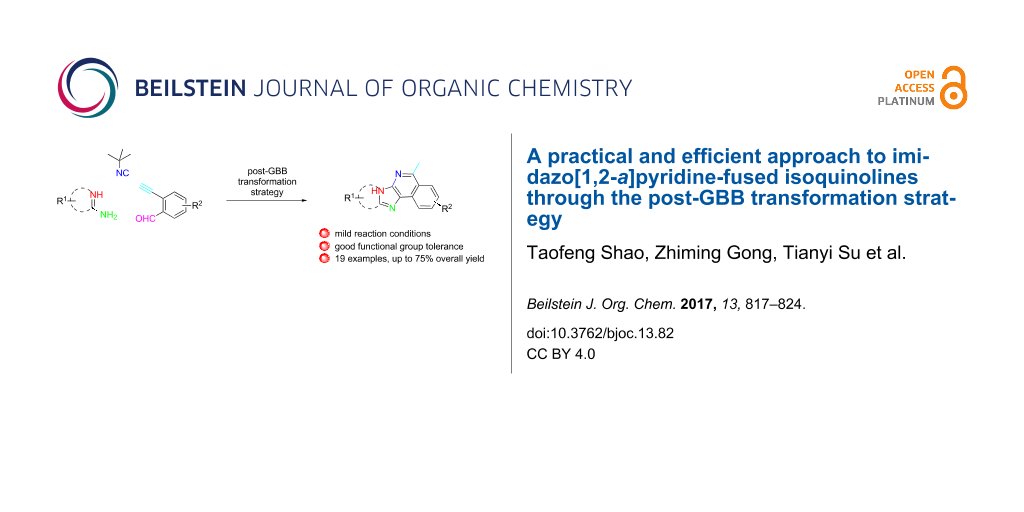
Abstract
Diversity-oriented synthesis of the biologically intriguing imidazo[1,2-a]pyridine-fused isoquinoline systems from readily available starting materials was achieved through the Groebke–Blackburn–Bienaymé reaction followed by a gold-catalyzed cyclization strategy. The synthetic approach is characterized by mild reaction conditions and a broad substrate scope, allowing for the rapid construction of structurally complex and diverse heterocycles in moderate to good yields.
Graphical Abstract
Introduction
Imidazo[1,2-a]pyridines have been reported to display a wide range of biological activities [1-5], and these skeletons are found in various clinical drugs such as zolpidem (I), alpidem (II), and olprinone (III), which were approved for the treatment of insomnia, anxiety and acute heart failure, respectively (Figure 1) [6]. Furthermore, the isoquinoline motif represents a privileged medicinal skeleton widely found in a number of natural alkaloids and pharmaceutically active compounds [7]. Some of them exhibit diversified biological properties, including anti-inflammatory [8], antibacterial [9], antiviral [10], and antitumor activities [11]. For example, the natural alkaloids berberine (IV) and narciclasine (V) possess antiplasmodial and antiviral activity, respectively [12,13]. Indotecan (VI) and its analog idimitecan (VII) were identified as topoisomerase I inhibitors, and were promoted into phase I clinical trials [14].
Figure 1: Representative bioactive imidazo[1,2-a]pyridine and isoquinoline-containing derivatives.
Figure 1: Representative bioactive imidazo[1,2-a]pyridine and isoquinoline-containing derivatives.
Multicomponent reactions (MCRs) [15-19], comprising three or more components, provide straightforward approaches to a wide range of heterocycles through the formation of various bonds in a one-pot process. These reactions not only greatly accelerate chemical syntheses [20], but also allow access to diverse chemical structures [21] from readily accessible building blocks. In the past decades, considerable efforts have been made towards the development of new MCRs and their application to the diversity-oriented synthesis of biologically relevant molecules for drug discovery [22-27].
The Ugi reaction [28], an elegant pioneer of a multicomponent reaction, represents a powerful synthetic tool to assemble versatile peptide-like compounds. It has found many applications in the facile synthesis of natural products and biologically interesting molecules [29,30]. Although the Ugi-4CR generates linear α-acylamino-amides, a wide range of heterocycles are accessible through the combination with other transformations (post-transformation strategy) [31]. For example, the Ugi/Diels–Alder process leads to the formation of benzofurans and indoles [32] as well as to structurally complex polycyclic ring systems [33]; an Ugi/aza-Wittig process allowed for the synthesis of 2,5-disubstituted 1,3,4-oxadiazoles [34]; the Ugi/Pictet–Spengler sequence provided a rapid and efficient approach to polycyclic natural product-like alkaloids [35]. Accordingly, the combination of the Ugi reaction with other transformations proved to be powerful strategies for the efficient synthesis of novel heterocycles. In 1998, the Groebke–Blackburn–Bienaymé (GBB) reaction, an Ugi-3CR variant was discovered by three groups independently [36-38]. The GBB reaction of an amidine, an aldehyde and an isocyanide proceeds through the isocyanide-involving formal [4 + 1] cycloaddition [39] affording the biologically important imidazo[1,2-a]pyridine scaffold. Due to the atom and step economy, high efficiency and intriguing biological profiles of the products, the GBB reaction has attracted broad attention in the field of organic synthesis [40-42]. In order to expand the structural diversity of GBB products, further investigation of GBB-based synthetic strategies remains highly desirable.
In continuation of our research on the development of MCR strategies for the rapid library synthesis of biologically interesting heterocycles [43-47], we were interested in a practical synthetic strategy towards imidazo[1,2-a]pyridine-fused isoquinoline systems. We believe that this type of polycyclic systems may have interesting biological profiles [48]. Herein, we report our recent efforts on the development of a post-GBB transformation strategy for the concise synthesis of diverse imidazo[1,2-a]pyridine-fused isoquinoline systems.
Results and Discussion
From a design perspective, we envisioned that the imidazo[1,2-a]pyridine-fused isoquinoline 6a [49,50] could be constructed through a GBB reaction/cyclization strategy (Scheme 1). The intermediate GBB product 4a could be constructed starting from 2-ethynylbenzaldehyde (2a) through an imine formation/formal [4 + 1] cycloaddition/[1,3]-H shift. The so obtained GBB product imidazo[1,2-a]pyridine 4a bearing an amino group and an acetylene unit may then undergo a sequential 6-exo-dig cyclization/retro-ene reaction to form the desired imidazo[1,2-a]pyridine-fused isoquinoline 6a. The cyclization reaction could be realized with the aid of silver or gold catalysts [51,52].
Scheme 1: GBB-based MCR strategy for the imidazo[1,2-a]pyridine-fused isoquinoline derivatives.
Scheme 1: GBB-based MCR strategy for the imidazo[1,2-a]pyridine-fused isoquinoline derivatives.
With this idea in mind, we commenced our studies by investigating the GBB reaction of 2-aminopyridine (1a), 2-ethynylbenzaldehyde (2a) and tert-butylisocyanide (3). The GBB reaction proceeded smoothly in MeOH in the presence of catalytic PTSA or HClO4 at room temperature to afford imidazo[1,2-a]pyridine 4a in 90% yield, and the cyclized product 6a was not detected under these mild conditions. Subsequent heating of 4a in refluxing 1,4-dioxane or toluene failed to deliver the expected product 6a, even under acidic or basic conditions.
Then, we turned to Ag and Au catalysts and investigated the metal-catalyzed intramolecular cyclization reaction of 4a and the results are collected in Table 1. First, we investigated AgOTf as the catalyst, which afforded the cyclized product 6a in 12% yield in refluxing CH2Cl2 in the presence of 10 mol % of catalyst. The yield was increased to 45% when replacing CH2Cl2 with CHCl3, whereas only a trace amount of the desired product was obtained in refluxing CH3CN or 1,4-dioxane (Table 1, entries 1–4). It revealed that the solvent plays a key role in this cyclization reaction. For comparison, we tested also AgSbF6 as the catalyst and it was found to be less effective than AgOTf (Table 1, entry 5). To improve the reaction efficiency, we next evaluated the cyclization reaction in refluxing CHCl3 in the presence of a range of Au catalysts. Although almost no reaction took place with Au(PPh3)Cl as the catalyst, the use of Au(PPh3)NTf2 resulted in a satisfactory yield (70%) of the product (Table 1, entries 6–9). Motivated by this result, other Au catalysts were further surveyed, and Au(JohnPhos)Cl was found to be the most efficient, delivering 6a in 78% yield (Table 1, entries 10–14). Next, the effect of the solvent on the reaction was tested and replacement of CHCl3 with CH3CN led to a slightly enhanced yield (83%) (Table 1, entries 15 and 16). Additionally, in refluxing CH3CN no other Au catalysts afforded better results than Au(JohnPhos)Cl (Table 1, entries 17 and 18). Overall, the optimal conditions for the cyclization reaction are as follows: Au(JohnPhos)Cl (10 mol %), CH3CN, reflux, 24 h.
Table 1: Optimization of the cyclization reaction conditions.a
|
|
|||
| Entry | Catalyst | Solvent | Yieldb (%) |
| 1 | AgOTf | CH2Cl2 | 12 |
| 2 | AgOTf | CHCl3 | 45 |
| 3 | AgOTf | CH3CN | trace |
| 4 | AgOTf | dioxane | trace |
| 5 | AgSbF6 | CHCl3 | 38 |
| 6 | Au(PPh3)Cl | CHCl3 | trace |
| 7 | Au(PPh3)OTf | CHCl3 | 42 |
| 8 | Au(PPh3)SbF6 | CHCl3 | 21 |
| 9 | Au(PPh3)NTf2 | CHCl3 | 70 |
| 10 | Au2(dppe) (SbF6)2 | CHCl3 | 51 |
| 11 | Au2(binap)( SbF6)2 | CHCl3 | 53 |
| 12 | Au(JohnPhos)Cl | CHCl3 | 78 |
| 13 | Au(JohnPhos)OTf | CHCl3 | 42 |
| 14 | Au(JohnPhos)SbF6 | CHCl3 | 74 |
| 15 | Au(JohnPhos)Cl | CH3CN | 83 |
| 16 | Au(JohnPhos)Cl | dioxane | 49 |
| 17 | Au2(dppe) Cl2 | CH3CN | 34 |
| 18 | Au2(binap)Cl2 | CH3CN | 42 |
aGeneral conditions: substrate 4a (0.2 mmol), catalyst (10 mol %), solvent (2 mL) at reflux temperature for 24 h. bIsolated yield.
With the optimal conditions at hand, we then set out to explore the reaction scope for the library generation of structurally diverse imidazo[1,2-a]pyridine-fused isoquinolines and the results are collected in Table 2. Initially, several GBB adducts 4 were synthesized through GBB reaction of amidines 1, substituted 2-ethynylbenzaldehydes 2 and tert-butylisocyanide (3). Indeed, the acetylene group in the aldehyde component had no obvious steric effect on the efficiency of the GBB reaction affording the GBB product in good to excellent yields in most cases. On the other hand, the substituent ortho to the amino group in the amidine component had a negative effect on the GBB reaction efficiency due to steric hindrance (Table 2, entries 6, 11, 13 and 17).
Table 2: Substrate scope for the syntheses of compounds 4 and 6.a
| Entry | Starting materials | GBB product 4 | Yieldb (%) | Cyclized product 6 | Yieldb (%) |
|---|---|---|---|---|---|
| 1 |
|
4b |
94 |
6b |
72 |
| 2 |
|
4c |
80 |
6c |
75 |
| 3 |
|
4d |
62 |
6d |
61 |
| 4 |
|
4e |
96 |
6e |
63 |
| 5 |
|
4f |
89 |
6f |
78 |
| 6 |
|
4g |
61 |
6g |
80 |
| 7 |
|
4h |
85 |
6h |
56 |
| 8 |
|
4i |
88 |
6i |
58 |
| 9 |
|
4j |
64 |
6j |
78 |
| 10 |
|
4k |
75 |
6k |
87 |
| 11 |
|
4l |
49 |
6l |
79 |
| 12 |
|
4m |
71 |
6m |
48 |
| 13 |
|
4n |
47 |
6n |
55 |
| 14 |
|
4o |
54 |
6o |
62 |
| 15 |
|
4p |
95 |
6p |
63 |
| 16 |
|
4q |
74 |
6q |
58 |
| 17 |
|
4r |
43 |
6r |
67 |
| 18 |
|
4s |
57 |
6s |
59 |
aGBB reaction conditions: 1 (0.5 mmol), 2 (0.5 mmol), 3 (0.6 mmol), MeOH (1 mL); PTSA (5%), room temperature, 12h; annulation conditions: substrate 4 (0.2 mmol), Au(JohnPhos)Cl (10 mol %), CH3CN (2 mL) at reflux temperature for 24 h. bIsolated yields.
Then, the newly generated GBB adducts 4b–s were exposed to the established cyclization conditions to deliver the corresponding imidazo[1,2-a]pyridine-fused isoquinolines 6b–s in moderate to good yields, and their structures were unambiguously confirmed by 1H NMR, 13C NMR, and HRMS analysis. Various functionalities related to the amidine and aldehyde components, including electron-donating methoxy and methyl groups or electron-withdrawing halides, were well tolerated. Generally, the substitution pattern of the amidine moiety had little effect on the Au-catalyzed annulation reaction, whereas neutral or electron-donating groups on the aldehyde moiety gave a higher yield in comparison with the electron-withdrawing halides. Notably, bromo-substituted substrates were also tolerated the reaction conditions, allowing for the further manipulation through various cross-coupling reaction (Table 2, entry 9).
Conclusion
In conclusion, we have developed a practical and efficient synthetic approach to structurally diverse imidazo[1,2-a]pyridine-fused isoquinolines with moderate to good yields through the GBB multicomponent reaction/Au-catalyzed cyclization strategy. The described method provides a new tool for a rapid compound library generation from readily accessible starting materials. Further, the protocol tolerates a broad substrate scope, which will make it attractive for the application in parallel synthesis and combinatorial chemistry.
Experimental
Typical procedure for the GBB multicomponent reaction. To a solution of 2-aminopyridine (1a, 0.5 mmol), 2-ethynylbenzaldehyde (2a, 0.5 mmol), and tert-butylisocyanide (3, 0.6 mmol) in 1 mL of methanol were added p-toluenesulfonic acid (4.7 mg, 0.025 mmol) and the reaction mixture was stirred at rt for 12 h. The mixture was diluted with 15 mL of dichloromethane and washed successively with water (10 mL), saturated NaHCO3 solution (10 mL) and brine (10 mL). After drying over anhydrous Na2SO4, the mixture was concentrated under vacuum and the resulting residue was purified by flash chromatography (hexane/ethyl acetate 8:1) to afford GBB adduct 4a (90% yield).
Typical procedure for the Au-catalyzed cyclization reaction. To a solution of the GBB adduct 4 (0.2 mmol) in 2 mL of acetonitrile was added Au(JohnPhos)Cl (0.02 mmol) and the resulting mixture was stirred under inert atmosphere at reflux temperature for 24 h. Then, the solvent was removed under vacuum and the residue purified by flash chromatography (hexane/ethyl acetate 5:1) to afford the desired product 6.
Supporting Information
| Supporting Information File 1: Characterization data for all compounds and copies of NMR spectra for compounds 6a–s. | ||
| Format: PDF | Size: 2.9 MB | Download |
Acknowledgements
We thank the Shenzhen Basic Research Project (Grants No. JCYJ20140417144423193, and JSGG20140717102922014), the National Natural Science Foundation of China (Grant No. 21102006) and the Natural Science Foundation of Guangdong Province (Grant No. 2014A030312004) for financial support.
References
-
Tantry, S. J.; Markad, S. D.; Shinde, V.; Bhat, J.; Balakrishnan, G.; Gupta, A. K.; Ambady, A.; Raichurkar, A.; Kedari, C.; Sharma, S.; Mudugal, N. V.; Narayan, A.; Kumar, C. N. N.; Nanduri, R.; Bharath, S.; Reddy, J.; Panduga, V.; Prabhakar, K. R.; Kandaswamy, K.; Saralaya, R.; Kaur, P.; Dinesh, N.; Guptha, S.; Rich, K.; Murray, D.; Plant, H.; Preston, M.; Ashton, H.; Plant, D.; Walsh, J.; Alcock, P.; Naylor, K.; Collier, M.; Whiteaker, J.; McLaughlin, R. E.; Mallya, M.; Panda, M.; Rudrapatna, S.; Ramachandran, V.; Shandil, R.; Sambandamurthy, V. K.; Mdluli, K.; Cooper, C. B.; Rubin, H.; Yano, T.; Iyer, P.; Narayanan, S.; Kavanagh, S.; Mukherjee, K.; Balasubramanian, V.; Hosagrahara, V. P.; Solapure, S.; Ravishankar, S.; Hammed, P. S. J. Med. Chem. 2017, 60, 1379–1399. doi:10.1021/acs.jmedchem.6b01358
Return to citation in text: [1] -
Shukla, N. M.; Salunke, D. B.; Yoo, E.; Mutz, C. A.; Balakrishna, R.; David, S. A. Bioorg. Med. Chem. 2012, 20, 5850–5863. doi:10.1016/j.bmc.2012.07.052
Return to citation in text: [1] -
Hamdouchi, C.; de Blas, J.; del Prado, M.; Gruber, J.; Heinz, B. A.; Vance, L. J. Med. Chem. 1999, 42, 50–59. doi:10.1021/jm9810405
Return to citation in text: [1] -
Frett, B.; McConnell, N.; Smith, C. C.; Wang, Y.; Shah, N. P.; Li, H.-y. Eur. J. Med. Chem. 2015, 94, 123–131. doi:10.1016/j.ejmech.2015.02.052
Return to citation in text: [1] -
Bode, M. L.; Gravestock, D.; Moleele, S. S.; van der Westhuyzen, C. W.; Pelly, S. C.; Steenkamp, P. A.; Hoppe, H. C.; Khan, T.; Nkabinde, L. A. Bioorg. Med. Chem. 2011, 19, 4227–4237. doi:10.1016/j.bmc.2011.05.062
Return to citation in text: [1] -
Enguehard-Gueiffier, C.; Gueiffier, A. Mini-Rev. Med. Chem. 2007, 7, 888–899. doi:10.2174/138955707781662645
Return to citation in text: [1] -
Bentley, K. W. Nat. Prod. Rep. 1998, 15, 341–362. doi:10.1039/a815341y
Return to citation in text: [1] -
Charpiot, B.; Bitsch, F.; Buchheit, K.-H.; Channez, P.; Mazzoni, L.; Mueller, T.; Vachier, I.; Naef, R. Bioorg. Med. Chem. 2001, 9, 1793–1805. doi:10.1016/S0968-0896(01)00077-3
Return to citation in text: [1] -
Kim, S.-H.; Shin, D.-S.; Oh, M.-N.; Chung, S.-C.; Lee, J.-S.; Oh, K.-B. Biosci., Biotechnol., Biochem. 2004, 68, 421–424. doi:10.1271/bbb.68.421
Return to citation in text: [1] -
Kartsev, V. G. Med. Chem. Res. 2004, 13, 325–336. doi:10.1007/s00044-004-0038-2
Return to citation in text: [1] -
Chaniyara, R.; Kapuriya, N.; Dong, H.; Lee, P.-C.; Suman, S.; Marvania, B.; Chou, T.-C.; Lee, T.-C.; Kakadiya, R.; Shah, A.; Su, T.-S. Bioorg. Med. Chem. 2011, 19, 275–286. doi:10.1016/j.bmc.2010.11.030
Return to citation in text: [1] -
Wright, C. W.; Marshall, S. J.; Russell, P. F.; Anderson, M. M.; Phillipson, J. D.; Kirby, G. C.; Warhurst, D. C.; Schiff, P. L., Jr. J. Nat. Prod. 2000, 63, 1638–1640. doi:10.1021/np000144r
Return to citation in text: [1] -
Gabrielsen, B.; Monath, T. P.; Huggins, J. W.; Kefauver, D. F.; Pettit, G. R.; Groszek, G.; Hollingshead, M.; Kirsi, J. J.; Shannon, W. M.; Schubert, E. M.; DaRe, J.; Ugarkar, B.; Ussery, M. A.; Phelan, M. J. J. Nat. Prod. 1992, 55, 1569–1581. doi:10.1021/np50089a003
Return to citation in text: [1] -
Peterson, K. E.; Cinelli, M. A.; Morrell, A. E.; Mehta, A.; Dexheimer, T. S.; Agama, K.; Antony, S.; Pommier, Y.; Cushman, M. J. Med. Chem. 2011, 54, 4937–4953. doi:10.1021/jm101338z
Return to citation in text: [1] -
Dömling, A.; Ugi, I. Angew. Chem., Int. Ed. 2000, 39, 3168–3210. doi:10.1002/1521-3773(20000915)39:18<3168::AID-ANIE3168>3.0.CO;2-U
Return to citation in text: [1] -
Dömling, A. Chem. Rev. 2006, 106, 17–89. doi:10.1021/cr0505728
Return to citation in text: [1] -
Zhu, J.; Bienaymé, H., Eds. Multicomponent Reactions; Wiley-VCH: Weinheim, Germany, 2005.
Return to citation in text: [1] -
Koopmanschap, G.; Ruijter, E.; Orru, R. V. A. Beilstein J. Org. Chem. 2014, 10, 544–598. doi:10.3762/bjoc.10.50
Return to citation in text: [1] -
Rotstein, B. H.; Zaretsky, S.; Rai, V.; Yudin, A. K. Chem. Rev. 2014, 114, 8323–8359. doi:10.1021/cr400615v
Return to citation in text: [1] -
Bienaymé, H.; Hulme, C.; Oddon, G.; Schmitt, P. Chem. – Eur. J. 2000, 6, 3321–3329. doi:10.1002/1521-3765(20000915)6:18<3321::AID-CHEM3321>3.0.CO;2-A
Return to citation in text: [1] -
Wess, J.; Urmann, M.; Sickenberger, B. Angew. Chem., Int. Ed. 2001, 40, 3341–3350. doi:10.1002/1521-3773(20010917)40:18<3341::AID-ANIE3341>3.0.CO;2-D
Return to citation in text: [1] -
Koszytkowska-Stawińska, M.; Buchowicz, W. Beilstein J. Org. Chem. 2014, 10, 1706–1732. doi:10.3762/bjoc.10.179
Return to citation in text: [1] -
Haji, M. Beilstein J. Org. Chem. 2016, 12, 1269–1301. doi:10.3762/bjoc.12.121
Return to citation in text: [1] -
Hulme, C.; Ayaz, M.; Martinez-Ariza, G.; Medda, F.; Shaw, A. Recent Advances in Multicomponent Reaction Chemistry: Applications in Small Molecule Drug Discovery. In Small Molecule Medicinal Chemistry: Strategies and Technologies; Czechtizky, W.; Hamley, P., Eds.; Wiley-VCH: Weinheim, Germany, 2015; pp 145–187. doi:10.1002/9781118771723.ch6
Return to citation in text: [1] -
Dömling, A.; Wang, W.; Wang, K. Chem. Rev. 2012, 112, 3083–3135. doi:10.1021/cr100233r
Return to citation in text: [1] -
Akritopoulou-Zanze, I. Curr. Opin. Chem. Biol. 2008, 12, 324–331. doi:10.1016/j.cbpa.2008.02.004
Return to citation in text: [1] -
Weber, L. Curr. Med. Chem. 2002, 9, 2085–2093. doi:10.2174/0929867023368719
Return to citation in text: [1] -
Ugi, I.; Steinbrückner, C. Angew. Chem. 1960, 72, 267–268. doi:10.1002/ange.19600720709
Return to citation in text: [1] -
Touré, B. B.; Hall, D. G. Chem. Rev. 2009, 109, 4439–4486. doi:10.1021/cr800296p
Return to citation in text: [1] -
Bauer, S. M.; Armstrong, R. W. J. Am. Chem. Soc. 1999, 121, 6355–6366. doi:10.1021/ja9811243
Return to citation in text: [1] -
Zhu, J. Eur. J. Org. Chem. 2003, 1133–1144. doi:10.1002/ejoc.200390167
Return to citation in text: [1] -
Lu, K.; Luo, T.; Xiang, Z.; You, Z.; Fathi, R.; Chen, J.; Yang, Z. J. Comb. Chem. 2005, 7, 958–967. doi:10.1021/cc050099b
Return to citation in text: [1] -
Lee, D.; Sello, J. K.; Schreiber, S. L. Org. Lett. 2000, 2, 709–712. doi:10.1021/ol005574n
Return to citation in text: [1] -
Ramazani, A.; Rezaei, A. Org. Lett. 2010, 12, 2852–2855. doi:10.1021/ol100931q
Return to citation in text: [1] -
Wang, W.; Ollio, S.; Herdtweck, E.; Dömling, A. J. Org. Chem. 2011, 76, 637–644. doi:10.1021/jo102058s
Return to citation in text: [1] -
Groebke, K.; Weber, L.; Mehlin, F. Synlett 1998, 661–663. doi:10.1055/s-1998-1721
Return to citation in text: [1] -
Blackburn, C.; Cuan, B.; Fleming, P.; Shiosaki, K.; Tsai, S. Tetrahedron Lett. 1998, 39, 3635–3638. doi:10.1016/S0040-4039(98)00653-4
Return to citation in text: [1] -
Bienaymé, H.; Bouzid, K. Angew. Chem., Int. Ed. 1998, 37, 2234–2237. doi:10.1002/(SICI)1521-3773(19980904)37:16<2234::AID-ANIE2234>3.0.CO;2-R
Return to citation in text: [1] -
Kruithof, A.; Ruijter, E.; Orru, R. V. A. Chem. – Asian J. 2015, 10, 508–520. doi:10.1002/asia.201403207
Return to citation in text: [1] -
Hulme, C.; Lee, Y.-S. Mol. Diversity 2008, 12, No. 1. doi:10.1007/s11030-008-9072-1
Return to citation in text: [1] -
Devi, N.; Rawal, R. K.; Singh, V. Tetrahedron 2015, 71, 183–232. doi:10.1016/j.tet.2014.10.032
Return to citation in text: [1] -
Shaaban, B.; Abdel-Wahab, B. F. Mol. Diversity 2016, 20, 233–254. doi:10.1007/s11030-015-9602-6
Return to citation in text: [1] -
Che, C.; Xiang, J.; Wang, G.-X.; Fathi, R.; Quan, J.-M.; Yang, Z. J. Comb. Chem. 2007, 9, 982–989. doi:10.1021/cc070058a
Return to citation in text: [1] -
Che, C.; Li, S.; Jiang, X.; Quan, J.; Lin, S.; Yang, Z. Org. Lett. 2010, 12, 4682–4685. doi:10.1021/ol1020477
Return to citation in text: [1] -
Che, C.; Li, S.; Yu, Z.; Li, F.; Xin, S.; Zhou, L.; Lin, S.; Yang, Z. ACS Comb. Sci. 2013, 15, 202–207. doi:10.1021/co400001h
Return to citation in text: [1] -
Che, C.; Yang, B.; Jiang, X.; Shao, T.; Yu, Z.; Tao, C.; Li, S.; Lin, S. J. Org. Chem. 2014, 79, 436–440. doi:10.1021/jo4024792
Return to citation in text: [1] -
Yang, B.; Tao, C.; Shao, T.; Gong, J.; Che, C. Beilstein J. Org. Chem. 2016, 12, 1487–1492. doi:10.3762/bjoc.12.145
Return to citation in text: [1] -
Xiang, J.; Yang, H.; Che, C.; Zou, H.; Yang, H.; Wei, Y.; Quan, J.; Zhang, H.; Yang, Z.; Lin, S. PLoS One 2009, 4, e4361. doi:10.1371/journal.pone.0004361
Return to citation in text: [1] -
Guchhait, S. K.; Chaudhary, V.; Madaan, C. Org. Biomol. Chem. 2012, 10, 9271–9277. doi:10.1039/c2ob26733k
Return to citation in text: [1] -
Chavignon, O.; Raihane, M.; Deplat, P.; Chabard, J. L.; Gueiffier, A.; Blache, Y.; Dauphin, G.; Teulade, J. C. Heterocycles 1995, 41, 2019–2026. doi:10.3987/COM-95-7126
Return to citation in text: [1] -
Miaskiewicz, S.; Gaillard, B.; Kern, N.; Weibel, J.-M.; Pale, P.; Blanc, A. Angew. Chem., Int. Ed. 2016, 55, 9088–9092. doi:10.1002/anie.201604329
Return to citation in text: [1] -
Mirabdolbaghi, R.; Dudding, T. Org. Lett. 2015, 17, 1930–1933. doi:10.1021/acs.orglett.5b00617
Return to citation in text: [1]
| 1. | Tantry, S. J.; Markad, S. D.; Shinde, V.; Bhat, J.; Balakrishnan, G.; Gupta, A. K.; Ambady, A.; Raichurkar, A.; Kedari, C.; Sharma, S.; Mudugal, N. V.; Narayan, A.; Kumar, C. N. N.; Nanduri, R.; Bharath, S.; Reddy, J.; Panduga, V.; Prabhakar, K. R.; Kandaswamy, K.; Saralaya, R.; Kaur, P.; Dinesh, N.; Guptha, S.; Rich, K.; Murray, D.; Plant, H.; Preston, M.; Ashton, H.; Plant, D.; Walsh, J.; Alcock, P.; Naylor, K.; Collier, M.; Whiteaker, J.; McLaughlin, R. E.; Mallya, M.; Panda, M.; Rudrapatna, S.; Ramachandran, V.; Shandil, R.; Sambandamurthy, V. K.; Mdluli, K.; Cooper, C. B.; Rubin, H.; Yano, T.; Iyer, P.; Narayanan, S.; Kavanagh, S.; Mukherjee, K.; Balasubramanian, V.; Hosagrahara, V. P.; Solapure, S.; Ravishankar, S.; Hammed, P. S. J. Med. Chem. 2017, 60, 1379–1399. doi:10.1021/acs.jmedchem.6b01358 |
| 2. | Shukla, N. M.; Salunke, D. B.; Yoo, E.; Mutz, C. A.; Balakrishna, R.; David, S. A. Bioorg. Med. Chem. 2012, 20, 5850–5863. doi:10.1016/j.bmc.2012.07.052 |
| 3. | Hamdouchi, C.; de Blas, J.; del Prado, M.; Gruber, J.; Heinz, B. A.; Vance, L. J. Med. Chem. 1999, 42, 50–59. doi:10.1021/jm9810405 |
| 4. | Frett, B.; McConnell, N.; Smith, C. C.; Wang, Y.; Shah, N. P.; Li, H.-y. Eur. J. Med. Chem. 2015, 94, 123–131. doi:10.1016/j.ejmech.2015.02.052 |
| 5. | Bode, M. L.; Gravestock, D.; Moleele, S. S.; van der Westhuyzen, C. W.; Pelly, S. C.; Steenkamp, P. A.; Hoppe, H. C.; Khan, T.; Nkabinde, L. A. Bioorg. Med. Chem. 2011, 19, 4227–4237. doi:10.1016/j.bmc.2011.05.062 |
| 9. | Kim, S.-H.; Shin, D.-S.; Oh, M.-N.; Chung, S.-C.; Lee, J.-S.; Oh, K.-B. Biosci., Biotechnol., Biochem. 2004, 68, 421–424. doi:10.1271/bbb.68.421 |
| 29. | Touré, B. B.; Hall, D. G. Chem. Rev. 2009, 109, 4439–4486. doi:10.1021/cr800296p |
| 30. | Bauer, S. M.; Armstrong, R. W. J. Am. Chem. Soc. 1999, 121, 6355–6366. doi:10.1021/ja9811243 |
| 8. | Charpiot, B.; Bitsch, F.; Buchheit, K.-H.; Channez, P.; Mazzoni, L.; Mueller, T.; Vachier, I.; Naef, R. Bioorg. Med. Chem. 2001, 9, 1793–1805. doi:10.1016/S0968-0896(01)00077-3 |
| 22. | Koszytkowska-Stawińska, M.; Buchowicz, W. Beilstein J. Org. Chem. 2014, 10, 1706–1732. doi:10.3762/bjoc.10.179 |
| 23. | Haji, M. Beilstein J. Org. Chem. 2016, 12, 1269–1301. doi:10.3762/bjoc.12.121 |
| 24. | Hulme, C.; Ayaz, M.; Martinez-Ariza, G.; Medda, F.; Shaw, A. Recent Advances in Multicomponent Reaction Chemistry: Applications in Small Molecule Drug Discovery. In Small Molecule Medicinal Chemistry: Strategies and Technologies; Czechtizky, W.; Hamley, P., Eds.; Wiley-VCH: Weinheim, Germany, 2015; pp 145–187. doi:10.1002/9781118771723.ch6 |
| 25. | Dömling, A.; Wang, W.; Wang, K. Chem. Rev. 2012, 112, 3083–3135. doi:10.1021/cr100233r |
| 26. | Akritopoulou-Zanze, I. Curr. Opin. Chem. Biol. 2008, 12, 324–331. doi:10.1016/j.cbpa.2008.02.004 |
| 27. | Weber, L. Curr. Med. Chem. 2002, 9, 2085–2093. doi:10.2174/0929867023368719 |
| 6. | Enguehard-Gueiffier, C.; Gueiffier, A. Mini-Rev. Med. Chem. 2007, 7, 888–899. doi:10.2174/138955707781662645 |
| 28. | Ugi, I.; Steinbrückner, C. Angew. Chem. 1960, 72, 267–268. doi:10.1002/ange.19600720709 |
| 14. | Peterson, K. E.; Cinelli, M. A.; Morrell, A. E.; Mehta, A.; Dexheimer, T. S.; Agama, K.; Antony, S.; Pommier, Y.; Cushman, M. J. Med. Chem. 2011, 54, 4937–4953. doi:10.1021/jm101338z |
| 20. | Bienaymé, H.; Hulme, C.; Oddon, G.; Schmitt, P. Chem. – Eur. J. 2000, 6, 3321–3329. doi:10.1002/1521-3765(20000915)6:18<3321::AID-CHEM3321>3.0.CO;2-A |
| 12. | Wright, C. W.; Marshall, S. J.; Russell, P. F.; Anderson, M. M.; Phillipson, J. D.; Kirby, G. C.; Warhurst, D. C.; Schiff, P. L., Jr. J. Nat. Prod. 2000, 63, 1638–1640. doi:10.1021/np000144r |
| 13. | Gabrielsen, B.; Monath, T. P.; Huggins, J. W.; Kefauver, D. F.; Pettit, G. R.; Groszek, G.; Hollingshead, M.; Kirsi, J. J.; Shannon, W. M.; Schubert, E. M.; DaRe, J.; Ugarkar, B.; Ussery, M. A.; Phelan, M. J. J. Nat. Prod. 1992, 55, 1569–1581. doi:10.1021/np50089a003 |
| 21. | Wess, J.; Urmann, M.; Sickenberger, B. Angew. Chem., Int. Ed. 2001, 40, 3341–3350. doi:10.1002/1521-3773(20010917)40:18<3341::AID-ANIE3341>3.0.CO;2-D |
| 11. | Chaniyara, R.; Kapuriya, N.; Dong, H.; Lee, P.-C.; Suman, S.; Marvania, B.; Chou, T.-C.; Lee, T.-C.; Kakadiya, R.; Shah, A.; Su, T.-S. Bioorg. Med. Chem. 2011, 19, 275–286. doi:10.1016/j.bmc.2010.11.030 |
| 10. | Kartsev, V. G. Med. Chem. Res. 2004, 13, 325–336. doi:10.1007/s00044-004-0038-2 |
| 15. | Dömling, A.; Ugi, I. Angew. Chem., Int. Ed. 2000, 39, 3168–3210. doi:10.1002/1521-3773(20000915)39:18<3168::AID-ANIE3168>3.0.CO;2-U |
| 16. | Dömling, A. Chem. Rev. 2006, 106, 17–89. doi:10.1021/cr0505728 |
| 17. | Zhu, J.; Bienaymé, H., Eds. Multicomponent Reactions; Wiley-VCH: Weinheim, Germany, 2005. |
| 18. | Koopmanschap, G.; Ruijter, E.; Orru, R. V. A. Beilstein J. Org. Chem. 2014, 10, 544–598. doi:10.3762/bjoc.10.50 |
| 19. | Rotstein, B. H.; Zaretsky, S.; Rai, V.; Yudin, A. K. Chem. Rev. 2014, 114, 8323–8359. doi:10.1021/cr400615v |
| 34. | Ramazani, A.; Rezaei, A. Org. Lett. 2010, 12, 2852–2855. doi:10.1021/ol100931q |
| 32. | Lu, K.; Luo, T.; Xiang, Z.; You, Z.; Fathi, R.; Chen, J.; Yang, Z. J. Comb. Chem. 2005, 7, 958–967. doi:10.1021/cc050099b |
| 33. | Lee, D.; Sello, J. K.; Schreiber, S. L. Org. Lett. 2000, 2, 709–712. doi:10.1021/ol005574n |
| 49. | Guchhait, S. K.; Chaudhary, V.; Madaan, C. Org. Biomol. Chem. 2012, 10, 9271–9277. doi:10.1039/c2ob26733k |
| 50. | Chavignon, O.; Raihane, M.; Deplat, P.; Chabard, J. L.; Gueiffier, A.; Blache, Y.; Dauphin, G.; Teulade, J. C. Heterocycles 1995, 41, 2019–2026. doi:10.3987/COM-95-7126 |
| 51. | Miaskiewicz, S.; Gaillard, B.; Kern, N.; Weibel, J.-M.; Pale, P.; Blanc, A. Angew. Chem., Int. Ed. 2016, 55, 9088–9092. doi:10.1002/anie.201604329 |
| 52. | Mirabdolbaghi, R.; Dudding, T. Org. Lett. 2015, 17, 1930–1933. doi:10.1021/acs.orglett.5b00617 |
| 43. | Che, C.; Xiang, J.; Wang, G.-X.; Fathi, R.; Quan, J.-M.; Yang, Z. J. Comb. Chem. 2007, 9, 982–989. doi:10.1021/cc070058a |
| 44. | Che, C.; Li, S.; Jiang, X.; Quan, J.; Lin, S.; Yang, Z. Org. Lett. 2010, 12, 4682–4685. doi:10.1021/ol1020477 |
| 45. | Che, C.; Li, S.; Yu, Z.; Li, F.; Xin, S.; Zhou, L.; Lin, S.; Yang, Z. ACS Comb. Sci. 2013, 15, 202–207. doi:10.1021/co400001h |
| 46. | Che, C.; Yang, B.; Jiang, X.; Shao, T.; Yu, Z.; Tao, C.; Li, S.; Lin, S. J. Org. Chem. 2014, 79, 436–440. doi:10.1021/jo4024792 |
| 47. | Yang, B.; Tao, C.; Shao, T.; Gong, J.; Che, C. Beilstein J. Org. Chem. 2016, 12, 1487–1492. doi:10.3762/bjoc.12.145 |
| 48. | Xiang, J.; Yang, H.; Che, C.; Zou, H.; Yang, H.; Wei, Y.; Quan, J.; Zhang, H.; Yang, Z.; Lin, S. PLoS One 2009, 4, e4361. doi:10.1371/journal.pone.0004361 |
| 39. | Kruithof, A.; Ruijter, E.; Orru, R. V. A. Chem. – Asian J. 2015, 10, 508–520. doi:10.1002/asia.201403207 |
| 40. | Hulme, C.; Lee, Y.-S. Mol. Diversity 2008, 12, No. 1. doi:10.1007/s11030-008-9072-1 |
| 41. | Devi, N.; Rawal, R. K.; Singh, V. Tetrahedron 2015, 71, 183–232. doi:10.1016/j.tet.2014.10.032 |
| 42. | Shaaban, B.; Abdel-Wahab, B. F. Mol. Diversity 2016, 20, 233–254. doi:10.1007/s11030-015-9602-6 |
| 35. | Wang, W.; Ollio, S.; Herdtweck, E.; Dömling, A. J. Org. Chem. 2011, 76, 637–644. doi:10.1021/jo102058s |
| 36. | Groebke, K.; Weber, L.; Mehlin, F. Synlett 1998, 661–663. doi:10.1055/s-1998-1721 |
| 37. | Blackburn, C.; Cuan, B.; Fleming, P.; Shiosaki, K.; Tsai, S. Tetrahedron Lett. 1998, 39, 3635–3638. doi:10.1016/S0040-4039(98)00653-4 |
| 38. | Bienaymé, H.; Bouzid, K. Angew. Chem., Int. Ed. 1998, 37, 2234–2237. doi:10.1002/(SICI)1521-3773(19980904)37:16<2234::AID-ANIE2234>3.0.CO;2-R |
© 2017 Shao et al.; licensee Beilstein-Institut.
This is an Open Access article under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
The license is subject to the Beilstein Journal of Organic Chemistry terms and conditions: (http://www.beilstein-journals.org/bjoc)