Abstract
A highly efficient, simple and environmentally friendly synthesis of 3-arylquinolines has been developed in the presence of Al2O3/MeSO3H via one-pot reaction of anilines and styrene oxide. This methodology provides very rapid access to 3-arylquinolines in good to excellent yields under solvent-free conditions at room temperature in air.
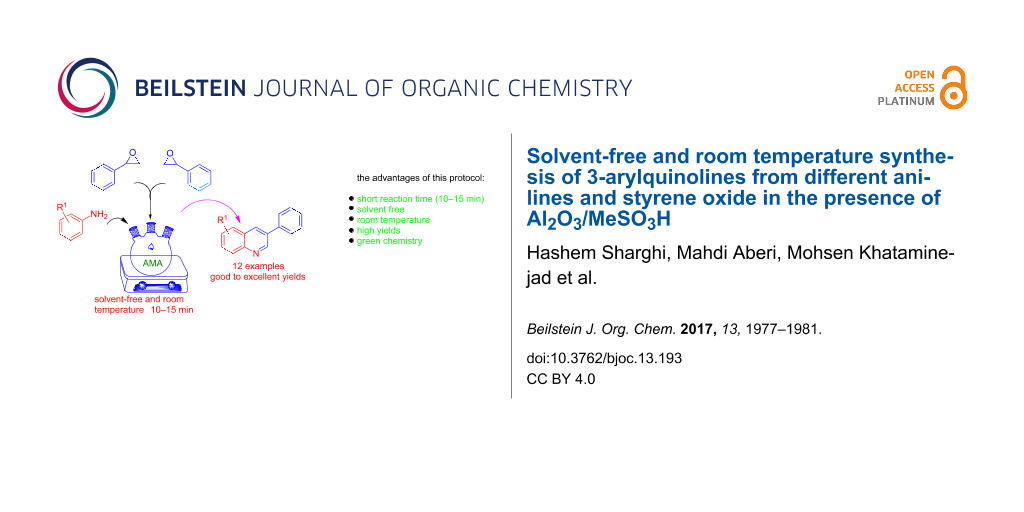
Graphical Abstract
Introduction
Quinoline derivatives have received considerable interest because they are found in numerous natural products with many biological activities. They have also played an important role in medicinal chemistry due to their pharmacological properties [1-4]. Transition metal-catalyzed processes [5-8] and metal-free paths [9-11] are two general approaches for the synthesis of this type of compounds. However, the existing methods suffer from complicated multistep processes, limited availability of substrates, toxic organic solvents, long reaction times, expensive catalyst and low regioselectivity in some cases.
One current area of modern synthetic organic chemistry is the development of powerful and effective practical procedures that minimize the requisite time, temperature, labour, and cost for the desired transformations [12,13]. The tandem reaction of anilines with styrene oxide via C–C cleavage is the efficient synthetic route to quinolones [1]. The reaction was performed using FeCl3 as catalyst in 1,4-dioxane as solvent at 110 °C for 12 h. According to the significance of this progress, we have decided to re-optimize it. The mixture of Al2O3 and MeSO3H has been previously used as an effective and mild reagent for organic transformations [14].
In continuation of our studies to develop new synthetic methods for heterocycles [15-19], herein, we disclose a novel route to the synthesis of 3-arylquinolines from aniline derivatives and styrene oxide at room temperature under solvent-free conditions (Scheme 1).
Scheme 1: Synthesis of 3-arylquinolines from anilines and styrene oxide. AMA = Al2O3/methanesulfonic acid
Scheme 1: Synthesis of 3-arylquinolines from anilines and styrene oxide. AMA = Al2O3/methanesulfonic acid
Scheme 1 briefly compares the procedure reported by Wang and co-workers and our method. As it can be seen, the reaction can be performed under very short reaction time and low temperature.
Results and Discussion
To exploit optimized conditions for the synthesis of quinolines, the reaction of 3,4-dimethylaniline (1, 1.0 mmol) and styrene oxide (2, 2.0 mmol) in an open atmosphere was chosen as a model reaction (Table 1).
Control experiments showed that in the absence of Al2O3 and MeSO3H, no quinoline 3a was observed (Table 1, entry 1). The results also showed the importance of using both of Al2O3 and MeSO3H. In the absence of MeSO3H no product was obtained (Table 1, entry 2). In the presence of MeSO3H, 6,7-dimethyl-3-phenylquinoline (3a) was obtained in 75% yield (Table 1, entry 3). Finally, the best result was obtained using a mixture of Al2O3 (0.1 g) and MeSO3H (0.3 mL) under solvent-free conditions at room temperature for 10 min (Table 1, entry 6). Various anilines with electron-withdrawing and electron-donating functional groups such as o-Me, m-Me, p-Me, m-Et, p-MeO, m-Br, o-Cl, 3,4-dimethyl and p-OEt were treated with styrene oxide to form the desired products (Table 2). In continuation of our study, aliphatic epoxides were also checked; unfortunately, they were not applicable for the preparation of quinolines. All novel and known compounds were characterized by their melting points, IR, 1H NMR, 13C NMR and mass spectra.
Table 1: Optimization studies for the synthesis of 6,7-dimethyl-3-phenylquinoline (3a) from 3,4-dimethylaniline (1a, 1.0 mmol) with styrene oxide (2, 2.0 mmol) in open air.
|
|
||||
| Entry | Catalyst | Conditions | Time (min) | Yield (%)a |
|---|---|---|---|---|
| 1 | None | solvent-free/rt | 60 | no reaction |
| 2 | Al2O3 (0.1 g) | solvent-free/rt | 60 | no reaction |
| 3 | MeSO3H (0.3 mL) | solvent-free/rt | 10 | 75 |
| 4 | Al2O3 (0.1 g) + MeSO3H (0.1 mL) | solvent-free/rt | 10 | 35 |
| 5 | Al2O3 (0.1 g) + MeSO3H (0.2 mL) | solvent-free/rt | 10 | 79 |
| 6 | Al2O3 (0.1 g) + MeSO3H (0.3 mL) | solvent-free/rt | 10 | 91 |
| 7 | Al2O3 (0.2 g) + MeSO3H (0.3 mL) | solvent-free/rt | 10 | 85 |
aIsolated yield.
Table 2: One-pot synthesis of 3-arylquinolines from the reaction of different anilines (1.0 mmol) with styrene oxide (2.0 mmol) in the presence of Al2O3 and MeSO3H at room temperature under solvent-free conditions.
|
|
||||
| Entry | Aniline | Product | Time (min) | Yield (%)a |
|---|---|---|---|---|
| 1 |
1a |
3a |
10 | 91 |
| 2 |
1b |
3b |
12 | 89 |
| 3 |
1c |
3c |
12 | 88 |
| 4 |
1d |
3d |
15 | 86 |
| 5 |
1e |
3e |
10 | 85 |
| 6 |
1f |
3f |
15 | 84 |
| 7 |
1g |
3g |
10 | 83 |
| 8 |
1h |
3h |
10 | 82 |
| 9 |
1i |
3i |
12 | 81 |
| 10 |
1j |
3j |
15 | 84 |
| 11 |
1k |
3k |
10 | 82 |
| 12 |
1l |
3l |
15 | 80 |
aIsolated yield.
In order to recover Al2O3, the mixture was diluted with ethyl acetate and filtered. The solid on the filter paper was washed by ethyl acetate and evaporated. It should be noted that only Al2O3 was reused and it is necessary to add MeSO3H again for each cycle with recovered Al2O3. The recycled catalyst could be reused five times without any significant loss in activity (Figure 1).
![[1860-5397-13-193-1]](/bjoc/content/figures/1860-5397-13-193-1.png?scale=2.0&max-width=1024&background=FFFFFF)
Figure 1: Investigation of the reusability of Al2O3.
Figure 1: Investigation of the reusability of Al2O3.
Conclusion
In conclusion, Al2O3 and MeSO3H exhibited an excellent reactivity in the one-pot synthesis of 3-arylquinolines using anilines and styrene oxide. The methodology has the advantages of good to excellent yields, readily available starting materials, short reaction time, mild and solvent-free conditions. The method utilizes nonexpensive reagents and starting materials, as well. Further work is in progress to extend the scope and to investigate mechanism aspects of this reaction.
Experimental
Instrumentation, analysis and starting material
Starting materials and solvents were purchased from Aldrich, Fluka, and Merck. IR spectra were obtained using a Shimadzu Fourier transform infrared (FTIR) 8300 spectrophotometer. Melting points were determined in open capillary tubes in a Büchi-535 circulating oil melting point apparatus. Mass spectra were determined on a Shimadzu GCMS-QP 1000 EX instrument at 70 or 20 eV. NMR spectra were recorded on a Bruker Avance DPX-250 (1H NMR 250 MHz and 13C NMR 62.9 MHz) spectrometer in pure deuterated solvents with tetramethylsilane (TMS) as an internal standard. The used methanesulfonic acid 98% and acidic alumina (Al2O3) type 540 C were purchased from Fluka. The elemental analyses were performed with a Thermo Finnigan CHNS-O analyzer, 1112 series. The purity determination of the substrates and reaction monitoring were accomplished by TLC on silica gel PolyGram SILG/UV 254 plates. Column chromatography was carried out on short columns of silica gel 60 (70–230 mesh) in glass columns.
General procedure for the synthesis of quinolines in the presence of Al2O3/MeSO3H
Aniline (1.0 mmol) and styrene oxide (2.0 mmol) were added to a mixture of MeSO3H (0.3 mL) and Al2O3 (0.1 g). The mixture was stirred at room temperature in solvent-free conditions for the period of time reported in Table 2. After completion of the reaction, the mixture was diluted with ethyl acetate, and filtered. The filtrate was washed with a solution of NaHCO3 (5%; 3 × 30 mL) and then 30 mL deionized water. The solution was dried over magnesium sulfate; the solvent was evaporated to give the crude product, which was purified by silica gel column chromatography employing n-hexane/ethyl acetate (10:1) as eluent.
Supporting Information
| Supporting Information File 1: Additional experimental and analytical data and NMR spectra. | ||
| Format: PDF | Size: 1.3 MB | Download |
References
-
Zhang, Y.; Wang, M.; Li, P.; Wang, L. Org. Lett. 2012, 14, 2206–2209. doi:10.1021/ol300391t
Return to citation in text: [1] [2] -
Michael, J. P. Nat. Prod. Rep. 2001, 18, 543–559. doi:10.1039/B005387M
Return to citation in text: [1] -
Funayama, S.; Murata, K.; Noshita, T. Heterocycles 2001, 54, 1139–1148. doi:10.3987/REV-00-SR(I)8
Return to citation in text: [1] -
Sawada, Y.; Kayakiri, H.; Abe, Y.; Imai, K.; Mizutani, T.; Inamura, N.; Asano, M.; Aramori, I.; Hatori, C.; Katayama, A. J. Med. Chem. 2004, 47, 1617–1630. doi:10.1021/jm030159x
Return to citation in text: [1] -
Zhang, Z.; Tan, J.; Wang, Z. Org. Lett. 2008, 10, 173–175. doi:10.1021/ol702153x
Return to citation in text: [1] -
Liu, X.-Y.; Ding, P.; Huang, J.-S.; Che, C.-M. Org. Lett. 2007, 9, 2645–2648. doi:10.1021/ol070814l
Return to citation in text: [1] -
Gabriele, B.; Mancuso, R.; Salerno, G.; Ruffolo, G.; Plastina, P. J. Org. Chem. 2007, 72, 6873–6877. doi:10.1021/jo071094z
Return to citation in text: [1] -
Gaddam, V.; Ramesh, S.; Nagarajan, R. Tetrahedron 2010, 66, 4218–4222. doi:10.1016/j.tet.2010.03.095
Return to citation in text: [1] -
Zhao, Y.-L.; Zhang, W.; Wang, S.; Liu, Q. J. Org. Chem. 2007, 72, 4985–4988. doi:10.1021/jo070069q
Return to citation in text: [1] -
Tanaka, S.-y.; Yasuda, M.; Baba, A. J. Org. Chem. 2006, 71, 800–803. doi:10.1021/jo052004y
Return to citation in text: [1] -
Hessian, K. O.; Flynn, B. L. Org. Lett. 2006, 8, 243–246. doi:10.1021/ol052518j
Return to citation in text: [1] -
Zhang, Y.; Li, P.; Wang, L. J. Heterocycl. Chem. 2011, 48, 153–157. doi:10.1002/jhet.417
Return to citation in text: [1] -
Trost, B. M. Acc. Chem. Res. 2002, 35, 695–705. doi:10.1021/ar010068z
Return to citation in text: [1] -
Sharghi, H.; Beni, A. R. S. Synthesis 2004, 2900–2904. doi:10.1055/s-2004-831253
Return to citation in text: [1] -
Sharghi, H.; Aberi, M.; Shiri, P. Appl. Organomet. Chem. 2017, in press. doi:10.1002/aoc.3761
Return to citation in text: [1] -
Sharghi, H.; Shiri, P. Synthesis 2015, 47, 1131–1146. doi:10.1055/s-0034-1379951
Return to citation in text: [1] -
Sharghi, H.; Shiri, P.; Aberi, M. Mol. Diversity 2014, 18, 559–575. doi:10.1007/s11030-014-9527-5
Return to citation in text: [1] -
Sharghi, H.; Shiri, P.; Aberi, M. Synthesis 2014, 46, 2489–2498. doi:10.1055/s-0034-1378206
Return to citation in text: [1] -
Sharghi, H.; Aberi, M.; Doroodmand, M. M.; Shiri, P. J. Iran. Chem. Soc. 2017, 14, 1557–1573. doi:10.1007/s13738-017-1097-x
Return to citation in text: [1]
| 1. | Zhang, Y.; Wang, M.; Li, P.; Wang, L. Org. Lett. 2012, 14, 2206–2209. doi:10.1021/ol300391t |
| 2. | Michael, J. P. Nat. Prod. Rep. 2001, 18, 543–559. doi:10.1039/B005387M |
| 3. | Funayama, S.; Murata, K.; Noshita, T. Heterocycles 2001, 54, 1139–1148. doi:10.3987/REV-00-SR(I)8 |
| 4. | Sawada, Y.; Kayakiri, H.; Abe, Y.; Imai, K.; Mizutani, T.; Inamura, N.; Asano, M.; Aramori, I.; Hatori, C.; Katayama, A. J. Med. Chem. 2004, 47, 1617–1630. doi:10.1021/jm030159x |
| 1. | Zhang, Y.; Wang, M.; Li, P.; Wang, L. Org. Lett. 2012, 14, 2206–2209. doi:10.1021/ol300391t |
| 12. | Zhang, Y.; Li, P.; Wang, L. J. Heterocycl. Chem. 2011, 48, 153–157. doi:10.1002/jhet.417 |
| 13. | Trost, B. M. Acc. Chem. Res. 2002, 35, 695–705. doi:10.1021/ar010068z |
| 9. | Zhao, Y.-L.; Zhang, W.; Wang, S.; Liu, Q. J. Org. Chem. 2007, 72, 4985–4988. doi:10.1021/jo070069q |
| 10. | Tanaka, S.-y.; Yasuda, M.; Baba, A. J. Org. Chem. 2006, 71, 800–803. doi:10.1021/jo052004y |
| 11. | Hessian, K. O.; Flynn, B. L. Org. Lett. 2006, 8, 243–246. doi:10.1021/ol052518j |
| 5. | Zhang, Z.; Tan, J.; Wang, Z. Org. Lett. 2008, 10, 173–175. doi:10.1021/ol702153x |
| 6. | Liu, X.-Y.; Ding, P.; Huang, J.-S.; Che, C.-M. Org. Lett. 2007, 9, 2645–2648. doi:10.1021/ol070814l |
| 7. | Gabriele, B.; Mancuso, R.; Salerno, G.; Ruffolo, G.; Plastina, P. J. Org. Chem. 2007, 72, 6873–6877. doi:10.1021/jo071094z |
| 8. | Gaddam, V.; Ramesh, S.; Nagarajan, R. Tetrahedron 2010, 66, 4218–4222. doi:10.1016/j.tet.2010.03.095 |
| 15. | Sharghi, H.; Aberi, M.; Shiri, P. Appl. Organomet. Chem. 2017, in press. doi:10.1002/aoc.3761 |
| 16. | Sharghi, H.; Shiri, P. Synthesis 2015, 47, 1131–1146. doi:10.1055/s-0034-1379951 |
| 17. | Sharghi, H.; Shiri, P.; Aberi, M. Mol. Diversity 2014, 18, 559–575. doi:10.1007/s11030-014-9527-5 |
| 18. | Sharghi, H.; Shiri, P.; Aberi, M. Synthesis 2014, 46, 2489–2498. doi:10.1055/s-0034-1378206 |
| 19. | Sharghi, H.; Aberi, M.; Doroodmand, M. M.; Shiri, P. J. Iran. Chem. Soc. 2017, 14, 1557–1573. doi:10.1007/s13738-017-1097-x |
| 14. | Sharghi, H.; Beni, A. R. S. Synthesis 2004, 2900–2904. doi:10.1055/s-2004-831253 |
© 2017 Sharghi et al.; licensee Beilstein-Institut.
This is an Open Access article under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
The license is subject to the Beilstein Journal of Organic Chemistry terms and conditions: (http://www.beilstein-journals.org/bjoc)