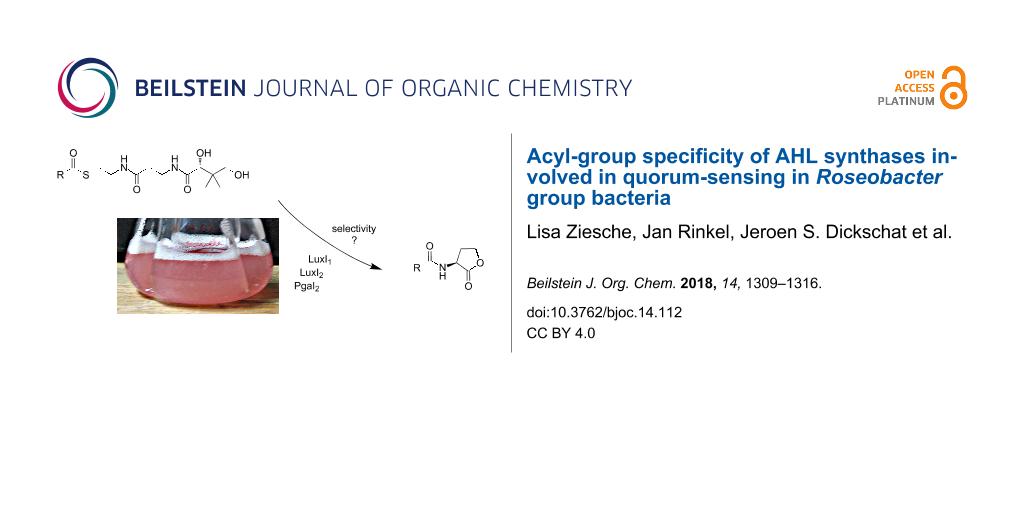
Abstract
N-Acylhomoserine lactones (AHLs) are important bacterial messengers, mediating different bacterial traits by quorum sensing in a cell-density dependent manner. AHLs are also produced by many bacteria of the marine Roseobacter group, which constitutes a large group within the marine microbiome. Often, specific mixtures of AHLs differing in chain length and oxidation status are produced by bacteria, but how the biosynthetic enzymes, LuxI homologs, are selecting the correct acyl precursors is largely unknown. We have analyzed the AHL production in Dinoroseobacter shibae and three Phaeobacter inhibens strains, revealing strain-specific mixtures. Although large differences were present between the species, the fatty acid profiles, the pool for the acyl precursors for AHL biosynthesis, were very similar. To test the acyl-chain selectivity, the three enzymes LuxI1 and LuxI2 from D. shibae DFL-12 as well as PgaI2 from P. inhibens DSM 17395 were heterologously expressed in E. coli and the enzymes isolated for in vitro incubation experiments. The enzymes readily accepted shortened acyl coenzyme A analogs, N-pantothenoylcysteamine thioesters of fatty acids (PCEs). Fifteen PCEs were synthesized, varying in chain length from C4 to C20, the degree of unsaturation and also including unusual acid esters, e.g., 2E,11Z-C18:2-PCE. The latter served as a precursor of the major AHL of D. shibae DFL-12 LuxI1, 2E,11Z-C18:2-homoserine lactone (HSL). Incubation experiments revealed that PgaI2 accepts all substrates except C4 and C20-PCE. Competition experiments demonstrated a preference of this enzyme for C10 and C12 PCEs. In contrast, the LuxI enzymes of D. shibae are more selective. While 2E,11Z-C18:2-PCE is preferentially accepted by LuxI1, all other PCEs were not, except for the shorter, saturated C10–C14-PCEs. The AHL synthase LuxI2 accepted only C14 PCE and 3-hydroxydecanoyl-PCE. In summary, chain-length selectivity in AHLs can vary between different AHL enzymes. Both, a broad substrate acceptance and tuned specificity occur in the investigated enzymes.
Graphical Abstract
Introduction
The Roseobacter group, a subgroup of the Rhodobacteraceae family, constitutes an important class of Gram-negative marine bacteria, occurring in many different habitats [1,2], in fresh water as well as on surfaces [3]. They can produce a variety of secondary metabolites, including antibiotics [4,5], volatile compounds [6,7], oligohydroxybutyrates [8] and a range of N-acylhomoserine lactones (AHLs) [8-10]. AHLs are quorum-sensing signaling compounds that are used for cell–cell communication to regulate several physiological traits regulated by cell density, the ‘quorum’ [11-16], in roseobacters, e.g., in the production of the antibiotic tropodithietic acid in Phaeobacter inhibens [15] and cell differentiation in Dinoroseobacter shibae [14]. Roseobacter group AHLs are characterized by saturated, unsaturated and sometimes oxygenated acyl chains ranging in length between C8 and C18 [8] with the exception of the aromatic p-coumaroylhomoserine lactone produced by Rugeria pomeroyi DSS-3 [17].
In a recent analysis we showed the AHL presence in 19 out of 24 Roseobacter group bacterial strains isolated from macroalgal surfaces [8]. The most widespread AHL was 7-tetradecenoylhomoserine lactone (7-C14:1-HSL), present in seven strains. No clear correlation between phylogeny and AHL occurrence was observed. In some strains only one AHL was detected, while others such as P. gallaeciensis BS107 produced eight different AHLs [8].
The biosynthesis of AHLs is mediated by the enzyme LuxI or its homologs, and often accompanied by a regulator protein, LuxR [18,19]. An ACP-bound fatty acid acyl group 1 is transferred onto the amino group of S-adenosylmethionine (SAM, 2) that is followed by substitution of the good leaving group 5’-deoxy-5’-thiomethyladenosine (5) of the thioester group, leading to homoserine lactone 4 formation (Scheme 1). Recently a LuxI-homolog, BjaI [20] preferring acyl-coenzyme A (CoA) substrates instead of the common ACP precursors, was characterized [21].
Scheme 1: Biosynthesis of AHLs by ACP-dependent LuxI type enzymes.
Scheme 1: Biosynthesis of AHLs by ACP-dependent LuxI type enzymes.
The LuxI-type enzymes are the most widespread and best understood AHL synthases. Four structures of LuxI-type enzymes have been published, covering both ACP and CoA-dependent structures with various chain lengths and different oxidation states of the acyl chain at C-3 [21-24]. A great diversity among AHL synthases is observed. The preference for unsubstituted, 3-oxo or 3-hydroxyacyl precursors is mediated by binding interactions inside the active site of AHL synthases [18,21]. Investigations on the chain-length selectivity of the AHL synthases are limited. BjaI can accept substrates ranging from isovaleryl-CoA, the native substrate, up to isononanoyl-CoA [21].
Three different LuxI homologs, LuxI1, LuxI2, and LuxI3, occur in Dinoroseobacter shibae DFL-12 [14]. Recently, we were surprised to find that the structures of AHLs synthesized by a LuxI homolog from D. shibae DFL-12 depended on the host in which the enzyme was expressed [10]. Expression of LuxI1 in E. coli led to a predominant formation of a 2:1:0.3 mixture of 9-C18:1-homoserine lactone (HSL), C16:0-HSL and C14:0-HSL, while the overexpression in its parent strain furnished the native product, 2E,11Z-C18:2-HSL, accompanied by 4% each of 9-C18:1-HSL and 2,9-C16:2-HSL. While the native substrates of LuxI2 and LuxI3 were not detected because of their low concentration, their overexpression in E. coli led to the production of a 6:1 mixture of 7Z-C14:1-HSL and C14:0-HSL for LuxI2 and no AHL formation for LuxI3 [10]. The differences between the AHLs in terms of chain length and degree of unsaturation prompted us to investigate the acyl-chain selectivity of LuxI-type enzymes in roseobacters. Does the enzyme have an inherent selectivity for a specific acyl-chain precursor or does it react unselectively with every acyl-precursor available? In the latter case the presence of the acyl precursors would determine the structure of the final AHL. To answer this question, the fatty acid composition of the native roseobacters was determined and compared to the AHLs produced. In addition, LuxI-type enzymes were heterologously expressed in E. coli and the purified recombinant enzymes were tested with different precursors to probe their selectivity. Both model organisms of the Roseobacter group, P. inhibens (formerly P. gallaeciensis [25]) DSM17395 and Dinoroseobacter shibae DFL-12, were investigated, together with closely related P. inhibens strains T5 and 2.10 [26], to investigate strain variability. Previously, the LuxI homolog PgaI1 from P. inhibens DSM 17395 has been characterized, producing R-3-OH-C10:0-HSL [15,27]. This strain produced additionally long chain AHLs such as C18:1-HSL [9] and contains a second AHL synthase, PgaI2 [28], probably involved in the biosynthesis of the long chain AHLs. Here we report on the characterization of PgaI2 from P. inhibens and of LuxI1 and LuxI2 from D. shibae by in vitro incubation experiments.
Results and Discussion
The AHL production of four Roseobacter group strains was analyzed by a GC/MS-based method using XAD-16 as adsorbent in marine broth, developed by us [8]. The bacteria were isolated from different habitats: D. shibae DFL-12 was isolated from the dinoflagellate Prorocentrum lima [29], P. inhibens T5 was collected from a water sample of the German Wadden Sea [30], P. inhibens DSM17395 was isolated from seawater of larval cultures of the scallop Pecten maximus in Spain [25] and P. inhibens 2.10 stemmed from the surface of the green macroalga Ulva australis in Australia [31].
The results showed that P. inhibens 2.10 and P. inhibens DSM17395 produce the same four AHLs, 3-OH-C10:0-HSL as major components and known from previous analyses of P. inhibens [9,32], C16:0-HSL, C16:1-HSL, and C18:1-HSL (Table 1). P. inhibens T5 additionally produced 3-oxo-C10:0-HSL and C12:2-HSL with unknown location of the double bonds. D. shibae DFL-12 released C14:1-HSL, 3-oxo-C14-HSL, C18:1-HSL, and C18:2-HSL, similar to previous results [10,16].
Table 1: Presence of different AHLs in four strains of Roseobacter group bacteria.a
| strain |
3-OH-
C10:0-HSL |
3-oxo-
C10:0-HSL |
C12:2-
HSL |
C14:1-
HSL |
3-oxo-
C14:0-HSL |
C16:0-
HSL |
C16:1-
HSL |
C18:1-
HSL |
C18:2-
HSL |
|---|---|---|---|---|---|---|---|---|---|
| P. inhibens 2.10 | 40.5 | 15.9 | 12.9 | 30.6 | |||||
| P. inhibens DSM17395 | 87.1 | 3.9 | 2.8 | 6.2 | |||||
| P. inhibens T5 | 37.3 | 5.0 | 16.2 | 4.9 | 10.0 | 26.6 | |||
| D. shibae DFL-12 | 5.8 | 13.6 | 9.3 | 71.3 | |||||
aRelative amounts of AHLs for each strain in %.
In addition, the fatty acid profile of the four strains was determined. Therefore, bacterial colonies from agar plates were added to 20 μL of methanolic trimethylsulfonium hydroxide (TMSH) solution. This procedure lyses the bacteria and concomitantly transfers any bound or free fatty acid into its methyl ester (FAME) [33]. The extracts were analyzed by GC/MS (Figure 1). Short and long FAMEs were detected, ranging from methyl octanoate to methyl icosanoate (Table 2). The three Phaeobacter strains produced identical fatty acids. We identified FAMEs with a C8:0, C12:0, C12:1, C16:0, C16:1, C17:0, C18:2, C18:0, C18:1, and C19:1 chain, the three last ones being the most abundant. D. shibae DFL-12 showed a similar fatty acid production, but no FAMEs with C8 or C12 chains were detected. Instead, 3-OH-C10:0-HSL, C14:0, and C20:0 FAMEs occurred in addition.
![[1860-5397-14-112-1]](/bjoc/content/figures/1860-5397-14-112-1.png?scale=2.0&max-width=1024&background=FFFFFF)
Figure 1: Total ion chromatograms of the FAME extracts of A) P. inhibens 2.10, B) P. inhibens DSM17395, C) P. inhibens T5 and D) D. shibae DFL-12. *other compounds.
Figure 1: Total ion chromatograms of the FAME extracts of A) P. inhibens 2.10, B) P. inhibens DSM17395, C) P....
Table 2: Presence of bound or free fatty acids in four different Roseobacter group strains detected as methyl esters.a
| P. inhibens | D. shibae | P. inhibens | D. shibae | |||||
| agar plate | liquid culture | |||||||
| 2.10 | DSM17395 | T5 | DFL-12 | 2.10 | DSM17395 | T5 | DFL 12 | |
| C8:0 | 5.0 | 1.8 | 1.0 | 0.1 | 0.9 | 0.2 | ||
| 3-OH-C10:0-HSL | 0.1 | 0.5 | ||||||
| C12:0 | 0.6 | 0.4 | 0.2 | 0.1 | 0.2 | 0.1 | ||
| 5Z-C12:1 | 8.2 | 2.9 | 2.2 | 2.3 | 0.5 | 2.2 | 0.9 | 0.8 |
| C14:0 | <0.1 | 0.1 | ||||||
| C16:0 | 3.8 | 7.5 | 3.0 | 0.6 | 4.3 | 2.6 | 2.2 | 0.3 |
| 9Z-C16:1 | 0.1 | <0.1 | <0.1 | 0.5 | 0.1 | 0.1 | <0.1 | 0.2 |
| C17:0 | 0.4 | 0.2 | 0.2 | <0.1 | 0.1 | 0.1 | 0.1 | <0.1 |
| C18:0 | 3.9 | 8.6 | 4.6 | 2.6 | 2.1 | 1.8 | 1.8 | 3.4 |
| 11Z-C18:1 | 60.4 | 66.7 | 83.1 | 91.9 | 61.0 | 67.8 | 67.3 | 92.4 |
| 11E-C18:1 | 0.3 | 0.1 | 0.1 | <0.1 | 0.1 | 0.1 | 0.1 | 0.1 |
| 13Z-C18:1 | 0.4 | <0.1 | <0.1 | 0.1 | 1.2 | 1.0 | 1.0 | 0.3 |
| 2E,11Z-C18:2 | 1.2 | 0.3 | 1.2 | <0.1 | 3.2 | 1.8 | 2.9 | <0.1 |
| 11Me-12E-C19:1 | 15.7 | 11.4 | 4.3 | 0.6 | 27.2 | 21.5 | 23.4 | 1.0 |
| 13Z-C20:1 | 1.2 | 0.9 | ||||||
aRelative peak areas of FAMEs for each strain in %.
The location of the double bond of the major acids was determined by dimethyl disulfide (DMDS) derivatization [32,34]. The fragment ions at m/z 145 and 161 of the DMDS-derivative and the secondary fragments obtained by loss of the methyl ester group (m/z 129) located the position of the double bond in C12:1-FAME at C-5. Similarly, 9-C16:1 (m/z 145, 185, 217) and 11-C18:1-FAMEs (m/z 145, 213, 245) were assigned. The three Phaeobacter strains showed also a small peak with identical mass spectrum compared to 11-C18:1-FAME eluting slightly earlier than the major compound, indicating minor amounts of 11E-C18:1-FAME next to the major 11Z-C18:1-FAME. DMDS adducts derived from E-configured double bonds elute slightly earlier than their Z-configured counterparts on apolar GC phases [34]. All four strains additionally contained 13-C18:1-FAME (m/z 61, 117, 241 and 273) in small amounts. The mass spectrum of C19:1-FAME differed from that of methyl nonadecenoate, but was identical to that of methyl 11-methyl-12-octadecenoate [29,35,36], as was that of its DMDS derivative (m/z 131, 241, 273, see Figures S1 and S2 in the Supporting Information File 1). Similarly, 13-C20:1 was identified in D. shibae DFL-12. Small amounts of a DMDS adduct of C18:2 were detected that added only one equivalent of DMDS. This reactivity is observed when a double bond is conjugated with a carbonyl group [37,38]. The ion at m/z 145 located one double bond at C-11, while the ions at m/z 211 and 243 revealed another unsaturation in the alkyl chain towards the carboxy terminus. These data indicate this FAME to be 2,11-C18:1, the parent acid of the major D. shibae AHL, 2E,11Z-C18:2-HSL [10]. The analysis performed with bacteria grown in liquid medium led to comparable results, indicating that the fatty acid composition does not depend on the culture method.
By comparing the fatty acid profiles and AHL production no direct correlation between fatty acids and AHLs can be observed. The major acid C18:1 is only reflected by a minor component in the AHL profile of the four strains. Small amounts of 2,11-C18:2 occur in all strains, only the D. shibae strain uses this acid as precursor for its major 2,11-C18:2-HSL. In contrast, the precursor acid 3-OH-C10:0-HSL is produced by D. shibae, but not present in the profiles of P. inhibens, which produces large amounts of 3-OH-C10:0-HSL. Furthermore, the prominent acid 11Me-12-C18:1 is not used for AHL formation. Acids used for production of minor AHLs such as C14:1 or C12:2 were not detected.
These results show that the fatty acid pool and AHL formation are indeed uncoupled. Although the fatty acid composition of the investigated strains is very similar, the AHL production differs largely. The complete absence or presence of only minor amounts of precursor acids of AHLs such as 2,11-C18:2 or 3-OH-C10:0-HSL might indicate that they are available only for AHL biosynthesis, but are not used for other physiological purposes. Such acids may be immediately transformed after their biosynthesis into an AHL, or are stored in a form not cleavable by the TMSH method used. These precursor acids may also originate from fatty acid degradation, a pathway that proceeds via free coenzyme A intermediates and not via acyl carrier protein-bound substrates like in the fatty acid biosynthesis.
These results led to the question whether the acyl-chain selectivity is an inherent property of the AHL synthase itself or whether this is determined by other factors, e.g., precursor availability. Therefore, LuxI-type synthases from D. shibae (LuxI1, LuxI2) and from P. inhibens DSM17395 (PgaI2) were cloned and expressed in E. coli to allow in vitro experiments with suitable acyl precursors to probe AHL formation. After protein purification of the AHL synthases and incubation with the precursors S-adenosyl methionine (SAM) and different acyl derivatives (free fatty acids, SNAC esters, PCEs) the AHL production was determined using GC/MS [9,10,32]. Coenzyme A or abbreviated ACP analogs, N-pantothenoylcysteamine thioesters of fatty acids (PCEs) were synthesized (Scheme 2) to serve as substrate substitutes for the native precursors.
Scheme 2: Synthesis of N-pantothenoylcysteamine thioesters (PCEs) for feeding experiments with AHL synthases.
Scheme 2: Synthesis of N-pantothenoylcysteamine thioesters (PCEs) for feeding experiments with AHL synthases.
Calcium pantothenate (6) was protected with acetone forming acid 7 that was transformed with cysteamine into the protected thiol 8 [39]. Steglich esterification [40] with different free acids led to nine saturated PCEs 10a–i, four monounsaturated acids (11a–d), 3-OH-C10:0-HSL PCE (12), and 2E,11Z-C18:2-PCE (13) after deprotection with acetic acid [41]. Although compounds 10–13 can be further purified by HPLC, the crude products proved to be pure enough for the next experiments.
The incubation experiments were performed with the three recombinant AHL synthases, SAM, and the different precursors 10–13. The AHL-synthase PgaI2 of P. inhibens showed a higher activity compared to the two D. shibae synthases. It accepted all substrates, including unsaturated ones, with the exclusion of the very short C4:0 and very long C20:0-PCEs.
The AHL-synthase LuxI2 was able to produce C14:0-HSL and 3-OH-C10-HSL in low concentration from the respective precursors. It is likely responsible for the formation of C14:0-HSL and 3-oxo-C14:0-HSL in D. shibae DFL-12. The AHL synthase LuxI1 used five precursors to synthesize C8:0, C10:0, C12:0 and C14:0-HSL in low amounts, while 2E,11Z-C18:2-HSL, its native product, is formed in high concentration.
To further evaluate the selectivity of the promiscuous enzyme PgaI2 from P. inhibens, competition experiments were performed. Targeting the optimal chain length of the fully saturated substrates first, a mixture with equal molar concentrations of the substrates 10a–h was offered to the recombinant protein. GC/MS analysis of the resulting extract (Figure 2A) revealed a distribution of AHL products around the chain length of C10 and C12, which were shown to be the most prominent products. In a second experiment with substrates 10a–i, 11a–c, and 12 also unsaturated substrates and the hydroxylated precursor were tested (Figure 2B). It turned out that the same distribution of the saturated AHLs as for the first experiment was observed with none of the additional substrates showing a significantly higher conversion. These results point to a very flexible active site of the investigated AHL synthase PgaI2, which converts a variety of substrates. The highest conversion efficiency in the competition experiments was found for the saturated substrates 10c and 10d with lower abundance of any AHL products deviating from this chain length. It should be noted that the amount of added SAM was not sufficient to convert all substrates, so the product spectrum likely reflects different enzyme kinetics for the PCE substrates. In contrast, in the single-substrate incubation experiments (Table 3) an excess of SAM was used, and this may have led to the formation even of products that are disfavored in the competition experiments.
![[1860-5397-14-112-2]](/bjoc/content/figures/1860-5397-14-112-2.png?scale=2.5&max-width=1024&background=FFFFFF)
Figure 2: Total ion chromatograms of the extracts from competition experiments using recombinant PgaI2 from P. inhibens with SAM and a mixture of equally concentrated substrates A) 10a–h (saturated chains C4–C18) and B) 10a–i, 11a–c and 12. Prominent contaminants are indicated by asterisks.
Figure 2: Total ion chromatograms of the extracts from competition experiments using recombinant PgaI2 from P...
Table 3: Results of incubation experiments of single precursors 10–13 with E. coli constructs with recombinant AHL synthases PgaI2, LuxI1 and LuxI2 from different Roseobacter group bacteria.a
| Precursor | AHL |
P. inhibens
PgaI2 |
D. shibae
LuxI1 |
D. shibae
LuxI2 |
| 10a | C4:0 | – | – | – |
| 10b | C6:0 | x | – | – |
| 10c | C8:0 | xx | x | – |
| 10d | C10:0 | xx | x | – |
| 10e | C12:0 | xx | x | – |
| 10f | C14:0 | xx | x | x |
| 10g | C16:0 | x | – | – |
| 10h | C18:0 | x | – | – |
| 10i | C20:0 | – | – | – |
| 12 | 3-OH-C10:0 | xx | – | x |
| 11a | 7Z-C14:1 | xx | – | – |
| 11b | 9Z-C16:1 | xx | – | – |
| 11c | 9Z-C18:1 | xx | – | – |
| 11d | 11Z-C18:1 | xx | – | – |
| 13 | 2E,11Z-C18:2 | xx | xx | – |
axx: high production, x: low production, –: no production.
Conclusion
The results showed that the enzymes exhibit varying substrate plasticity. While the P. inhibens synthase PgaI2 accepted most precursors, the best performance was observed with the saturated substrates harboring C10 or C12 chain lengths. In P. inhibens this enzyme is most likely responsible for the biosynthesis of long-chain AHLs. In contrast, D. shibae synthase LuxI1 showed a high selectivity for 2E,11Z-C18:2-HSL and did not even accept similar substrates such as 11c or 11d. Interestingly, considerably shorter saturated substrates, e.g., 10e, are accepted. The D. shibae synthase LuxI2 synthase was even more selective. It seems likely that other factors than AHL synthase substrate specificity influence the observed formation of only certain AHLs by these wild-type enzymes. These factors might include selectivity found in enzymes activating or transporting acids to AHL synthases, or interact with the LuxI enzyme, either directly or indirectly. The combination of the different selectivity levels may eventually lead to the specific mixtures observed in the different AHL producing bacterial strains.
Supporting Information
| Supporting Information File 1: Experimental, mass spectra, SDS page and NMR spectra. | ||
| Format: PDF | Size: 781.8 KB | Download |
References
-
Giebel, H.-A.; Kalhoefer, D.; Lemke, A.; Thole, S.; Gahl-Janssen, R.; Simon, M.; Brinkhoff, T. ISME J. 2011, 5, 8–19. doi:10.1038/ismej.2010.87
Return to citation in text: [1] -
Selje, N.; Simon, M.; Brinkhoff, T. Nature 2004, 427, 445–448. doi:10.1038/nature02272
Return to citation in text: [1] -
Freese, H. M.; Methner, A.; Overmann, J. Front. Microbiol. 2017, 8, No. 1659. doi:10.3389/fmicb.2017.01659
Return to citation in text: [1] -
Brock, N. L.; Nikolay, A.; Dickschat, J. S. Chem. Commun. 2014, 50, 5487–5489. doi:10.1039/c4cc01924e
Return to citation in text: [1] -
Seyedsayamdost, M. R.; Case, R. J.; Kolter, R.; Clardy, J. Nat. Chem. 2011, 3, 331–335. doi:10.1038/nchem.1002
Return to citation in text: [1] -
Thiel, V.; Brinkhoff, T.; Dickschat, J. S.; Wickel, S.; Grunenberg, J.; Wagner-Döbler, I.; Simon, M.; Schulz, S. Org. Biomol. Chem. 2010, 8, 234–246. doi:10.1039/B909133E
Return to citation in text: [1] -
Brock, N. L.; Menke, M.; Klapschinski, T. A.; Dickschat, J. S. Org. Biomol. Chem. 2014, 12, 4318–4323. doi:10.1039/c4ob00719k
Return to citation in text: [1] -
Ziesche, L.; Bruns, H.; Dogs, M.; Wolter, L.; Mann, F.; Wagner-Döbler, I.; Brinkhoff, T.; Schulz, S. ChemBioChem 2015, 16, 2094–2107. doi:10.1002/cbic.201500189
Return to citation in text: [1] [2] [3] [4] [5] [6] -
Wagner-Döbler, I.; Thiel, V.; Eberl, L.; Allgaier, M.; Bodor, A.; Meyer, S.; Ebner, S.; Hennig, A.; Pukall, R.; Schulz, S. ChemBioChem 2005, 6, 2195–2206. doi:10.1002/cbic.200500189
Return to citation in text: [1] [2] [3] [4] -
Neumann, A.; Patzelt, D.; Wagner-Döbler, I.; Schulz, S. ChemBioChem 2013, 14, 2355–2361. doi:10.1002/cbic.201300424
Return to citation in text: [1] [2] [3] [4] [5] [6] -
Buchan, A.; Mitchell, A.; Cude, W. N.; Campagna, S. Acyl-Homoserine Lactone-Based Quorum Sensing in Members of the Marine Bacterial Roseobacter Clade: Complex Cell-to-Cell Communication Controls Multiple Physiologies. In Stress and Environmental Regulation of Gene Expression and Adaptation in Bacteria; de Bruijn, F. J., Ed.; Wiley Blackwell, 2016; pp 225–233. doi:10.1002/9781119004813.ch19
Return to citation in text: [1] -
Zan, J.; Liu, Y.; Fuqua, C.; Hill, R. T. Int. J. Mol. Sci. 2014, 15, 654–669. doi:10.3390/ijms15010654
Return to citation in text: [1] -
Schulz, S.; Hötling, S. Nat. Prod. Rep. 2015, 32, 1042–1066. doi:10.1039/C5NP00006H
Return to citation in text: [1] -
Patzelt, D.; Wang, H.; Buchholz, I.; Rohde, M.; Gröbe, L.; Pradella, S.; Neumann, A.; Schulz, S.; Heyber, S.; Münch, K.; Münch, R.; Jahn, D.; Wagner-Döbler, I.; Tomasch, J. ISME J. 2013, 7, 2274–2286. doi:10.1038/ismej.2013.107
Return to citation in text: [1] [2] [3] -
Berger, M.; Neumann, A.; Schulz, S.; Simon, M.; Brinkhoff, T. J. Bacteriol. 2011, 193, 6576–6585. doi:10.1128/JB.05818-11
Return to citation in text: [1] [2] [3] -
Wang, H.; Ziesche, L.; Frank, O.; Michael, V.; Martin, M.; Petersen, J.; Schulz, S.; Wagner-Döbler, I.; Tomasch, J. BMC Genomics 2014, 15, No. 130. doi:10.1186/1471-2164-15-130
Return to citation in text: [1] [2] -
Schaefer, A. L.; Greenberg, E. P.; Oliver, C. M.; Oda, Y.; Huang, J. J.; Bittan-Banin, G.; Peres, C. M.; Schmidt, S.; Juhaszova, K.; Sufrin, J. R.; Harwood, C. S. Nature 2008, 454, 595–599. doi:10.1038/nature07088
Return to citation in text: [1] -
Churchill, M. E. A.; Herman, J. P. Acyl-homoserine lactone biosynthesis: structure and mechanism. In Chemical communication among bacteria; Winans, S. C.; Bassler, B. L., Eds.; ASM Press: Washington, DC, 2008; pp 275–289. doi:10.1128/9781555815578.ch17
Return to citation in text: [1] [2] -
Dickschat, J. S. Nat. Prod. Rep. 2010, 27, 343–369. doi:10.1039/b804469b
Return to citation in text: [1] -
Dong, S.-H.; Frane, N. D.; Christensen, Q. H.; Greenberg, E. P.; Nagarajan, R.; Nair, S. K. Proc. Natl. Acad. Sci. U. S. A. 2017, 114, 9092–9097. doi:10.1073/pnas.1705400114
Return to citation in text: [1] -
Lindemann, A.; Pessi, G.; Schaefer, A. L.; Mattmann, M. E.; Christensen, Q. H.; Kessler, A.; Hennecke, H.; Blackwell, H. E.; Greenberg, E. P.; Harwood, C. S. Proc. Natl. Acad. Sci. U. S. A. 2011, 108, 16765–16770. doi:10.1073/pnas.1114125108
Return to citation in text: [1] [2] [3] [4] -
Chung, J.; Goo, E.; Yu, S.; Choi, O.; Lee, J.; Kim, J.; Kim, H.; Igarashi, J.; Suga, H.; Moon, J. S.; Hwang, I.; Rhee, S. Proc. Natl. Acad. Sci. U. S. A. 2011, 108, 12089–12094. doi:10.1073/pnas.1103165108
Return to citation in text: [1] -
Gould, T. A.; Schweizer, H. P.; Churchill, M. E. A. Mol. Microbiol. 2004, 53, 1135–1146. doi:10.1111/j.1365-2958.2004.04211.x
Return to citation in text: [1] -
Watson, W. T.; Minogue, T. D.; Val, D. V.; Beck von Bodman, S.; Churchill, M. E. A. Mol. Cell 2002, 9, 685–694. doi:10.1016/S1097-2765(02)00480-X
Return to citation in text: [1] -
Buddruhs, N.; Pradella, S.; Göker, M.; Päuker, O.; Pukall, R.; Spröer, C.; Schumann, P.; Petersen, J.; Brinkhoff, T. Int. J. Syst. Evol. Microbiol. 2013, 63, 4340–4349. doi:10.1099/ijs.0.053900-0
Return to citation in text: [1] [2] -
Dogs, M.; Voget, S.; Teshima, H.; Petersen, J.; Davenport, K.; Dalingault, H.; Chen, A.; Pati, A.; Ivanova, N.; Goodwin, L. A.; Chain, P.; Detter, J. C.; Standfest, S.; Rohde, M.; Gronow, S.; Kyrpides, N. C.; Woyke, T.; Simon, M.; Klenk, H.-P.; Göker, M.; Brinkhoff, T. Stand. Genomic Sci. 2013, 9, 334–350. doi:10.4056/sigs.4448212
Return to citation in text: [1] -
Bruhn, J. B.; Nielsen, K. F.; Hjelm, M.; Hansen, M.; Bresciani, J.; Schulz, S.; Gram, L. Appl. Environ. Microbiol. 2005, 71, 7263–7270. doi:10.1128/AEM.71.11.7263-7270.2005
Return to citation in text: [1] -
Cude, W. N.; Buchan, A. Front. Microbiol. 2013, 4, No. 336. doi:10.3389/fmicb.2013.00336
Return to citation in text: [1] -
Biebl, H.; Allgaier, M.; Tindall, B. J.; Koblizek, M.; Lünsdorf, H.; Pukall, R.; Wagner-Döbler, I. Int. J. Syst. Evol. Microbiol. 2005, 55, 1089–1096. doi:10.1099/ijs.0.63511-0
Return to citation in text: [1] [2] -
Martens, T.; Heidorn, T.; Pukall, R.; Simon, M.; Tindall, B. J.; Brinkhoff, T. Int. J. Syst. Evol. Microbiol. 2006, 56, 1293–1304. doi:10.1099/ijs.0.63724-0
Return to citation in text: [1] -
Thole, S.; Kalhoefer, D.; Voget, S.; Berger, M.; Engelhardt, T.; Liesegang, H.; Wollherr, A.; Kjelleberg, S.; Daniel, R.; Simon, M.; Thomas, T.; Brinkhoff, T. ISME J. 2012, 6, 2229–2244. doi:10.1038/ismej.2012.62
Return to citation in text: [1] -
Thiel, V.; Kunze, B.; Verma, P.; Wagner-Döbler, I.; Schulz, S. ChemBioChem 2009, 10, 1861–1868. doi:10.1002/cbic.200900126
Return to citation in text: [1] [2] [3] -
Müller, K.-D.; Husmann, H.; Nalik, H. P.; Schomburg, G. Chromatographia 1990, 30, 245–248. doi:10.1007/BF02319701
Return to citation in text: [1] -
Scribe, P.; Guezennec, J.; Dagaut, J.; Pepe, C.; Saliot, A. Anal. Chem. 1988, 60, 928–931. doi:10.1021/ac00160a019
Return to citation in text: [1] [2] -
Rontani, J.-F.; Christodoulou, S.; Koblizek, M. Lipids 2005, 40, 97–108. doi:10.1007/s11745-005-1364-6
Return to citation in text: [1] -
Kerger, B. D.; Nichols, P. D.; Antworth, C. P.; Sand, W.; Bock, E.; Cox, J. C.; Langworthy, T. A.; White, D. C. FEMS Microbiol. Ecol. 1986, 38, 67–77. doi:10.1111/j.1574-6968.1986.tb01954.x
Return to citation in text: [1] -
Vincenti, M.; Guglielmetti, G.; Cassani, G.; Tonini, C. Anal. Chem. 1987, 59, 694–699. doi:10.1021/ac00132a003
Return to citation in text: [1] -
Bierl-Leonhardt, B. A.; DeVilbiss, E. D. Anal. Chem. 1981, 53, 936–938. doi:10.1021/ac00229a055
Return to citation in text: [1] -
Gaudelli, N. M.; Townsend, C. A. J. Org. Chem. 2013, 78, 6412–6426. doi:10.1021/jo4007893
Return to citation in text: [1] -
Neises, B.; Steglich, W. Angew. Chem., Int. Ed. Engl. 1978, 17, 522–524. doi:10.1002/anie.197805221
Return to citation in text: [1] -
Agarwal, V.; Diethelm, S.; Ray, L.; Garg, N.; Awakawa, T.; Dorrestein, P. C.; Moore, B. S. Org. Lett. 2015, 17, 4452–4455. doi:10.1021/acs.orglett.5b02113
Return to citation in text: [1]
| 8. | Ziesche, L.; Bruns, H.; Dogs, M.; Wolter, L.; Mann, F.; Wagner-Döbler, I.; Brinkhoff, T.; Schulz, S. ChemBioChem 2015, 16, 2094–2107. doi:10.1002/cbic.201500189 |
| 29. | Biebl, H.; Allgaier, M.; Tindall, B. J.; Koblizek, M.; Lünsdorf, H.; Pukall, R.; Wagner-Döbler, I. Int. J. Syst. Evol. Microbiol. 2005, 55, 1089–1096. doi:10.1099/ijs.0.63511-0 |
| 30. | Martens, T.; Heidorn, T.; Pukall, R.; Simon, M.; Tindall, B. J.; Brinkhoff, T. Int. J. Syst. Evol. Microbiol. 2006, 56, 1293–1304. doi:10.1099/ijs.0.63724-0 |
| 1. | Giebel, H.-A.; Kalhoefer, D.; Lemke, A.; Thole, S.; Gahl-Janssen, R.; Simon, M.; Brinkhoff, T. ISME J. 2011, 5, 8–19. doi:10.1038/ismej.2010.87 |
| 2. | Selje, N.; Simon, M.; Brinkhoff, T. Nature 2004, 427, 445–448. doi:10.1038/nature02272 |
| 8. | Ziesche, L.; Bruns, H.; Dogs, M.; Wolter, L.; Mann, F.; Wagner-Döbler, I.; Brinkhoff, T.; Schulz, S. ChemBioChem 2015, 16, 2094–2107. doi:10.1002/cbic.201500189 |
| 20. | Dong, S.-H.; Frane, N. D.; Christensen, Q. H.; Greenberg, E. P.; Nagarajan, R.; Nair, S. K. Proc. Natl. Acad. Sci. U. S. A. 2017, 114, 9092–9097. doi:10.1073/pnas.1705400114 |
| 34. | Scribe, P.; Guezennec, J.; Dagaut, J.; Pepe, C.; Saliot, A. Anal. Chem. 1988, 60, 928–931. doi:10.1021/ac00160a019 |
| 6. | Thiel, V.; Brinkhoff, T.; Dickschat, J. S.; Wickel, S.; Grunenberg, J.; Wagner-Döbler, I.; Simon, M.; Schulz, S. Org. Biomol. Chem. 2010, 8, 234–246. doi:10.1039/B909133E |
| 7. | Brock, N. L.; Menke, M.; Klapschinski, T. A.; Dickschat, J. S. Org. Biomol. Chem. 2014, 12, 4318–4323. doi:10.1039/c4ob00719k |
| 21. | Lindemann, A.; Pessi, G.; Schaefer, A. L.; Mattmann, M. E.; Christensen, Q. H.; Kessler, A.; Hennecke, H.; Blackwell, H. E.; Greenberg, E. P.; Harwood, C. S. Proc. Natl. Acad. Sci. U. S. A. 2011, 108, 16765–16770. doi:10.1073/pnas.1114125108 |
| 29. | Biebl, H.; Allgaier, M.; Tindall, B. J.; Koblizek, M.; Lünsdorf, H.; Pukall, R.; Wagner-Döbler, I. Int. J. Syst. Evol. Microbiol. 2005, 55, 1089–1096. doi:10.1099/ijs.0.63511-0 |
| 35. | Rontani, J.-F.; Christodoulou, S.; Koblizek, M. Lipids 2005, 40, 97–108. doi:10.1007/s11745-005-1364-6 |
| 36. | Kerger, B. D.; Nichols, P. D.; Antworth, C. P.; Sand, W.; Bock, E.; Cox, J. C.; Langworthy, T. A.; White, D. C. FEMS Microbiol. Ecol. 1986, 38, 67–77. doi:10.1111/j.1574-6968.1986.tb01954.x |
| 4. | Brock, N. L.; Nikolay, A.; Dickschat, J. S. Chem. Commun. 2014, 50, 5487–5489. doi:10.1039/c4cc01924e |
| 5. | Seyedsayamdost, M. R.; Case, R. J.; Kolter, R.; Clardy, J. Nat. Chem. 2011, 3, 331–335. doi:10.1038/nchem.1002 |
| 8. | Ziesche, L.; Bruns, H.; Dogs, M.; Wolter, L.; Mann, F.; Wagner-Döbler, I.; Brinkhoff, T.; Schulz, S. ChemBioChem 2015, 16, 2094–2107. doi:10.1002/cbic.201500189 |
| 33. | Müller, K.-D.; Husmann, H.; Nalik, H. P.; Schomburg, G. Chromatographia 1990, 30, 245–248. doi:10.1007/BF02319701 |
| 3. | Freese, H. M.; Methner, A.; Overmann, J. Front. Microbiol. 2017, 8, No. 1659. doi:10.3389/fmicb.2017.01659 |
| 18. | Churchill, M. E. A.; Herman, J. P. Acyl-homoserine lactone biosynthesis: structure and mechanism. In Chemical communication among bacteria; Winans, S. C.; Bassler, B. L., Eds.; ASM Press: Washington, DC, 2008; pp 275–289. doi:10.1128/9781555815578.ch17 |
| 19. | Dickschat, J. S. Nat. Prod. Rep. 2010, 27, 343–369. doi:10.1039/b804469b |
| 32. | Thiel, V.; Kunze, B.; Verma, P.; Wagner-Döbler, I.; Schulz, S. ChemBioChem 2009, 10, 1861–1868. doi:10.1002/cbic.200900126 |
| 34. | Scribe, P.; Guezennec, J.; Dagaut, J.; Pepe, C.; Saliot, A. Anal. Chem. 1988, 60, 928–931. doi:10.1021/ac00160a019 |
| 14. | Patzelt, D.; Wang, H.; Buchholz, I.; Rohde, M.; Gröbe, L.; Pradella, S.; Neumann, A.; Schulz, S.; Heyber, S.; Münch, K.; Münch, R.; Jahn, D.; Wagner-Döbler, I.; Tomasch, J. ISME J. 2013, 7, 2274–2286. doi:10.1038/ismej.2013.107 |
| 17. | Schaefer, A. L.; Greenberg, E. P.; Oliver, C. M.; Oda, Y.; Huang, J. J.; Bittan-Banin, G.; Peres, C. M.; Schmidt, S.; Juhaszova, K.; Sufrin, J. R.; Harwood, C. S. Nature 2008, 454, 595–599. doi:10.1038/nature07088 |
| 9. | Wagner-Döbler, I.; Thiel, V.; Eberl, L.; Allgaier, M.; Bodor, A.; Meyer, S.; Ebner, S.; Hennig, A.; Pukall, R.; Schulz, S. ChemBioChem 2005, 6, 2195–2206. doi:10.1002/cbic.200500189 |
| 32. | Thiel, V.; Kunze, B.; Verma, P.; Wagner-Döbler, I.; Schulz, S. ChemBioChem 2009, 10, 1861–1868. doi:10.1002/cbic.200900126 |
| 15. | Berger, M.; Neumann, A.; Schulz, S.; Simon, M.; Brinkhoff, T. J. Bacteriol. 2011, 193, 6576–6585. doi:10.1128/JB.05818-11 |
| 8. | Ziesche, L.; Bruns, H.; Dogs, M.; Wolter, L.; Mann, F.; Wagner-Döbler, I.; Brinkhoff, T.; Schulz, S. ChemBioChem 2015, 16, 2094–2107. doi:10.1002/cbic.201500189 |
| 10. | Neumann, A.; Patzelt, D.; Wagner-Döbler, I.; Schulz, S. ChemBioChem 2013, 14, 2355–2361. doi:10.1002/cbic.201300424 |
| 16. | Wang, H.; Ziesche, L.; Frank, O.; Michael, V.; Martin, M.; Petersen, J.; Schulz, S.; Wagner-Döbler, I.; Tomasch, J. BMC Genomics 2014, 15, No. 130. doi:10.1186/1471-2164-15-130 |
| 11. | Buchan, A.; Mitchell, A.; Cude, W. N.; Campagna, S. Acyl-Homoserine Lactone-Based Quorum Sensing in Members of the Marine Bacterial Roseobacter Clade: Complex Cell-to-Cell Communication Controls Multiple Physiologies. In Stress and Environmental Regulation of Gene Expression and Adaptation in Bacteria; de Bruijn, F. J., Ed.; Wiley Blackwell, 2016; pp 225–233. doi:10.1002/9781119004813.ch19 |
| 12. | Zan, J.; Liu, Y.; Fuqua, C.; Hill, R. T. Int. J. Mol. Sci. 2014, 15, 654–669. doi:10.3390/ijms15010654 |
| 13. | Schulz, S.; Hötling, S. Nat. Prod. Rep. 2015, 32, 1042–1066. doi:10.1039/C5NP00006H |
| 14. | Patzelt, D.; Wang, H.; Buchholz, I.; Rohde, M.; Gröbe, L.; Pradella, S.; Neumann, A.; Schulz, S.; Heyber, S.; Münch, K.; Münch, R.; Jahn, D.; Wagner-Döbler, I.; Tomasch, J. ISME J. 2013, 7, 2274–2286. doi:10.1038/ismej.2013.107 |
| 15. | Berger, M.; Neumann, A.; Schulz, S.; Simon, M.; Brinkhoff, T. J. Bacteriol. 2011, 193, 6576–6585. doi:10.1128/JB.05818-11 |
| 16. | Wang, H.; Ziesche, L.; Frank, O.; Michael, V.; Martin, M.; Petersen, J.; Schulz, S.; Wagner-Döbler, I.; Tomasch, J. BMC Genomics 2014, 15, No. 130. doi:10.1186/1471-2164-15-130 |
| 25. | Buddruhs, N.; Pradella, S.; Göker, M.; Päuker, O.; Pukall, R.; Spröer, C.; Schumann, P.; Petersen, J.; Brinkhoff, T. Int. J. Syst. Evol. Microbiol. 2013, 63, 4340–4349. doi:10.1099/ijs.0.053900-0 |
| 8. | Ziesche, L.; Bruns, H.; Dogs, M.; Wolter, L.; Mann, F.; Wagner-Döbler, I.; Brinkhoff, T.; Schulz, S. ChemBioChem 2015, 16, 2094–2107. doi:10.1002/cbic.201500189 |
| 9. | Wagner-Döbler, I.; Thiel, V.; Eberl, L.; Allgaier, M.; Bodor, A.; Meyer, S.; Ebner, S.; Hennig, A.; Pukall, R.; Schulz, S. ChemBioChem 2005, 6, 2195–2206. doi:10.1002/cbic.200500189 |
| 10. | Neumann, A.; Patzelt, D.; Wagner-Döbler, I.; Schulz, S. ChemBioChem 2013, 14, 2355–2361. doi:10.1002/cbic.201300424 |
| 8. | Ziesche, L.; Bruns, H.; Dogs, M.; Wolter, L.; Mann, F.; Wagner-Döbler, I.; Brinkhoff, T.; Schulz, S. ChemBioChem 2015, 16, 2094–2107. doi:10.1002/cbic.201500189 |
| 31. | Thole, S.; Kalhoefer, D.; Voget, S.; Berger, M.; Engelhardt, T.; Liesegang, H.; Wollherr, A.; Kjelleberg, S.; Daniel, R.; Simon, M.; Thomas, T.; Brinkhoff, T. ISME J. 2012, 6, 2229–2244. doi:10.1038/ismej.2012.62 |
| 21. | Lindemann, A.; Pessi, G.; Schaefer, A. L.; Mattmann, M. E.; Christensen, Q. H.; Kessler, A.; Hennecke, H.; Blackwell, H. E.; Greenberg, E. P.; Harwood, C. S. Proc. Natl. Acad. Sci. U. S. A. 2011, 108, 16765–16770. doi:10.1073/pnas.1114125108 |
| 21. | Lindemann, A.; Pessi, G.; Schaefer, A. L.; Mattmann, M. E.; Christensen, Q. H.; Kessler, A.; Hennecke, H.; Blackwell, H. E.; Greenberg, E. P.; Harwood, C. S. Proc. Natl. Acad. Sci. U. S. A. 2011, 108, 16765–16770. doi:10.1073/pnas.1114125108 |
| 22. | Chung, J.; Goo, E.; Yu, S.; Choi, O.; Lee, J.; Kim, J.; Kim, H.; Igarashi, J.; Suga, H.; Moon, J. S.; Hwang, I.; Rhee, S. Proc. Natl. Acad. Sci. U. S. A. 2011, 108, 12089–12094. doi:10.1073/pnas.1103165108 |
| 23. | Gould, T. A.; Schweizer, H. P.; Churchill, M. E. A. Mol. Microbiol. 2004, 53, 1135–1146. doi:10.1111/j.1365-2958.2004.04211.x |
| 24. | Watson, W. T.; Minogue, T. D.; Val, D. V.; Beck von Bodman, S.; Churchill, M. E. A. Mol. Cell 2002, 9, 685–694. doi:10.1016/S1097-2765(02)00480-X |
| 37. | Vincenti, M.; Guglielmetti, G.; Cassani, G.; Tonini, C. Anal. Chem. 1987, 59, 694–699. doi:10.1021/ac00132a003 |
| 38. | Bierl-Leonhardt, B. A.; DeVilbiss, E. D. Anal. Chem. 1981, 53, 936–938. doi:10.1021/ac00229a055 |
| 18. | Churchill, M. E. A.; Herman, J. P. Acyl-homoserine lactone biosynthesis: structure and mechanism. In Chemical communication among bacteria; Winans, S. C.; Bassler, B. L., Eds.; ASM Press: Washington, DC, 2008; pp 275–289. doi:10.1128/9781555815578.ch17 |
| 21. | Lindemann, A.; Pessi, G.; Schaefer, A. L.; Mattmann, M. E.; Christensen, Q. H.; Kessler, A.; Hennecke, H.; Blackwell, H. E.; Greenberg, E. P.; Harwood, C. S. Proc. Natl. Acad. Sci. U. S. A. 2011, 108, 16765–16770. doi:10.1073/pnas.1114125108 |
| 10. | Neumann, A.; Patzelt, D.; Wagner-Döbler, I.; Schulz, S. ChemBioChem 2013, 14, 2355–2361. doi:10.1002/cbic.201300424 |
| 9. | Wagner-Döbler, I.; Thiel, V.; Eberl, L.; Allgaier, M.; Bodor, A.; Meyer, S.; Ebner, S.; Hennig, A.; Pukall, R.; Schulz, S. ChemBioChem 2005, 6, 2195–2206. doi:10.1002/cbic.200500189 |
| 10. | Neumann, A.; Patzelt, D.; Wagner-Döbler, I.; Schulz, S. ChemBioChem 2013, 14, 2355–2361. doi:10.1002/cbic.201300424 |
| 32. | Thiel, V.; Kunze, B.; Verma, P.; Wagner-Döbler, I.; Schulz, S. ChemBioChem 2009, 10, 1861–1868. doi:10.1002/cbic.200900126 |
| 9. | Wagner-Döbler, I.; Thiel, V.; Eberl, L.; Allgaier, M.; Bodor, A.; Meyer, S.; Ebner, S.; Hennig, A.; Pukall, R.; Schulz, S. ChemBioChem 2005, 6, 2195–2206. doi:10.1002/cbic.200500189 |
| 28. | Cude, W. N.; Buchan, A. Front. Microbiol. 2013, 4, No. 336. doi:10.3389/fmicb.2013.00336 |
| 26. | Dogs, M.; Voget, S.; Teshima, H.; Petersen, J.; Davenport, K.; Dalingault, H.; Chen, A.; Pati, A.; Ivanova, N.; Goodwin, L. A.; Chain, P.; Detter, J. C.; Standfest, S.; Rohde, M.; Gronow, S.; Kyrpides, N. C.; Woyke, T.; Simon, M.; Klenk, H.-P.; Göker, M.; Brinkhoff, T. Stand. Genomic Sci. 2013, 9, 334–350. doi:10.4056/sigs.4448212 |
| 15. | Berger, M.; Neumann, A.; Schulz, S.; Simon, M.; Brinkhoff, T. J. Bacteriol. 2011, 193, 6576–6585. doi:10.1128/JB.05818-11 |
| 27. | Bruhn, J. B.; Nielsen, K. F.; Hjelm, M.; Hansen, M.; Bresciani, J.; Schulz, S.; Gram, L. Appl. Environ. Microbiol. 2005, 71, 7263–7270. doi:10.1128/AEM.71.11.7263-7270.2005 |
| 10. | Neumann, A.; Patzelt, D.; Wagner-Döbler, I.; Schulz, S. ChemBioChem 2013, 14, 2355–2361. doi:10.1002/cbic.201300424 |
| 41. | Agarwal, V.; Diethelm, S.; Ray, L.; Garg, N.; Awakawa, T.; Dorrestein, P. C.; Moore, B. S. Org. Lett. 2015, 17, 4452–4455. doi:10.1021/acs.orglett.5b02113 |
| 25. | Buddruhs, N.; Pradella, S.; Göker, M.; Päuker, O.; Pukall, R.; Spröer, C.; Schumann, P.; Petersen, J.; Brinkhoff, T. Int. J. Syst. Evol. Microbiol. 2013, 63, 4340–4349. doi:10.1099/ijs.0.053900-0 |
| 14. | Patzelt, D.; Wang, H.; Buchholz, I.; Rohde, M.; Gröbe, L.; Pradella, S.; Neumann, A.; Schulz, S.; Heyber, S.; Münch, K.; Münch, R.; Jahn, D.; Wagner-Döbler, I.; Tomasch, J. ISME J. 2013, 7, 2274–2286. doi:10.1038/ismej.2013.107 |
| 39. | Gaudelli, N. M.; Townsend, C. A. J. Org. Chem. 2013, 78, 6412–6426. doi:10.1021/jo4007893 |
| 10. | Neumann, A.; Patzelt, D.; Wagner-Döbler, I.; Schulz, S. ChemBioChem 2013, 14, 2355–2361. doi:10.1002/cbic.201300424 |
| 40. | Neises, B.; Steglich, W. Angew. Chem., Int. Ed. Engl. 1978, 17, 522–524. doi:10.1002/anie.197805221 |
© 2018 Ziesche et al.; licensee Beilstein-Institut.
This is an Open Access article under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
The license is subject to the Beilstein Journal of Organic Chemistry terms and conditions: (https://www.beilstein-journals.org/bjoc)