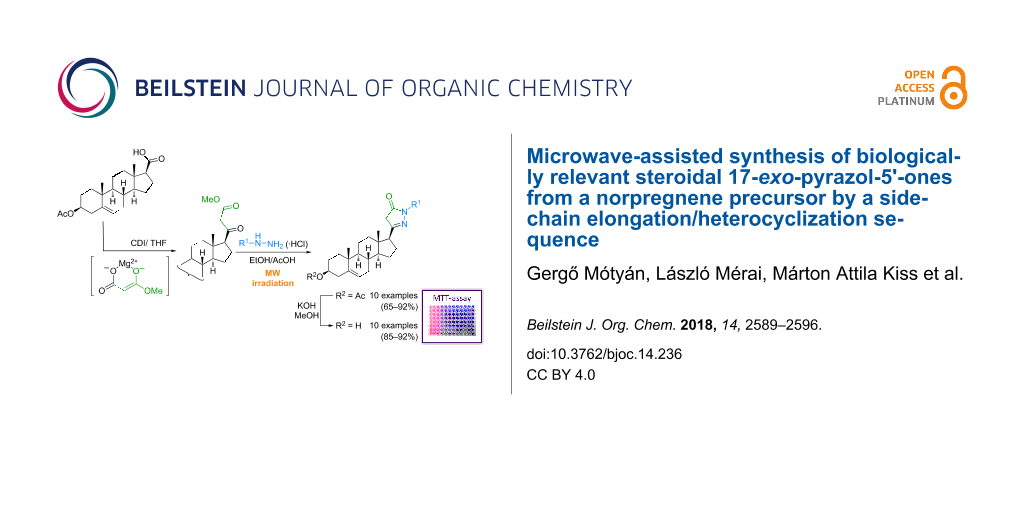
Abstract
Multistep syntheses of novel 17β-pyrazol-5'-ones in the Δ5-androstane series were efficiently carried out from pregnenolone acetate. A steroidal 17-carboxylic acid was first synthesized as a norpregnene precursor by the bromoform reaction and subsequent acetylation. Its CDI-activated acylimidazole derivative was then converted to a β-ketoester containing a two carbon atom-elongated side chain than that of the starting material. A Knorr cyclization of the bifunctional 1,3-dicarbonyl compound with hydrazine and its monosubstituted derivatives in AcOH under microwave heating conditions led to the regioselective formation of 17-exo-heterocycles in good to excellent yields. The suppression of an acid-catalyzed thermal decarboxylation of the β-ketoester and thus a significant improvement in the yield of the desired heterocyclic products could be achieved by the preliminary liberation of the arylhydrazines from their hydrochloride salts in EtOH in the presence of NaOAc. The reaction rates were found to depend on the electronic character of the substituent present in the phenylhydrazine applied. The antiproliferative activities of the structurally related steroidal pyrazol-5'-ones and their deacetylated analogs were screened on three human adherent breast cancer cell lines (MCF7, T47D and MDA-MB-231): the microculture tetrazolium assay revealed that some of the presented derivatives exerted cell growth inhibitory effects on some of these cell lines comparable to those of the reference compound, cisplatin.
Graphical Abstract
Introduction
17-exo-Heterocyclic androstanes with five or six-membered heterocyclic rings connected directly to C-17 of the sterane core represent a remarkable subclass of semisynthetic sex hormone analogs in consequence of their dual pharmacological importance. A number of these derivatives display an inhibitory effect on 17α-hydroxylase-C17,20-lyase (P45017α) enzyme, which, acting as an important regulator, plays an essential role in the endogenous production of androgen hormones, and therefore, in the development of prostate cancer [1]. According to extensive structure–activity relationship and docking studies, a potent steroidal inhibitor should possess certain structural characteristics for efficient P45017α inhibition [1-3], such as (i) a five or six-membered non-bulky heterocycle containing O, N or S atoms attached to position C-17 of the sterane skeleton with the lone electron pairs capable of coordinating with the heme iron at the active site; (ii) a N atom at either position 3′ or 4′ relative to the atom through which the heterocyclic ring is connected to the sterane framework; (iii) a hydroxy or keto group at C-3 and (iv) the presence of a C16–C17 double bond, which facilitates the inhibitory effect but is not an essential requirement. Some 17-heterocycle-substituted androstanes, even those that lack the structural features described above, are also known to display cytotoxic effects on diverse cancer cells by inducing a disturbance in the cell cycle and promoting apoptosis without affecting normal cellular proliferation [4,5]. In these latter cases, detailed structural criteria are still not available owing to little information about the mode of action of these derivatives.
Amongst steroidal 17-exo-heterocycles, those containing a pyrazole heteroaromatic ring are of special relevance with respect to the above-mentioned bioactivities [6-9]. Interestingly, so far only a few examples of compounds in which a pyrazolone moiety is attached to the sterane skeleton have been published, but not to C-17 [10]. Nevertheless, this heterocyclic scaffold is also an important building block in many clinically relevant drugs, agrochemicals, dyes, pigments and chelating agents [11-13], and therefore its introduction to C-17 of androstanes may be of interest from a pharmacological point of view.
The first and probably most frequently used method for the synthesis of pyrazolones is based on the Knorr condensation of β-ketoesters with substituted or unsubstituted hydrazines. However, these reactions often suffer from certain disadvantages, such as the necessity of high temperature or prolonged reaction time and low yields of the desired products [14]. A rate acceleration and yield improvement could be achieved in some cases by performing the reactions under microwave (MW) conditions [15-17]. Especially with respect to pyrazol-5-ones, keto–enol tautomerism can be challenging and is of special importance in biological systems, chemical reactivity, and molecular recognition [18]. The tautomeric equilibrium in solution is strongly influenced by the substitution pattern of the heterocyclic ring, the polarity and protic nature of the solvent and, although to a lesser extent, by the temperature and concentration [19].
In view of the above-mentioned reasons, the main objective of the present study was to design and carry out the preparation of novel steroidal heterocycles containing pyrazol-5-one moieties attached to C-17 of the sterane core, using both conventional heating and MW irradiation. The reaction conditions were optimized in order to improve the yields of the desired products and the influences of substituents of the hydrazine reagents investigated. The in vitro antiproliferative activities of the synthesized compounds were also determined on three human breast malignant cell lines (MCF7, T47D, and MDA-MB-231) by means of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay [20]. Furthermore, the most promising molecules were additionally tested on mouse fibroblasts (NIH-3T3) in order to obtain preliminary results concerning the cancer selectivity of the selected agents.
Results and Discussion
Synthetic studies
The steroidal β-ketoester precursor 4, suitable for the attempted heterocyclization reaction with hydrazines was synthesized from commercially available pregnenolone acetate (1) via a multistep sequence (Scheme 1). First compound 1 was converted to the 17β-carboxylic acid 2b by the bromoform reaction and subsequent acetylation according to well-known literature procedures [21-23]. After the activation of 2b with 1,1′-carbonyldiimidazole (CDI) as coupling reagent in THF, the magnesium enolate of malonic acid half ester, prepared in situ from potassium methyl malonate, MgCl2 and triethylamine in acetonitrile, was added [24,25]. The acylation of magnesium methyl malonate by the preformed imidazole 3 led to the desired bifunctional starting material 4 in good yield (79%).
Scheme 1: Multistep synthesis of steroidal β-ketoesters 4 and 4' from pregnenolone acetate (1) and pregnadienolone acetate (1').
Scheme 1: Multistep synthesis of steroidal β-ketoesters 4 and 4' from pregnenolone acetate (1) and pregnadien...
Analogously, β-ketoester 4' could be obtained from pregnadienolone acetate 1' through a Δ5,16-carboxylic acid intermediate [23,26] under identical conditions albeit in disappointing low yield (33%) which is presumably caused by the decreased propensity of the conjugated carbonyl compound to react with the magnesium enolate. Although the presence of a C16–C17 double bond as in 4' is assumed to be beneficial for a P45017α-inhibitory effect, further transformations of this compound were abandoned because of the insufficient yield and its potential tendency to react with monosubstituted hydrazines – not only with its β-ketoester moiety to give 17-exo-heterocycles, but also with its enone part to provide ring D-condensed pyrazolines [27].
Therefore, the ring-closure reactions of 4 with unsubstituted and monosubstituted hydrazines as binucleophilic reagents were investigated next. First, compound 4 was reacted with hydrazine hydrate (5a) in refluxing ethanol containing a catalytic amount of AcOH (Scheme 2).
Scheme 2: Cyclization of compound 4 with hydrazine hydrate (5a), phenylhydrazine (5b) and methylhydrazine (5c).
Scheme 2: Cyclization of compound 4 with hydrazine hydrate (5a), phenylhydrazine (5b) and methylhydrazine (5c...
TLC monitoring of the reaction indicated full conversion of 4 within 4 h reaction time to afford a fairly polar product insoluble or only slightly soluble in all commonly used NMR solvents. However, a subsequent derivatization with acetic anhydride in pyridine to afford 8, allowed its structure verification indirectly. This derivatization did not only improve the solubility of the compound, but also eliminated the possibility of prototropic tautomerism through acetylation of both the amino and hydroxy groups present in the heterocyclic ring in 6a. The 1H and 13C NMR spectroscopical analysis confirmed the structure of 8, which at the same time supported the formation of 6a in a yield of 84% during the previous reaction step. While N-unsubstituted pyrazolones such as 6a theoretically may have eight tautomeric forms [28], their N(1')-substituted derivatives, lacking a functionality on pyrazole C-4, can only exist in three equilibrating tautomers (OH, CH and NH) [18,29]. Therefore, further heterocyclizations of 4 were performed with monosubstituted hydrazine derivatives. The reaction with phenylhydrazine (5b) was completed within 7 h in refluxing EtOH in the presence of an acid catalyst. A reduction of the reaction time to 3 h could be achieved by changing the solvent to AcOH affording the desired product 6b in high yield (86%, Scheme 2). On the other hand, the reaction of 4 with methylhydrazine (5c) required a longer reaction time (5 h) in refluxing AcOH to furnish the purified product (6c) in a diminished yield (61%). This may be attributed to the weaker nucleophilic character of the external N compared to the internal one in 5c [27], in contrast to phenylhydrazine (5b), making the first condensation step more difficult. The regioselectivity of the reactions with monosubstituted hydrazines is controlled by the higher reactivity of the ketone moiety over the ester towards nucleophiles, and the least hindered terminal nitrogen atom of the binucleophiles. Both reactions were repeated in AcOH under microwave conditions at 120 °C furnishing products 6b and 6c within shorter time (20 min and 40 min), however, without a substantial improvement in the yields.
Since commercially available arylhydrazine hydrochlorides were intended to be applied for further transformations, the reactions of 4 with 5b·HCl using an equivalent amount of NaOAc in AcOH, both under conventional heating and MW irradiation, were also carried out. Although similar reaction conditions have been described in the literature for the reactions of methyl acetoacetate with arylhydrazine hydrochlorides to afford the corresponding pyrazol-5-ones in 50–70% yields within 5–10 h [30], no full conversion of 4 could be achieved. Even after refluxing the mixture for 24 h the desired product 6b was obtained only in low yield (≈30%). At the same time, the use of the MW-assisted method at 120 °C shortened the conversion time significantly (80 min). However, in addition to 6b (≈50%), the conventional, and even more the MW-promoted transformations led to a considerable amount (20–25%) of pregnenolone acetate (1) as a byproduct. The latter is thought to be produced by an acid-catalyzed thermal decarboxylation of 4 during the relatively long heating period. Further the unwanted side reaction was attributed to the poor solubility of 5b·HCl in AcOH resulting in a heterogeneous reaction mixture even at high temperature and therefore a slow liberation of 5b from its salt upon the addition of NaOAc. In order to circumvent this issue, the reaction was repeated with in situ-liberated phenylhydrazine (5b) by dissolving 5b·HCl and NaOAc in a small amount of EtOH under mild heating for 10 min. To this solution, containing NaCl as the only solid substance, the steroidal dicarbonyl compound 4 dissolved in AcOH was added. The so obtained mixture was then irradiated in a MW reactor at 120 °C for 20 min. Under these conditions, the corresponding product 6b was obtained in 85% yield after chromatographic purification without notable formation of 1.
After optimizing the conditions for the MW-assisted synthesis of 6b from 4 with 5b·HCl, analogous heterocyclization reactions were carried out with different substituted phenylhydrazine hydrochlorides 5d–j. All reactions furnished the corresponding 17-exo-heterocycles 6d–j in good to excellent yields (83–92%, Table 1).
Table 1: Synthesis of steroidal N(1')-aryl-substituted pyrazol-5'-onesa.
|
|
|||||
| entry | arylhydrazine hydrochloride | R | pyrazol-5-one | irradiation time (min) | yieldb (%) |
|---|---|---|---|---|---|
| 1 | 5d | Me | 6d | 10 | 86 |
| 2 | 5e | iPr | 6e | 10 | 90 |
| 3 | 5f | t-Bu | 6f | 10 | 87 |
| 4 | 5g | OMe | 6g | 5 | 92 |
| 5 | 5h | F | 6h | 30 | 85 |
| 6 | 5i | Cl | 6i | 30 | 83 |
| 7 | 5j | Br | 6j | 30 | 85 |
aReagents and conditions: arylhydrazine hydrochloride 5d–j·HCl (1.2 equiv), NaOAc (1.2 equiv), EtOH (10 mL), 40 °C, 10 min, then compound 4 (1.0 mmol) in AcOH (20 mL), MW, 120 °C, 5–30 min. bAfter purification by column chromatography.
The electronic features of the substituents on the aromatic ring of 5 had a notable influence on the reaction rates. The ring-closure of 4 with 5d–g containing electron-donating groups (Table 1, entries 1–4) took place within shorter reaction times (5–10 min) compared to phenylhydrazine (5b). On the other hand the presence of electron-withdrawing halogens on the aromatic ring of 5 (Table 1, entries 5–7) lengthened the reaction time to 30 min. This observation can be explained by the enhanced or diminished nucleophilic character of the nitrogen atoms caused by the different groups on the aromatic ring in 5, favoring or hampering their nucleophilic attack during the intermolecular heterocyclization. In order to enlarge the compound library available for pharmacological studies, the 3β-OH analogs 7a–j of the primary products 6a–j were also synthesized through simple alkaline deacetylation (Scheme 2, Table 1).
The structures of all synthesized compounds were characterized by 1H and 13C NMR spectroscopy supplemented by IR and MS measurements. The dependence of the tautomeric equilibrium on the polarity of the applied solvent was observed during the NMR experiments. For example, in the 1H NMR spectrum of compound 7f recorded immediately after dissolution in CDCl3, the pyrazolone heterocyclic ring mainly exists as the CH-tautomeric form (Figure 1). The characteristic signals of the 4'-methylene hydrogens appear at 3.36 and 3.46 ppm in the 1H NMR spectrum. However, in the more polar solvent DMSO-d6, the equilibrium mixture of the NH- and OH-tautomers of 7f predominates. As the activation barrier between these latter isomers is low and their interconversion is rapid on the NMR timescale, only average signals can be observed for the 4'-H and NH/OH protons [31].
![[1860-5397-14-236-1]](/bjoc/content/figures/1860-5397-14-236-1.png?scale=2.0&max-width=1024&background=FFFFFF)
Figure 1: 1H NMR spectra of compound 7f in CDCl3 (top; # solvent signal) and in DMSO-d6 (bottom; # solvent signal).
Figure 1: 1H NMR spectra of compound 7f in CDCl3 (top; # solvent signal) and in DMSO-d6 (bottom; # solvent si...
Pharmacological studies
The pharmacological activities of the synthesized 17-exo-heterocycles 6a–j and 7a–j were studied in vitro. Their antiproliferative effects were determined by means of the MTT assay [20] on a panel of adherent breast cancer cell lines (MCF7, T47D and MDA-MB-231) after treatment for 72 h (Table 2). All compounds were initially screened at 10 and 30 μM concentrations and for those compounds that elicited growth inhibition of at least 50% at 10 μM and around 90% at 30 μM, IC50 values were calculated by using a set of dilutions (Figure S1 in Supporting Information File 1). The viability assays were repeated with the most potent agents 6h, 7f, 7i and 7j against NIH-3T3 mouse fibroblasts to generate preliminary data concerning the cancer selectivity. Since the molecular site of action of the tested compounds is not known, cisplatin (a clinically used DNA-binding anticancer agent) was applied as the reference. The results indicated that the 3β-acetates 6a–j exhibited weak or only modest antiproliferative activities, typically eliciting 20–30% growth inhibition at 10 μM. However, the p-fluoro derivative 6h, proved to be more effective on MCF7 and T47D cells. The deacetylated analogs 7a–j generally inhibited cancer cell growth more efficiently. The character of the substituent on the aromatic ring was crucial for the antiproliferative actions on the different cell lines. A tert-butyl group at the para position (7f) appeared favorable against T47D cells resulting in an IC50 value lower than that of the reference cisplatin. While the chloro and bromo-substituted derivatives exerted more pronounced effects on MCF7 cells in their 3-OH form (7i and 7j), the fluoro compound 7h proved to be less active than its ester 6h. Although the performed viability assay on animal fibroblasts cannot be considered as an appropriate toxicological evaluation, the obtained results are promising and point to a less growth inhibiting action of the compounds on fibroblasts than on cancer cells. All of them displayed less than 20% growth inhibition at 10 μM and their calculated IC50 values proved to be higher than those obtained on the malignant cell lines.
Table 2: Antiproliferative effects of the synthesized compounds on gynecological cell lines and NIH-3T3 fibroblasts.a
| compound | conc. (μM) |
inhibition of cell proliferation (%) ± SEM
[calculated IC50 (μM)] |
|||
|---|---|---|---|---|---|
| MCF7 | MDA-MB-231 | T47D | NIH-3T3 | ||
| 6a | 10 | 25.7 ± 1.1 | – | – | n.d. |
| 30 | 70.0 ± 1.0 | 30.9 ± 2.0 | 58.9 ± 1.0 | ||
| 6b | 10 | – | – | 38.2 ± 1.5 | n.d. |
| 30 | 76.8 ± 0.3 | 72.1 ± 0.4 | 90.9 ± 0.6 | ||
| 6c | 10 | – | – | – | n.d. |
| 30 | 52.2 ± 2.8 | 26.8 ± 1.2 | 83.8 ± 1,3 | ||
| 6d | 10 | – | – | – | n.d. |
| 30 | 35.8 ± 1.1 | – | 51.4 ± 1.7 | ||
| 6e | 10 | – | – | – | n.d. |
| 30 | – | – | 41.7 ± 2.6 | ||
| 6f | 10 | – | – | – | n.d. |
| 30 | 73.8 ± 0.6 | 29.3 ± 3.0 | 72.2 ± 1.7 | ||
| 6g | 10 | – | – | – | n.d. |
| 30 | 47.4 ± 1.5 | – | 60.7 ± 2.2 | ||
| 6h | 10 | 65.1 ± 0.9 | – | 74.9 ± 2.4 | – |
| 30 | 95.1 ± 1.1 | 90.8 ± 0.5 | 84.2 ± 1.6 | 88.3 ± 0.8 | |
| [9.0] | [6.5] | [18.1] | |||
| 6i | 10 | – | – | 22.2 ± 1.3 | n.d. |
| 30 | 87.8 ± 0.7 | 29.2 ± 1.2 | 78.0 ± 0.6 | ||
| 6j | 10 | 31.0 ± 1.7 | – | 31.1 ± 2.2 | n.d. |
| 30 | 86.4 ± 1.4 | 27.0 ± 1.0 | 77.5 ± 1.1 | ||
| 7a | 10 | – | – | 57.0 ± 0.9 | n.d. |
| 30 | – | – | 65.8 ± 1.3 | ||
| 7b | 10 | – | – | – | n.d. |
| 30 | 85.8 ± 1.0 | 40.6 ± 1.7 | 89.6 ± 0.7 | ||
| 7c | 10 | – | – | – | n.d. |
| 30 | 23.1 ± 2.6 | 57.9 ± 2.0 | – | ||
| 7d | 10 | – | – | 31.1 ± 1.7 | n.d. |
| 30 | 27.9 ± 2.7 | – | 60.3 ± 1.9 | ||
| 7e | 10 | – | – | – | n.d. |
| 30 | 66.3 ± 1.5 | – | 95.8 ± 0.2 | ||
| 7f | 10 | 21.1 ± 2.8 | 31.3 ± 1.3 | 83.1 ± 1.3 | – |
| 30 | 95.8 ± 0.3 | 89.6 ± 0.8 | 87.5 ± 0.5 | 84.6 ± 1.3 | |
| [4.3] | [18.2] | ||||
| 7g | 10 | – | – | 22.3 ± 1.3 | n.d. |
| 30 | 40.9 ± 1.6 | 39.6 ± 1.7 | 55.7 ± 2.2 | ||
| 7h | 10 | – | – | – | n.d. |
| 30 | 91.3 ± 0.8 | 96.8 ± 0.2 | 85.2 ± 1.1 | ||
| 7i | 10 | 66.8 ± 1.7 | 31.3 ± 1.5 | 32.8 ± 1.3 | – |
| 30 | 96.3 ± 0.2 | 96.8 ± 0.2 | 87.8 ± 0.5 | 94.0 ± 0.5 | |
| [6.9] | [15.3] | ||||
| 7j | 10 | 58.6 ± 1.2 | 42.0 ± 0.8 | 48.3 ± 1.9 | – |
| 30 | 89.5 ± 1.1 | 96.1 ± 0.2 | 85.5 ± 1.2 | 91.1 ± 1.0 | |
| [8.1] | [17.3] | ||||
| cisplatin | 10 | 66.9 ± 1.8 | – | 51.0 ± 2.0 | 94.2 ± 0.4 |
| 30 | 96.8 ± 0.4 | 71.5 ± 1.2 | 55.0 ± 1.5 | 96.4 ± 0.2 | |
| [5.8] | [19.1] | [9.8] | [3.2] | ||
aCompounds eliciting less than 20% inhibition of proliferation were considered ineffective and the exact results are not given, for simplicity. n.d.: not determined.
Conclusion
In summary, a microwave-assisted one-pot method for the facile and efficient synthesis of novel steroidal 17-exo-pyrazol-5'-ones from a β-ketoester precursor with arylhydrazine hydrochlorides has been developed. An acid-catalyzed thermal decarboxylation of the starting material as an unwanted side reaction could be avoided by applying a one-pot two-step protocol involving the in situ liberation of the reagent from its salt in EtOH followed by the heterocyclization reaction through the addition of AcOH. Some of the presented compounds 6h, 7f, 7i and 7j exerted considerable antiproliferative activity with promising cancer selectivity on a panel of human breast cancer cell lines. This indicates that the pyrazolone heterocyclic ring at the 17β position is a promising scaffold for the design of anticancer agents of the Δ5 androstene series.
Supporting Information
Experimental procedures for compounds 6a–j, 7a–j and 8, their 1H, 13C NMR, MS, IR, elemental analysis data and the copies of their NMR spectra.
| Supporting Information File 1: Experimental part. | ||
| Format: PDF | Size: 3.2 MB | Download |
References
-
Salvador, J. A. R.; Pinto, R. M. A.; Silvestre, S. M. J. Steroid Biochem. Mol. Biol. 2013, 137, 199–222. doi:10.1016/j.jsbmb.2013.04.006
Return to citation in text: [1] [2] -
DeVore, N. M.; Scott, E. E. Nature 2012, 482, 116–119. doi:10.1038/nature10743
Return to citation in text: [1] -
Njar, V. C. O.; Brodie, A. M. H. J. Med. Chem. 2015, 58, 2077–2087. doi:10.1021/jm501239f
Return to citation in text: [1] -
Frank, É.; Schneider, G. J. Steroid Biochem. Mol. Biol. 2013, 137, 301–315. doi:10.1016/j.jsbmb.2013.02.018
Return to citation in text: [1] -
Kovács, D.; Mótyán, G.; Wölfling, J.; Kovács, I.; Zupkó, I.; Frank, É. Bioorg. Med. Chem. Lett. 2014, 24, 1265–1268. doi:10.1016/j.bmcl.2014.01.069
Return to citation in text: [1] -
Iványi, Z.; Wölfling, J.; Görbe, T.; Szécsi, M.; Wittmann, T.; Schneider, G. Steroids 2010, 75, 450–456. doi:10.1016/j.steroids.2010.02.013
Return to citation in text: [1] -
Ling, Y.-z.; Li, J.-s.; Liu, Y.; Kato, K.; Klus, G. T.; Brodie, A. J. Med. Chem. 1997, 40, 3297–3304. doi:10.1021/jm970337k
Return to citation in text: [1] -
Kovács, D.; Wölfling, J.; Szabó, N.; Szécsi, M.; Schelz, Z.; Zupkó, I.; Frank, É. Eur. J. Med. Chem. 2016, 120, 284–295. doi:10.1016/j.ejmech.2016.05.006
Return to citation in text: [1] -
Li, J.; Zhao, X.; Li, L.; Yuan, Z.; Tan, F.; Shi, B.; Zhang, J. Steroids 2016, 107, 45–54. doi:10.1016/j.steroids.2015.12.018
Return to citation in text: [1] -
Shamsuzzaman; Mashrai, A.; Ahmad, A.; Dar, A. M.; Khanam, H.; Danishuddin, M.; Khan, A. U. Med. Chem. Res. 2014, 23, 348–362. doi:10.1007/s00044-013-0636-y
Return to citation in text: [1] -
Prajuli, R.; Banerjee, J.; Khana, H. Orient. J. Chem. 2015, 31, 2099–2106. doi:10.13005/ojc/310430
Return to citation in text: [1] -
Ma, R.; Zhu, J.; Liu, J.; Chen, L.; Shen, X.; Jiang, H.; Li, J. Molecules 2010, 15, 3593–3601. doi:10.3390/molecules15053593
Return to citation in text: [1] -
Ekekwe, N. D.; Arinze, A. J.; Nnanna, L. A.; Ukpabi, C. F.; Agwu, A.; Ogwuegbu, M. O. C. Am. J. Chem. 2012, 2, 52–56. doi:10.5923/j.chemistry.20120202.10
Return to citation in text: [1] -
Tarabová, D.; Soralová, S.; Breza, M.; Fronc, M.; Holzer, W.; Milata, V. Beilstein J. Org. Chem. 2014, 10, 752–760. doi:10.3762/bjoc.10.70
Return to citation in text: [1] -
Pal, S.; Mareddy, J.; Devi, N. S. J. Braz. Chem. Soc. 2008, 19, 1207–1214. doi:10.1590/S0103-50532008000600023
Return to citation in text: [1] -
Bagley, M. C.; Baashen, M.; Paddock, V. L.; Kipling, D.; Davis, T. Tetrahedron 2013, 69, 8429–8438. doi:10.1016/j.tet.2013.07.055
Return to citation in text: [1] -
Vaddula, B. R.; Varma, R. S.; Leazer, J. Tetrahedron Lett. 2013, 54, 1538–1541. doi:10.1016/j.tetlet.2013.01.029
Return to citation in text: [1] -
Holzer, W.; Kautsch, C.; Laggner, C.; Claramunt, R. M.; Pérez-Torralba, M.; Alkorta, I.; Elguero, J. Tetrahedron 2004, 60, 6791–6805. doi:10.1016/j.tet.2004.06.039
Return to citation in text: [1] [2] -
Claramunt, R. M.; López, C.; Santa María, M. D.; Sanz, D.; Elguero, J. Prog. Nucl. Magn. Reson. Spectrosc. 2006, 49, 169–206. doi:10.1016/j.pnmrs.2006.07.001
Return to citation in text: [1] -
Mosmann, T. J. Immunol. Methods 1983, 65, 55–63. doi:10.1016/0022-1759(83)90303-4
Return to citation in text: [1] [2] -
Zhu, N.; Ling, Y.; Lei, X.; Handratta, V.; Brodie, A. M. H. Steroids 2003, 68, 603–611. doi:10.1016/S0039-128X(03)00082-5
Return to citation in text: [1] -
Kovács, D.; Wölfling, J.; Szabó, N.; Szécsi, M.; Kovács, I.; Zupkó, I.; Frank, É. Eur. J. Med. Chem. 2013, 70, 649–660. doi:10.1016/j.ejmech.2013.10.038
Return to citation in text: [1] -
Kovács, D.; Wölfling, J.; Szabó, N.; Szécsi, M.; Minorics, R.; Zupkó, I.; Frank, É. Eur. J. Med. Chem. 2015, 98, 13–29. doi:10.1016/j.ejmech.2015.05.010
Return to citation in text: [1] [2] -
Magano, J.; Nanninga, T. N.; Winkle, D. D. Tetrahedron Lett. 2008, 49, 2956–2959. doi:10.1016/j.tetlet.2008.03.004
Return to citation in text: [1] -
Allan, K. M.; Hong, B. D.; Stoltz, B. M. Org. Biomol. Chem. 2009, 7, 4960–4964. doi:10.1039/B913336D
Return to citation in text: [1] -
Lu, Y.; Chen, J.; Janjetovic, Z.; Michaels, P.; Tang, E. K. Y.; Wang, J.; Tuckey, R. C.; Slominski, A. T.; Li, W.; Miller, D. D. J. Med. Chem. 2012, 55, 3573–3577. doi:10.1021/jm201478e
Return to citation in text: [1] -
Mótyán, G.; Kovács, F.; Wölfling, J.; Gyovai, A.; Zupkó, I.; Frank, É. Steroids 2016, 112, 36–46. doi:10.1016/j.steroids.2016.04.014
Return to citation in text: [1] [2] -
Enchev, V.; Neykov, G. D. J. Mol. Struct.: THEOCHEM 1992, 258, 217–234. doi:10.1016/0166-1280(92)85065-S
Return to citation in text: [1] -
Gupta, P.; Gupta, J. K.; Halve, A. K. Int. J. Pharm. Sci. Res. 2015, 6, 2291–2310. doi:10.13040/IJPSR.0975-8232.6(6).2291-10
Return to citation in text: [1] -
Sheng, X.; Hua, K.; Yang, C.; Wang, X.; Ji, H.; Xu, J.; Huang, Z.; Zhang, Y. Bioorg. Med. Chem. Lett. 2015, 25, 3535–3540. doi:10.1016/j.bmcl.2015.06.090
Return to citation in text: [1] -
Alkorta, I.; Goya, P.; Elguero, J.; Singh, S. P. Natl. Acad. Sci. Lett. (India) 2007, 30, 139–159.
Return to citation in text: [1]
| 30. | Sheng, X.; Hua, K.; Yang, C.; Wang, X.; Ji, H.; Xu, J.; Huang, Z.; Zhang, Y. Bioorg. Med. Chem. Lett. 2015, 25, 3535–3540. doi:10.1016/j.bmcl.2015.06.090 |
| 18. | Holzer, W.; Kautsch, C.; Laggner, C.; Claramunt, R. M.; Pérez-Torralba, M.; Alkorta, I.; Elguero, J. Tetrahedron 2004, 60, 6791–6805. doi:10.1016/j.tet.2004.06.039 |
| 29. | Gupta, P.; Gupta, J. K.; Halve, A. K. Int. J. Pharm. Sci. Res. 2015, 6, 2291–2310. doi:10.13040/IJPSR.0975-8232.6(6).2291-10 |
| 27. | Mótyán, G.; Kovács, F.; Wölfling, J.; Gyovai, A.; Zupkó, I.; Frank, É. Steroids 2016, 112, 36–46. doi:10.1016/j.steroids.2016.04.014 |
| 1. | Salvador, J. A. R.; Pinto, R. M. A.; Silvestre, S. M. J. Steroid Biochem. Mol. Biol. 2013, 137, 199–222. doi:10.1016/j.jsbmb.2013.04.006 |
| 10. | Shamsuzzaman; Mashrai, A.; Ahmad, A.; Dar, A. M.; Khanam, H.; Danishuddin, M.; Khan, A. U. Med. Chem. Res. 2014, 23, 348–362. doi:10.1007/s00044-013-0636-y |
| 27. | Mótyán, G.; Kovács, F.; Wölfling, J.; Gyovai, A.; Zupkó, I.; Frank, É. Steroids 2016, 112, 36–46. doi:10.1016/j.steroids.2016.04.014 |
| 6. | Iványi, Z.; Wölfling, J.; Görbe, T.; Szécsi, M.; Wittmann, T.; Schneider, G. Steroids 2010, 75, 450–456. doi:10.1016/j.steroids.2010.02.013 |
| 7. | Ling, Y.-z.; Li, J.-s.; Liu, Y.; Kato, K.; Klus, G. T.; Brodie, A. J. Med. Chem. 1997, 40, 3297–3304. doi:10.1021/jm970337k |
| 8. | Kovács, D.; Wölfling, J.; Szabó, N.; Szécsi, M.; Schelz, Z.; Zupkó, I.; Frank, É. Eur. J. Med. Chem. 2016, 120, 284–295. doi:10.1016/j.ejmech.2016.05.006 |
| 9. | Li, J.; Zhao, X.; Li, L.; Yuan, Z.; Tan, F.; Shi, B.; Zhang, J. Steroids 2016, 107, 45–54. doi:10.1016/j.steroids.2015.12.018 |
| 28. | Enchev, V.; Neykov, G. D. J. Mol. Struct.: THEOCHEM 1992, 258, 217–234. doi:10.1016/0166-1280(92)85065-S |
| 4. | Frank, É.; Schneider, G. J. Steroid Biochem. Mol. Biol. 2013, 137, 301–315. doi:10.1016/j.jsbmb.2013.02.018 |
| 5. | Kovács, D.; Mótyán, G.; Wölfling, J.; Kovács, I.; Zupkó, I.; Frank, É. Bioorg. Med. Chem. Lett. 2014, 24, 1265–1268. doi:10.1016/j.bmcl.2014.01.069 |
| 24. | Magano, J.; Nanninga, T. N.; Winkle, D. D. Tetrahedron Lett. 2008, 49, 2956–2959. doi:10.1016/j.tetlet.2008.03.004 |
| 25. | Allan, K. M.; Hong, B. D.; Stoltz, B. M. Org. Biomol. Chem. 2009, 7, 4960–4964. doi:10.1039/B913336D |
| 1. | Salvador, J. A. R.; Pinto, R. M. A.; Silvestre, S. M. J. Steroid Biochem. Mol. Biol. 2013, 137, 199–222. doi:10.1016/j.jsbmb.2013.04.006 |
| 2. | DeVore, N. M.; Scott, E. E. Nature 2012, 482, 116–119. doi:10.1038/nature10743 |
| 3. | Njar, V. C. O.; Brodie, A. M. H. J. Med. Chem. 2015, 58, 2077–2087. doi:10.1021/jm501239f |
| 23. | Kovács, D.; Wölfling, J.; Szabó, N.; Szécsi, M.; Minorics, R.; Zupkó, I.; Frank, É. Eur. J. Med. Chem. 2015, 98, 13–29. doi:10.1016/j.ejmech.2015.05.010 |
| 26. | Lu, Y.; Chen, J.; Janjetovic, Z.; Michaels, P.; Tang, E. K. Y.; Wang, J.; Tuckey, R. C.; Slominski, A. T.; Li, W.; Miller, D. D. J. Med. Chem. 2012, 55, 3573–3577. doi:10.1021/jm201478e |
| 18. | Holzer, W.; Kautsch, C.; Laggner, C.; Claramunt, R. M.; Pérez-Torralba, M.; Alkorta, I.; Elguero, J. Tetrahedron 2004, 60, 6791–6805. doi:10.1016/j.tet.2004.06.039 |
| 20. | Mosmann, T. J. Immunol. Methods 1983, 65, 55–63. doi:10.1016/0022-1759(83)90303-4 |
| 15. | Pal, S.; Mareddy, J.; Devi, N. S. J. Braz. Chem. Soc. 2008, 19, 1207–1214. doi:10.1590/S0103-50532008000600023 |
| 16. | Bagley, M. C.; Baashen, M.; Paddock, V. L.; Kipling, D.; Davis, T. Tetrahedron 2013, 69, 8429–8438. doi:10.1016/j.tet.2013.07.055 |
| 17. | Vaddula, B. R.; Varma, R. S.; Leazer, J. Tetrahedron Lett. 2013, 54, 1538–1541. doi:10.1016/j.tetlet.2013.01.029 |
| 21. | Zhu, N.; Ling, Y.; Lei, X.; Handratta, V.; Brodie, A. M. H. Steroids 2003, 68, 603–611. doi:10.1016/S0039-128X(03)00082-5 |
| 22. | Kovács, D.; Wölfling, J.; Szabó, N.; Szécsi, M.; Kovács, I.; Zupkó, I.; Frank, É. Eur. J. Med. Chem. 2013, 70, 649–660. doi:10.1016/j.ejmech.2013.10.038 |
| 23. | Kovács, D.; Wölfling, J.; Szabó, N.; Szécsi, M.; Minorics, R.; Zupkó, I.; Frank, É. Eur. J. Med. Chem. 2015, 98, 13–29. doi:10.1016/j.ejmech.2015.05.010 |
| 14. | Tarabová, D.; Soralová, S.; Breza, M.; Fronc, M.; Holzer, W.; Milata, V. Beilstein J. Org. Chem. 2014, 10, 752–760. doi:10.3762/bjoc.10.70 |
| 31. | Alkorta, I.; Goya, P.; Elguero, J.; Singh, S. P. Natl. Acad. Sci. Lett. (India) 2007, 30, 139–159. |
| 11. | Prajuli, R.; Banerjee, J.; Khana, H. Orient. J. Chem. 2015, 31, 2099–2106. doi:10.13005/ojc/310430 |
| 12. | Ma, R.; Zhu, J.; Liu, J.; Chen, L.; Shen, X.; Jiang, H.; Li, J. Molecules 2010, 15, 3593–3601. doi:10.3390/molecules15053593 |
| 13. | Ekekwe, N. D.; Arinze, A. J.; Nnanna, L. A.; Ukpabi, C. F.; Agwu, A.; Ogwuegbu, M. O. C. Am. J. Chem. 2012, 2, 52–56. doi:10.5923/j.chemistry.20120202.10 |
| 19. | Claramunt, R. M.; López, C.; Santa María, M. D.; Sanz, D.; Elguero, J. Prog. Nucl. Magn. Reson. Spectrosc. 2006, 49, 169–206. doi:10.1016/j.pnmrs.2006.07.001 |
| 20. | Mosmann, T. J. Immunol. Methods 1983, 65, 55–63. doi:10.1016/0022-1759(83)90303-4 |
© 2018 Mótyán et al.; licensee Beilstein-Institut.
This is an Open Access article under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/4.0). Please note that the reuse, redistribution and reproduction in particular requires that the authors and source are credited.
The license is subject to the Beilstein Journal of Organic Chemistry terms and conditions: (https://www.beilstein-journals.org/bjoc)