abstract
Chiral binaphthyl-modified squaramide-catalyzed enantioselective addition of diphenyl phosphonate to ketimines derived from isatins has been achieved. This method affords practical and efficient access to chiral 3-amino-3-phosphonyl-substituted oxindole derivatives in high yields with excellent enantioselectivities (up to 99% ee).
Graphical Abstract
Introduction
α-Aminophosphonate derivatives are important compounds as structural mimics of natural α-amino acids [1-3]. Chiral α-aminophosphonates have been shown a wide range of biological activities including antibacterial [4] and anticancer properties [5], enzyme inhibition [6], peptide mimetic function [7], and herbicidal properties [8]. Since the biological activity of α-aminophosphonate derivatives is dependent upon the chirality of the α-position to the phosphorus atom, asymmetric synthesis of α-aminophosphonates has received considerable attention, and numerous catalytic enantioselective methods using chiral catalysts have been reported [9-13].
Oxindole and its derivatives can be exploited as important synthons to synthesize various alkaloid natural products and biologically active compounds [14-16]. In particular, 3,3-disubstituted oxindoles bearing a quaternary stereogenic center at the C3-position have been reported to be biologically active against a variety of targets [17-19]. Consequently, the asymmetric synthesis of 3,3-disubstituted oxindole derivatives has received significant research attention over the past few decades [20-22]. General approaches for the synthesis of chiral 3-substituted-3-aminooxindole derivatives include the amination of various 3-monosubstituted oxindoles [23-27] and the nucleophilic addition to ketimines derived from isatin derivatives [28-35]. Recently, there were a few reports on the synthesis of chiral 3-amino-3-phosphonyl-substituted oxindole derivatives by the catalytic enantioselective hydrophosphonation of ketimines [36,37]. The previous synthetic procedures suffered from several drawbacks, such as a high catalyst loading, long reaction time, and low temperature required for good enantioselectivity. Thus, new approaches for the organocatalytic enantioselective addition of diphenyl phosphonate to isatin imines are highly desired.
In connection with our ongoing research program on the design and application in asymmetric catalysis of organocatalysts [38-45], we have reported the catalytic asymmetric decarboxylative aldol addition reaction of isatins with benzoylacetic acids catalyzed by chiral binaphthyl-based squaramide [46]. Here we wish to report the enantioselective addition reaction of diphenyl phosphonate to ketimines derived from isatins catalyzed by binaphthyl-modified bifunctional organocatalysts (Figure 1).
Figure 1: Structure of chiral bifunctional organocatalysts.
Figure 1: Structure of chiral bifunctional organocatalysts.
Results and Discussion
To determine suitable reaction conditions for the organocatalytic enantioselective addition reaction of diphenyl phosphonate to ketimines derived from isatins, we initially investigated a reaction system with ketimine 1a derived from N-allylisatin and diphenyl phosphonate (2) with organocatalyst in the presence of 4 Å molecular sieves. We first surveyed the effect of the structure of bifunctional organocatalysts I–VI (Figure 1) on enantioselectivity in ethyl acetate at room temperature (Table 1, entries 1–6). Catalyst III, which is a binaphthyl-modified squaramide bifunctional organocatalyst, was the best catalyst for this enantioselective addition reaction (90% ee, Table 1, entry 3). In order to improve the selectivity, different solvents were tested in the presence of 10 mol % of catalyst III together with ketimine 1a and diphenyl phosphonate (2). We obtained excellent results in ethyl acetate (85% yield, 90% ee, Table 1, entry 3), while a slight decrease in enationselectivities was observed when dichloromethane, chloroform, tetrahydrofuran, toluene, and methanol were used as the solvent (Table 1, entries 7–11). Under low catalyst loading of 2.5 mol %, this enantioselective addition reaction proceeded successfully to give 3a without compromising the reactivity and enantioselectivity (Table 1, entries 3 and 12–14). Finally, lowering the reaction temperature to 0 °C with catalyst III improved the enantioselectivity (93% ee, Table 1, entry 15). Performing the reaction without 4 Å molecular sieves generated a lower yield (Table 1, entry 16).
Table 1: Optimization of the reaction conditions. a
|
|
|||||
| entry | cat. | solvent | time (h) | yieldb (%) | eec (%) |
|---|---|---|---|---|---|
| 1 | I | EtOAc | 9 | 3a, 85 | 73 |
| 2 | II | EtOAc | 11 | 3a, 94 | 62 |
| 3 | III | EtOAc | 9 | 3a, 85 | 90 |
| 4 | IV | EtOAc | 12 | 3a, 85 | 54 |
| 5 | V | EtOAc | 12 | 3a, 85 | 78 |
| 6 | VI | EtOAc | 9 | 3a, 95 | 74 |
| 7 | III | CH2Cl2 | 3 | 3a, 92 | 87 |
| 8 | III | CHCl3 | 7 | 3a, 82 | 80 |
| 9 | III | THF | 3 | 3a, 88 | 85 |
| 10 | III | PhMe | 6 | 3a, 75 | 87 |
| 11 | III | MeOH | 8 | 3a, 54 | 84 |
| 12d | III | EtOAc | 16 | 3a, 82 | 90 |
| 13e | III | EtOAc | 19 | 3a, 80 | 90 |
| 14f | III | EtOAc | 25 | 3a, 76 | 81 |
| 15e,g | III | EtOAc | 21 | 3a, 80 | 93 |
| 16e,h | III | EtOAc | 21 | 3a, 58 | 93 |
aReaction conditions: ketimine (1a, 0.3 mmol), diphenyl phosphonate (2, 0.45 mmol), catalyst (0.03 mmol), solvent (3.0 mL) in the presence of 150 mg molecular sieves. bIsolated yield. cEnantiopurity was determined by HPLC analysis using Chiralpak IB column. d5 mol % catalyst loading. e2.5 mol % catalyst loading. f1.3 mol % catalyst loading. gReaction was performed at 0 °C. hReaction was performed without 4 Å molecular sieves.
With the optimized conditions in hand, we proceeded to investigate the scope of the enantioselective addition of diphenyl phosphonate (2) with various ketimines 1 in the presence of 2.5 mol % of binaphthyl-modified squaramide-tertiary amine catalyst III in ethyl acetate at 0 °C (Table 2). The corresponding addition products 3a–l were formed in high yields (74–94%) with excellent enantioselectivities (up to 99% ee). The reaction of diphenyl phosphonate (2) with N-allylated and 5-halo-N-allylated isatin imines provided adducts 3a–d in good yields (80–94%) with excellent enantioselectivities (93–97% ee, Table 2, entry 1–4). The addition of diphenyl phosphonate (2) to 5-chloro-N-substituted isatin imines 1e and 1f provided 3-amino-3-phosphonyl-substituted oxindole derivatives 3e and 3f in high yields (84% and 70%) with good enantioselectivities (99% ee and 88% ee, Table 2, entries 5 and 6). N-Benzylisatin imine 1g and 5-halogen-N-benzylisatin imines 1h–j reacted well with diphenyl phosphonate (2), giving 3-amino-3-phosphonyl-substituted oxindole derivatives 3g–j in high yields (78–88%) with excellent enantioselectivities (98–99% ee) (Table 2, entries 7–10). Ketimine 1k containing an electron donating group gave the desired product 3k in high yield (79%) with excellent enantioselectivity (99% ee, Table 2, entry 11). The nucleophilic addition of diphenyl phosphonate (2) to ketimine 2l derived from N-unprotected isatin was also studied. The adduct 3l was isolated in 74% yield with 73% ee (Table 2, entry 12). Unfortunately, the reaction of diphenyl phosphonate (2) with N-Boc-ketimine 2m provided adduct 3m with low yield and enantioselectivity (Table 2, entry 13). The absolute configuration of adducts 3 was determined to be R by comparison of the specific rotations and HPLC properties with literature values [36.37].
Table 2: Substrate scope.a
|
|
||||
| entry | 1 (R1, R2) | time (h) | yield (%)b | ee (%)c |
|---|---|---|---|---|
| 1 | 1a (R1 = CH2CH=CH2, R2 = H) | 21 | 3a, 80 | 93 |
| 2 | 1b (R1 = CH2CH=CH2, R2 = F) | 15 | 3b, 94 | 94 |
| 3 | 1c (R1 = CH2CH=CH2, R2 = Cl) | 12 | 3c, 90 | 94 |
| 4 | 1d (R1 = CH2CH=CH2, R2 = Br) | 19 | 3d, 84 | 97 |
| 5 | 1e (R1 = CH2C(CH3)=CH2, R2 = Cl) | 48 | 3e, 84 | 99 |
| 6 | 1f (R1 = CH2CH=CHCH3, R2 = Cl) | 47 | 3f, 70 | 88 |
| 7 | 1g (R1 = CH2C6H5, R2 = H) | 21 | 3g, 87 | 99 |
| 8 | 1h (R1 = CH2C6H5, R2 = F) | 20 | 3h, 88 | 99 |
| 9 | 1i (R1 = CH2C6H5, R2 = Cl) | 16 | 3i, 78 | 98 |
| 10 | 1j (R1 = CH2C6H5, R2 = Br) | 32 | 3j, 84 | 99 |
| 11 | 1k (R1 = CH2C6H5, R2 = OMe) | 48 | 3k, 79 | 99 |
| 12 | 1l (R1 = H, R2 = Cl) | 31 | 3l, 74 | 73 |
| 13 | 1m (R1 = Boc, R2 = H) | 48 | 3m, 45 | 26 |
aReaction conditions: ketimines (1, 0.3 mmol), diphenyl phosphonate (2, 0.45 mmol), catalyst (III, 7.5 μmol), EtOAc (3.0 mL) at 0 °C in the presence of 150 mg molecular sieve. bIsolated yield. cEnantiopurity was determined by HPLC analysis using Chiralpak IA (for 3f), IB (for 3a), IC (for 3b–e, 3g–j), and AD-H (for 3k, 3l) columns.
The stereochemical outcome in the above addition reaction was rationalized by a proposed stereochemical model. We propose that ketimine 1 is activated by the squaramide moiety through hydrogen bonding, and diphenyl phosphonate (2) is activated by the basic nitrogen atom in the tertiary amine of catalyst III. Then, diphenyl phosphonate (2) attacks the re-face of the carbon in ketimine 1 as shown in Figure 2.
To further demonstrate the synthetic potential of this method, we performed the addition reaction at the gram scale. As shown in Scheme 1, when ketimine 1a was treated with diphenyl phosphonate (2) in the presence of 2.5 mol % of catalyst III at 0 °C, the desired product 3a was obtained in 81% yield and 93% ee (Scheme 1).
Scheme 1: Gram scale addition of ketimine 1a and diphenyl phosphonate (2).
Scheme 1: Gram scale addition of ketimine 1a and diphenyl phosphonate (2).
Conclusion
In conclusion, we have developed a practical and efficient catalytic enantioselective addition reaction of diphenyl phosphonate (2) with various ketimines 1 derived from isatins. This transformation is catalyzed by binaphthyl-modified squaramide catalyst III with low catalyst loading (2.5 mol %). Chiral 3-amino-3-phosphonyl-substituted oxindole derivatives were obtained in high yields and excellent enantioselectivities were observed (up to 99% ee). This reaction affords valuable and easy access to chiral 3-amino-3-phosphonyl-substituted oxindole derivatives.
Experimental
General procedure for the enantioselective addition of diphenyl phosphonate (2) to ketimines derived from isatins 1: To a solution of ketimine 1 (0.3 mmol), diphenyl phosphonate (2, 0.45 mmol), and 4 Å molecular sieves (150 mg) in ethyl acetate (3 mL), the catalyst (III, 7.5 μmol) was added at 0 °C. The reaction mixture was stirred for 12–48 h. After completion of the reaction, the resulting solution was concentrated in vacuo and the obtained residue was purified by flash chromatography (EtOAc–hexane) to afford the corresponding adducts 3.
Supporting Information
| Supporting Information File 1: Experimental and analytical data. | ||
| Format: PDF | Size: 5.7 MB | Download |
References
-
Berlicki, L.; Kafarski, P. Curr. Org. Chem. 2005, 9, 1829–1850. doi:10.2174/138527205774913088
Return to citation in text: [1] -
Kafarski, P.; Lejczak, B. Curr. Med. Chem. 2001, 1, 301–312. doi:10.2174/1568011013354543
Return to citation in text: [1] -
Moonen, K.; Laureyn, I.; Stevens, C. V. Chem. Rev. 2004, 104, 6177–6216. doi:10.1021/cr030451c
Return to citation in text: [1] -
Xu, Y.; Yan, K.; Song, B.; Xu, G.; Yang, S.; Xue, W.; Hu, D.; Lu, P.; Ouyang, G.; Jin, L.; Chen, Z. Molecules 2006, 11, 666–676. doi:10.3390/11090666
Return to citation in text: [1] -
Yao, G.-y.; Ye, M.-y.; Huang, R.-z.; Li, Y.-j.; Pan, Y.-m.; Xu, Q.; Liao, Z.-X.; Wang, H.-s. Bioorg. Med. Chem. Lett. 2014, 24, 501–507. doi:10.1016/j.bmcl.2013.12.030
Return to citation in text: [1] -
Hu, D.-Y.; Wan, Q.-Q.; Yang, S.; Song, B.-A.; Bhadury, P. S.; Jin, L.-H.; Yan, K.; Liu, F.; Chen, Z.; Xue, W. J. Agric. Food Chem. 2008, 56, 998–1001. doi:10.1021/jf072394k
Return to citation in text: [1] -
Hirschmann, R.; Smith, A. B., III; Taylor, C. M.; Benkovic, P. A.; Taylor, S. D.; Yager, K. M.; Sprengeler, P. A.; Benkovic, S. J. Science 1994, 265, 234–237. doi:10.1126/science.8023141
Return to citation in text: [1] -
Barder, A. Aldrichimica Acta 1988, 21, 15.
Return to citation in text: [1] -
Mucha, A.; Kafarski, P.; Berlicki, L. J. Med. Chem. 2011, 54, 5955–5980. doi:10.1021/jm200587f
Return to citation in text: [1] -
Palacios, F.; Alonso, C.; de Los Santos, J. M. Chem. Rev. 2005, 105, 899–932. doi:10.1021/cr040672y
Return to citation in text: [1] -
Gröger, H.; Hammer, B. Chem. – Eur. J. 2000, 6, 943–948. doi:10.1002/(SICI)1521-3765(20000317)6:6<943::AID-CHEM943>3.0.CO;2-4
See for a review.
Return to citation in text: [1] -
Ordonez, M.; Viveros-Ceballos, J. L.; Cativiela, C.; Azerpe, A. Curr. Org. Synth. 2012, 9, 310–341. doi:10.2174/157017912801270595
Return to citation in text: [1] -
Vicario, J.; Ortiz, P.; Ezpeleta, J. M.; Palacios, F. J. Org. Chem. 2015, 80, 156–164. doi:10.1021/jo502233m
Return to citation in text: [1] -
Marti, C.; Carreira, E. M. Eur. J. Org. Chem. 2003, 12, 2209–2219. doi:10.1002/ejoc.200300050
Return to citation in text: [1] -
Dounay, A. B.; Overman, L. E. Chem. Rev. 2003, 103, 2945–2964. doi:10.1021/cr020039h
Return to citation in text: [1] -
Trost, B. M.; Brennan, M. K. Synthesis 2009, 3003–3025. doi:10.1055/s-0029-1216975
Return to citation in text: [1] -
Liu, Y.; Han, S.-J.; Liu, W.-B.; Stoltz, B. M. Acc. Chem. Res. 2015, 48, 740–751. doi:10.1021/ar5004658
Return to citation in text: [1] -
Galliford, C. V.; Scheidt, K. A. Angew. Chem., Int. Ed. 2007, 46, 8748–8758. doi:10.1002/anie.200701342
Return to citation in text: [1] -
Zhou, F.; Liu, Y.-L.; Zhou, J. Adv. Synth. Catal. 2010, 352, 1381–1407. doi:10.1002/adsc.201000161
Return to citation in text: [1] -
Kato, Y.; Furutachi, M.; Chen, Z.; Mitsunuma, H.; Matsunaga, S.; Shibasaki, M. J. Am. Chem. Soc. 2009, 131, 9168–9169. doi:10.1021/ja903566u
Return to citation in text: [1] -
Tomita, D.; Yamatsugu, K.; Kanai, M.; Shibasaki, M. J. Am. Chem. Soc. 2009, 131, 6946–6948. doi:10.1021/ja901995a
Return to citation in text: [1] -
Trost, B. M.; Czabaniuk, L. C. J. Am. Chem. Soc. 2010, 132, 15534–15536. doi:10.1021/ja1079755
Return to citation in text: [1] -
Cheng, L.; Liu, L.; Wang, D.; Chen, Y.-J. Org. Lett. 2009, 11, 3874–3877. doi:10.1021/ol901405r
Return to citation in text: [1] -
Qian, Z.-Q.; Zhou, F.; Du, T.-P.; Wang, B.-L.; Ding, M.; Zhao, X.-L.; Zhou, J. Chem. Commun. 2009, 6753–6755. doi:10.1039/B915257A
Return to citation in text: [1] -
Bui, T.; Hernández-Torres, G.; Milite, C.; Barbas, C. F., III. Org. Lett. 2010, 12, 5696–5699. doi:10.1021/ol102493q
Return to citation in text: [1] -
Mouri, S.; Chen, Z.; Mitsunuma, H.; Furutachi, M.; Matsunaga, S.; Shibasaki, M. J. Am. Chem. Soc. 2010, 132, 1255–1257. doi:10.1021/ja908906n
Return to citation in text: [1] -
Shen, K.; Liu, X.; Wang, G.; Lin, L.; Feng, X. Angew. Chem., Int. Ed. 2011, 50, 4684–4688. doi:10.1002/anie.201100758
Return to citation in text: [1] -
Montesinos-Magraner, M.; Vila, C.; Cantón, R.; Blay, G.; Fernández, I.; Muñoz, M. C.; Pedro, J. R. Angew. Chem., Int. Ed. 2015, 54, 6320–6324. doi:10.1002/anie.201501273
Return to citation in text: [1] -
Bao, X.; Wang, B.; Cui, L.; Zhu, G.; He, Y.; Qu, J.; Song, Y. Org. Lett. 2015, 17, 5168–5171. doi:10.1021/acs.orglett.5b02470
Return to citation in text: [1] -
Nakamura, S.; Takahashi, S. Org. Lett. 2015, 17, 2590–2593. doi:10.1021/acs.orglett.5b00805
Return to citation in text: [1] -
Arai, T.; Tsuchiya, K.; Matsumura, E. Org. Lett. 2015, 17, 2416–2419. doi:10.1021/acs.orglett.5b00928
Return to citation in text: [1] -
Takada, H.; Kumagai, N.; Shibasaki, M. Org. Lett. 2015, 17, 4762–4765. doi:10.1021/acs.orglett.5b02300
Return to citation in text: [1] -
Engl, O. D.; Fritz, S. P.; Wennemers, H. Angew. Chem., Int. Ed. 2015, 54, 8193–8197. doi:10.1002/anie.201502976
Return to citation in text: [1] -
Liu, T.; Liu, W.; Li, X.; Peng, F.; Shao, Z. J. Org. Chem. 2015, 80, 4950–4956. doi:10.1021/acs.joc.5b00302
Return to citation in text: [1] -
Zhao, J.; Fang, B.; Luo, W.; Hao, X.; Liu, X.; Lin, L.; Feng, X. Angew. Chem., Int. Ed. 2015, 54, 241–244. doi:10.1002/anie.201408730
Return to citation in text: [1] -
George, J.; Sridhar, B.; Reddy, B. V. S. Org. Biomol. Chem. 2014, 12, 1595–1602. doi:10.1039/C3OB42026D
Return to citation in text: [1] -
Kumar, A.; Sharma, V.; Kaur, J.; Kumar, V.; Mahajan, S.; Kumar, N.; Chimni, S. S. Tetrahedron 2014, 70, 7044–7049. doi:10.1016/j.tet.2014.06.013
Return to citation in text: [1] -
Kang, Y. K.; Kim, S. M.; Kim, D. Y. J. Am. Chem. Soc. 2010, 132, 11847–11849. doi:10.1021/ja103786c
Return to citation in text: [1] -
Kang, Y. K.; Lee, H. J.; Moon, H. W.; Kim, D. Y. RSC Adv. 2013, 3, 1332–1335. doi:10.1039/C2RA21945J
Return to citation in text: [1] -
Kang, Y. K.; Kim, D. Y. Adv. Synth. Catal. 2013, 355, 3131–3136. doi:10.1002/adsc.201300398
Return to citation in text: [1] -
Kang, Y. K.; Kim, D. Y. Chem. Commun. 2014, 50, 222–224. doi:10.1039/C3CC46710D
Return to citation in text: [1] -
Suh, C. W.; Woo, S. B.; Kim, D. Y. Asian J. Org. Chem. 2014, 3, 399–402. doi:10.1002/ajoc.201400022
Return to citation in text: [1] -
Suh, C. W.; Kim, D. Y. Org. Lett. 2014, 16, 5374–5377. doi:10.1021/ol502575f
Return to citation in text: [1] -
Sung, H. J.; Mang, J. Y.; Kim, D. Y. J. Fluorine Chem. 2015, 178, 40–46. doi:10.1016/j.jfluchem.2015.04.021
Return to citation in text: [1] -
Kwon, S. J.; Kim, D. Y. Chem. Rec. 2016, 16, 1191–1203. doi:10.1002/tcr.201600003
Return to citation in text: [1] -
Suh, C. W.; Chang, C. W.; Choi, K. W.; Lim, Y. J.; Kim, D. Y. Tetrahedron Lett. 2013, 54, 3651–3654. doi:10.1016/j.tetlet.2013.04.132
Return to citation in text: [1]
| 1. | Berlicki, L.; Kafarski, P. Curr. Org. Chem. 2005, 9, 1829–1850. doi:10.2174/138527205774913088 |
| 2. | Kafarski, P.; Lejczak, B. Curr. Med. Chem. 2001, 1, 301–312. doi:10.2174/1568011013354543 |
| 3. | Moonen, K.; Laureyn, I.; Stevens, C. V. Chem. Rev. 2004, 104, 6177–6216. doi:10.1021/cr030451c |
| 7. | Hirschmann, R.; Smith, A. B., III; Taylor, C. M.; Benkovic, P. A.; Taylor, S. D.; Yager, K. M.; Sprengeler, P. A.; Benkovic, S. J. Science 1994, 265, 234–237. doi:10.1126/science.8023141 |
| 46. | Suh, C. W.; Chang, C. W.; Choi, K. W.; Lim, Y. J.; Kim, D. Y. Tetrahedron Lett. 2013, 54, 3651–3654. doi:10.1016/j.tetlet.2013.04.132 |
| 6. | Hu, D.-Y.; Wan, Q.-Q.; Yang, S.; Song, B.-A.; Bhadury, P. S.; Jin, L.-H.; Yan, K.; Liu, F.; Chen, Z.; Xue, W. J. Agric. Food Chem. 2008, 56, 998–1001. doi:10.1021/jf072394k |
| 5. | Yao, G.-y.; Ye, M.-y.; Huang, R.-z.; Li, Y.-j.; Pan, Y.-m.; Xu, Q.; Liao, Z.-X.; Wang, H.-s. Bioorg. Med. Chem. Lett. 2014, 24, 501–507. doi:10.1016/j.bmcl.2013.12.030 |
| 36. | George, J.; Sridhar, B.; Reddy, B. V. S. Org. Biomol. Chem. 2014, 12, 1595–1602. doi:10.1039/C3OB42026D |
| 37. | Kumar, A.; Sharma, V.; Kaur, J.; Kumar, V.; Mahajan, S.; Kumar, N.; Chimni, S. S. Tetrahedron 2014, 70, 7044–7049. doi:10.1016/j.tet.2014.06.013 |
| 4. | Xu, Y.; Yan, K.; Song, B.; Xu, G.; Yang, S.; Xue, W.; Hu, D.; Lu, P.; Ouyang, G.; Jin, L.; Chen, Z. Molecules 2006, 11, 666–676. doi:10.3390/11090666 |
| 38. | Kang, Y. K.; Kim, S. M.; Kim, D. Y. J. Am. Chem. Soc. 2010, 132, 11847–11849. doi:10.1021/ja103786c |
| 39. | Kang, Y. K.; Lee, H. J.; Moon, H. W.; Kim, D. Y. RSC Adv. 2013, 3, 1332–1335. doi:10.1039/C2RA21945J |
| 40. | Kang, Y. K.; Kim, D. Y. Adv. Synth. Catal. 2013, 355, 3131–3136. doi:10.1002/adsc.201300398 |
| 41. | Kang, Y. K.; Kim, D. Y. Chem. Commun. 2014, 50, 222–224. doi:10.1039/C3CC46710D |
| 42. | Suh, C. W.; Woo, S. B.; Kim, D. Y. Asian J. Org. Chem. 2014, 3, 399–402. doi:10.1002/ajoc.201400022 |
| 43. | Suh, C. W.; Kim, D. Y. Org. Lett. 2014, 16, 5374–5377. doi:10.1021/ol502575f |
| 44. | Sung, H. J.; Mang, J. Y.; Kim, D. Y. J. Fluorine Chem. 2015, 178, 40–46. doi:10.1016/j.jfluchem.2015.04.021 |
| 45. | Kwon, S. J.; Kim, D. Y. Chem. Rec. 2016, 16, 1191–1203. doi:10.1002/tcr.201600003 |
| 17. | Liu, Y.; Han, S.-J.; Liu, W.-B.; Stoltz, B. M. Acc. Chem. Res. 2015, 48, 740–751. doi:10.1021/ar5004658 |
| 18. | Galliford, C. V.; Scheidt, K. A. Angew. Chem., Int. Ed. 2007, 46, 8748–8758. doi:10.1002/anie.200701342 |
| 19. | Zhou, F.; Liu, Y.-L.; Zhou, J. Adv. Synth. Catal. 2010, 352, 1381–1407. doi:10.1002/adsc.201000161 |
| 23. | Cheng, L.; Liu, L.; Wang, D.; Chen, Y.-J. Org. Lett. 2009, 11, 3874–3877. doi:10.1021/ol901405r |
| 24. | Qian, Z.-Q.; Zhou, F.; Du, T.-P.; Wang, B.-L.; Ding, M.; Zhao, X.-L.; Zhou, J. Chem. Commun. 2009, 6753–6755. doi:10.1039/B915257A |
| 25. | Bui, T.; Hernández-Torres, G.; Milite, C.; Barbas, C. F., III. Org. Lett. 2010, 12, 5696–5699. doi:10.1021/ol102493q |
| 26. | Mouri, S.; Chen, Z.; Mitsunuma, H.; Furutachi, M.; Matsunaga, S.; Shibasaki, M. J. Am. Chem. Soc. 2010, 132, 1255–1257. doi:10.1021/ja908906n |
| 27. | Shen, K.; Liu, X.; Wang, G.; Lin, L.; Feng, X. Angew. Chem., Int. Ed. 2011, 50, 4684–4688. doi:10.1002/anie.201100758 |
| 14. | Marti, C.; Carreira, E. M. Eur. J. Org. Chem. 2003, 12, 2209–2219. doi:10.1002/ejoc.200300050 |
| 15. | Dounay, A. B.; Overman, L. E. Chem. Rev. 2003, 103, 2945–2964. doi:10.1021/cr020039h |
| 16. | Trost, B. M.; Brennan, M. K. Synthesis 2009, 3003–3025. doi:10.1055/s-0029-1216975 |
| 28. | Montesinos-Magraner, M.; Vila, C.; Cantón, R.; Blay, G.; Fernández, I.; Muñoz, M. C.; Pedro, J. R. Angew. Chem., Int. Ed. 2015, 54, 6320–6324. doi:10.1002/anie.201501273 |
| 29. | Bao, X.; Wang, B.; Cui, L.; Zhu, G.; He, Y.; Qu, J.; Song, Y. Org. Lett. 2015, 17, 5168–5171. doi:10.1021/acs.orglett.5b02470 |
| 30. | Nakamura, S.; Takahashi, S. Org. Lett. 2015, 17, 2590–2593. doi:10.1021/acs.orglett.5b00805 |
| 31. | Arai, T.; Tsuchiya, K.; Matsumura, E. Org. Lett. 2015, 17, 2416–2419. doi:10.1021/acs.orglett.5b00928 |
| 32. | Takada, H.; Kumagai, N.; Shibasaki, M. Org. Lett. 2015, 17, 4762–4765. doi:10.1021/acs.orglett.5b02300 |
| 33. | Engl, O. D.; Fritz, S. P.; Wennemers, H. Angew. Chem., Int. Ed. 2015, 54, 8193–8197. doi:10.1002/anie.201502976 |
| 34. | Liu, T.; Liu, W.; Li, X.; Peng, F.; Shao, Z. J. Org. Chem. 2015, 80, 4950–4956. doi:10.1021/acs.joc.5b00302 |
| 35. | Zhao, J.; Fang, B.; Luo, W.; Hao, X.; Liu, X.; Lin, L.; Feng, X. Angew. Chem., Int. Ed. 2015, 54, 241–244. doi:10.1002/anie.201408730 |
| 9. | Mucha, A.; Kafarski, P.; Berlicki, L. J. Med. Chem. 2011, 54, 5955–5980. doi:10.1021/jm200587f |
| 10. | Palacios, F.; Alonso, C.; de Los Santos, J. M. Chem. Rev. 2005, 105, 899–932. doi:10.1021/cr040672y |
| 11. |
Gröger, H.; Hammer, B. Chem. – Eur. J. 2000, 6, 943–948. doi:10.1002/(SICI)1521-3765(20000317)6:6<943::AID-CHEM943>3.0.CO;2-4
See for a review. |
| 12. | Ordonez, M.; Viveros-Ceballos, J. L.; Cativiela, C.; Azerpe, A. Curr. Org. Synth. 2012, 9, 310–341. doi:10.2174/157017912801270595 |
| 13. | Vicario, J.; Ortiz, P.; Ezpeleta, J. M.; Palacios, F. J. Org. Chem. 2015, 80, 156–164. doi:10.1021/jo502233m |
| 20. | Kato, Y.; Furutachi, M.; Chen, Z.; Mitsunuma, H.; Matsunaga, S.; Shibasaki, M. J. Am. Chem. Soc. 2009, 131, 9168–9169. doi:10.1021/ja903566u |
| 21. | Tomita, D.; Yamatsugu, K.; Kanai, M.; Shibasaki, M. J. Am. Chem. Soc. 2009, 131, 6946–6948. doi:10.1021/ja901995a |
| 22. | Trost, B. M.; Czabaniuk, L. C. J. Am. Chem. Soc. 2010, 132, 15534–15536. doi:10.1021/ja1079755 |
© 2016 Jang et al.; licensee Beilstein-Institut.
This is an Open Access article under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
The license is subject to the Beilstein Journal of Organic Chemistry terms and conditions: (http://www.beilstein-journals.org/bjoc)