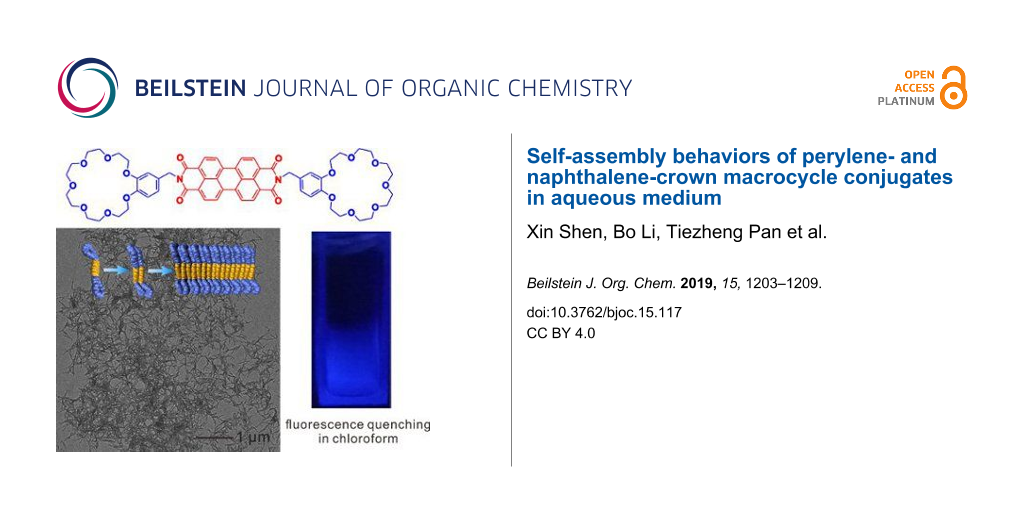
Abstract
The synthesis of conjugates of perylene diimide (PDI) and naphthalene diimide (NDI) modified with two benzo-21-crown-7 ethers (B21C7) are herein described. Their self-assembly behavior in various solvents was investigated particularly in aqueous medium, due to the recently discovered hydrophilic properties of B21C7 crown macrocycle. An unexpected fluorescence quenching phenomenon was observed in the PDI-B21C7 macrocycle conjugate in chloroform. The detailed UV–vis absorption and fluorescence spectra of these PDI/NDI derivatives in different solvents as well as their morphologies were investigated.

Graphical Abstract
Introduction
Owing to their unique physicochemical properties, self-assemblies have been recognized as a type of exciting nanomaterials with tremendous potential for research and commercial applications [1-6]. Thanks to the development of supramolecular chemistry, the ordered structures could be obtained through programmed self-assembly coupled with covalent chemical synthesis [7]. In fact, supramolecular technology has shown great importance in various kinds of functional materials, including natural protein complexes [8-10], hydrogels [11-13], carbon-based materials [14-17], self-healing materials [18-23], and composite materials [24-26]. Moreover, several new proof-of-concept applications of supramolecular assemblies are also of increasing interest, specifically in the fields of energy generation and storage [27], water treatment and environmental remediation [28,29], and healthcare and biomedical engineering [30-32].
Nowadays, as the research in supramolecular chemistry is expanding into the aqueous realm, a systematic understanding of the interaction relationship of supramolecular self-assemblies and aqueous medium is becoming increasingly important [33-36]. As the first generation of macrocyclic hosts, crown ethers, have been widely used as building blocks for supramolecular assemblies in the past two decades [37-53], mainly in the fabrication of crown ether-based threaded or interlocked assemblies in organic solvents [54]. However, we recently found that benzo-21-crown-7 (B21C7) displays impressive high water solubility in comparison with other macrocyclic hosts [55]. Moreover, the conjugation of B21C7s to the well-known supramolecular BTA core leads to the observation of special topological effects on ion selectivity in water [56]. Following this water-centered view, we further explored a new type of supramolecular polymeric adhesive based on B21C7 derived low–molecular weight (LMW) monomer, in which the water molecules serve as essential co-monomers to the polymerization process, rather than as solvent [57]. Compared with their linear glycol chain counterparts, B21C7 shows great potential to be an easy-to-accessed building block to probe the non-covalent interactions and chemical transformations influenced by water molecules.
Perylene diimide (PDI) and naphthalene diimide (NDI) are polycyclic aromatic chromophores widely used in the dye industry and have been applied as advanced materials in the fields of fluorescence labeling [58,59], organic semiconducting devices [60,61], and light harvesting [62-65]. PDI and NDI units have been extensively incorporated into functional supramolecular architectures through hydrogen bonding and metal ion coordination in nonpolar solvents [66-68]. Compared with NDI, PDI has more aromatic rings to generate stronger intermolecular π−π stacking, leading to molecular aggregates more easily, and these aggregates or supramolecular assemblies can give rise to desirable properties, such as charge transport and photophysical properties [69-74]. While the morphologies of these aggregates were greatly influenced by solvents, molecular structures and shapes, as well as the fraction of hydrophilic and/or hydrophobic parts [75-81]. By far, little was known to the self-assembly behaviors of B21C7 crown macrocycle functionalized PDIs/NDIs in aqueous solution, even though there have been several reports about crown ether-functionalized PDIs or NDIs, which mostly focused on their interactions with metal ions [82-85]. Herein, we attempted to combine these two classical organic moieties and crown macrocycle together and reported the PDI- and NDI-B21C7 conjugates. Their self-assemblies behaviors in aqueous medium were thus probed.
Results and Discussion
The synthetic route toward compounds 1 and 2 is shown in Scheme 1. Initially, the water-soluble crown macrocycle 3 was synthesized according to reported protocols, by using potassium cations as template [86,87]. The primary amine group in 3 offers diverse reaction pathways to design crown ethers appended LMW gelators, dendrimers and polymers [88-92]. In order to conjugate B21C7 onto PDI, compound 3 and 4-dimethylaminopyridine (DMAP) were dissolved in ethylene glycol, then 3,4,9,10-perylenetetracarboxylic dianhydride (PDI) was charged and suspended in the solution. The mixture was heated to 140 °C and stirred overnight. After cooling down to room temperature, the reaction mixture was dissolved in CH2Cl2. The solution was washed by diluted hydrochloric acid (0.05 M) and saturated brine, respectively. After the drying process, compound 1 was obtained with moderate yield. Compound 2 was prepared via a similar method except that NDI was used instead of PDI. All the final compounds were carefully characterized by 1H and 13C NMR spectroscopy and electrospray ionization mass spectroscopy (ESI-MS, Supporting Information File 1, Figures S1–S8).
Scheme 1: Synthetic route of perylene- and naphthalene-crown macrocycle conjugates 1 and 2.
Scheme 1: Synthetic route of perylene- and naphthalene-crown macrocycle conjugates 1 and 2.
UV–vis absorption and fluorescence spectra of 1 in different solvents, including CHCl3, MeCN, MeOH, H2O, and mixed solvent CHCl3/MeCN (v/v = 2:1), were recorded to investigate its self-assembly behavior. Figure 1a shows the UV–vis absorption spectra of 1 in solvents with different polarity. The spectrum of 1 in the less polar solvent CHCl3 shows a well-resolved vibronic structure (0–0, 0–1, and 0–2 transitions of PDI molecules) with three characteristic absorption bands at 530, 485, and 469 nm, respectively [67]. However, the ratio of intensities of the bands derived from 0–0 and 0–1 transitions is around 1.53, indicating that 1 in CHCl3 seems do not only exist in monomeric state, but also in kind of π–π aggregated state [93,94]. When more polar solvent MeCN or MeOH were used, merely a broad and very weak absorption band was observed. All the weak and disappearing bands indicate the overlapping of PDI chromophores to form J-type aggregates in polar solvents [84]. The intensities of the characteristic absorption bands of 1 in mixed solvents of CHCl3/MeCN (v/v = 2:1) are even higher than those of 1 in pure CHCl3, indicating a better solubility of 1 in the mixed solvents. We were encouraged to investigate the UV–vis absorption behavior of 1 in mixed solvents of CHCl3 and MeCN with different volume ratios (Supporting Information File 1, Figure S9). It is observed that when the volume proportion of MeCN in the mixed solvent is below 70%, the UV–vis absorption intensity of 1 is higher than that in pure CHCl3. However, when the volume proportion of MeCN increases to 80%, the UV–vis absorption intensities decrease dramatically. The variations of UV–vis absorbance of 1 in the mixed solvents with different ratios are correlative to its solubility changes, indicating that the solubility of 1 in solvents are probably influenced by the interactions between both PBI unit and crown ether units with solvents. The fluorescence spectra of 1 (λex = 490 nm) in different solvents also imply the varied self-assembly behavior (Figure 1b). The fluorescence of 1 in CHCl3 is strong with a broad emission band at 520–600 nm, and becomes even stronger when CHCl3/MeCN (v/v = 2:1) is used as solvent. But due to the aggregation-caused quenching (ACQ) effect, the fluorescence of 1 in MeCN decreased dramatically, and was almost totally quenched in MeOH and H2O. It is surprising to us that the fluorescence of 1 in CHCl3 is also quenched even at such a low concentration (5.0 × 10−6 M), as only a faint blue fluorescent emission of 1 could be detected (Figure 1b inset). According to previous reports, such a fluorescent quenching indicated the formation of a dimeric structure [67,95], and the electron rich substituents at the imide nitrogen should play an important role [96,97].
![[1860-5397-15-117-1]](/bjoc/content/figures/1860-5397-15-117-1.png?scale=2.0&max-width=1024&background=FFFFFF)
Figure 1: (a) UV–vis absorption spectra and (b) fluorescence spectra (λex = 490 nm) of 1 in different solvents (inset: photograph of 1 in CHCl3 irradiated under 365 nm); (c) UV–vis absorption spectra and (d) fluorescence spectra (λex = 360 nm) of 2 in different solvents (inset: photograph of 2 in CHCl3 irradiated under 365 nm). The concentration of 1 or 2 in the solutions is 5.0 × 10−6 M. All the measurements were performed at room temperature.
Figure 1: (a) UV–vis absorption spectra and (b) fluorescence spectra (λex = 490 nm) of 1 in different solvent...
For comparison, UV–vis absorption and fluorescence spectra of 2 in the above-tested five solvent systems were also recorded. Because of the relatively weaker π–π stacking effect of the NDI units comparing with PDI units, 2 has a better solubility in the organic solvents. Consequently, the UV–vis absorption bands of 2 in all the organic solvents can be clearly observed but with an overall hypsochromic shift (Figure 1c). The aggregation of 2 also takes place in the solvents with high polarity, such as MeOH and H2O, in which 2 shows weak or even no UV–vis absorption. Similar to 1, 2 also shows a strong fluorescence in CHCl3 and mixed CHCl3/MeCN (v/v = 2:1, Figure 1d). In the polar solvents MeCN and MeOH, fluorescence of 2 becomes much weaker, and is almost totally quenched in H2O.
In order to observe the aggregates formed by 1 and 2 visually in solutions, transmission electron microscopy (TEM) was utilized. First, the aggregates of 1 formed in CHCl3 and MeCN were investigated. As shown in Figure 2a, micelle-like assemblies of 1 were observed, while, because of the higher polarity of MeCN, the aggregation of 1 through π–π stacking was considerably enhanced forming linear assemblies (Figure 2b). Interestingly, when imposed to an aqueous medium through adding water slowly into MeCN solution under ultrasonication yielding a mixture solvent of H2O/MeCN = 10:1 (v/v), 1 assembled into a well-defined nanobelt in extremely polar aqueous environment, which further entangled into networks (Figure 2c) [68]. Previously, Würthner’s group reported the self-assembly of a trioligo(ethylene glycol)-conjugated PDI system which can self-assemble into similar nanobelt structures [79]. Therefore, we inferred that the B21C7 macrocycle units have a similar solubilizing and induction effect like oligo(ethylene glycol) chains for the self-assembly behavior of PDI derivatives. Due to the weaker π–π stacking of 2 leading to a less regular packing arrangement, no regular morphology was observed when CHCl3 or MeCN was used as solvent (Figure 2d and 2e), and only a short nanobelt was observed in H2O/MeCN (v/v = 10:1, Figure 2f). The TEM characterization result was corroborated well by a dynamic light scattering (DLS) measurement (Figure S10, Supporting Information File 1), which was proven to be a convenient method to study aggregation behavior in solvents [98,99]. It was found that the average diameter of aggregates of 1 in CHCl3 (100 μM) was around 31.1 nm. The size of aggregated 1 in MeCN was ranging from around 40 to 200 nm centered at 81.8 nm, while it further increased to 840.1 nm in H2O/MeCN (v/v = 10:1). However, the DLS measurement results of aggregates formed by 2 in either CHCl3 or MeCN was unrepeatable, possibly due to the irregular packing in these solvents. But DLS measurement result showed that the size of aggregates of 2 formed in H2O/MeCN (v/v = 10:1) was centered at 576.8 nm. It should be noted that the aggregates formed by 1 and 2 with specific shapes were obtained immediately after the preparation of solutions, without days’ evolution process [77].
![[1860-5397-15-117-2]](/bjoc/content/figures/1860-5397-15-117-2.jpg?scale=2.0&max-width=1024&background=FFFFFF)
Figure 2: TEM images of 1 aggregates in (a) CHCl3, (b) MeCN, and (c) H2O/MeCN (v/v = 10:1) (inset: schematic space-filling models illustrating the self-assembly of 1); TEM images of 2 aggregates in (d) CHCl3, (e) MeCN, and (f) H2O/MeCN (v/v = 10:1). Concentration of solutions for all the TEM analysis is 1 × 10−4 M.
Figure 2: TEM images of 1 aggregates in (a) CHCl3, (b) MeCN, and (c) H2O/MeCN (v/v = 10:1) (inset: schematic ...
Conclusion
In summary, we synthesized crown ether-functionalized PDI and NDI derivatives, and investigated their solvent-dependent solubility and self-assembly behaviors. It was found that the polarity of solvents have an important impact on their self-assembly behavior, thus inducing obvious changes in their UV–vis absorption and fluorescence properties. An unexpected fluorescence quenching phenomenon was observed in the PDI-B21C7 macrocycle conjugate in chloroform. Through TEM characterization, it was illustrated that in aqueous medium, both PDI and NDI derivatives containing crown ether units could quickly self-assembly into nanobelt aggregates, during which the crown ether units exhibited the similar solubilizing and induction effect like oligo(ethylene glycol) chains.
Supporting Information
| Supporting Information File 1: Experimental and analytical data. | ||
| Format: PDF | Size: 1.3 MB | Download |
Acknowledgements
We gratefully acknowledge financial support from the Thousand Talents Program of China, Postdoctoral Innovative Talents Supporting Project (BX20180255) of China, Key R&D Program of Shaanxi Province (2019KW-031, 2019KW-038), Natural Science Basic Research Plan in Shaanxi Province of China (2018JQ2017), Fundamental Research Funds for the Central Universities (3102018zy051, 3102018jcc007, 3102017OQD044, 3102017OQD045, 3102017OQD040, 3102017OQD115, 3102018zy051, 3102019ghxm005), and State Key Laboratory of Solidification Processing in NPU (SKLSP201817). We thank the Analytical & Testing Center of NPU for the characterization of materials.
References
-
Ariga, K.; Hill, J. P.; Lee, M. V.; Vinu, A.; Charvet, R.; Acharya, S. Adv. Mater. (Weinheim, Ger.) 2009, 9, 014109. doi:10.1088/1468-6996/9/1/014109
Return to citation in text: [1] -
Abbas, M.; Zou, Q.; Li, S.; Yan, X. Adv. Mater. (Weinheim, Ger.) 2017, 29, 1605021. doi:10.1002/adma.201605021
Return to citation in text: [1] -
Schacher, F. H.; Rupar, P. A.; Manners, I. Angew. Chem., Int. Ed. 2012, 51, 7898–7921. doi:10.1002/anie.201200310
Return to citation in text: [1] -
Li, X.; Iocozzia, J.; Chen, Y.; Zhao, S.; Cui, X.; Wang, W.; Yu, H.; Lin, S.; Lin, Z. Angew. Chem., Int. Ed. 2018, 57, 2046–2070. doi:10.1002/anie.201705019
Return to citation in text: [1] -
Chen, L.-J.; Yang, H.-B. Acc. Chem. Res. 2018, 51, 2699–2710. doi:10.1021/acs.accounts.8b00317
Return to citation in text: [1] -
Li, B.; Wang, B.; Huang, X.; Dai, L.; Cui, L.; Li, J.; Jia, X.; Li, C. Angew. Chem., Int. Ed. 2019, 58, 3885–3889. doi:10.1002/anie.201813972
Return to citation in text: [1] -
Lehn, J.-M. Angew. Chem., Int. Ed. Engl. 1988, 27, 89–112. doi:10.1002/anie.198800891
Return to citation in text: [1] -
Pandya, M. J.; Spooner, G. M.; Sunde, M.; Thorpe, J. R.; Rodger, A.; Woolfson, D. N. Biochemistry 2000, 39, 8728–8734. doi:10.1021/bi000246g
Return to citation in text: [1] -
Mulyasasmita, W.; Lee, J. S.; Heilshorn, S. C. Biomacromolecules 2011, 12, 3406–3411. doi:10.1021/bm200959e
Return to citation in text: [1] -
Luo, Q.; Hou, C.; Bai, Y.; Wang, R.; Liu, J. Chem. Rev. 2016, 116, 13571–13632. doi:10.1021/acs.chemrev.6b00228
Return to citation in text: [1] -
Xu, Y.; Sheng, K.; Li, C.; Shi, G. ACS Nano 2010, 4, 4324–4330. doi:10.1021/nn101187z
Return to citation in text: [1] -
Hauser, C. A. E.; Deng, R.; Mishra, A.; Loo, Y.; Khoe, U.; Zhuang, F.; Cheong, D. W.; Accardo, A.; Sullivan, M. B.; Riekel, C.; Ying, J. Y.; Hauser, U. A. Proc. Natl. Acad. Sci. U. S. A. 2011, 108, 1361–1366. doi:10.1073/pnas.1014796108
Return to citation in text: [1] -
Sutar, P.; Suresh, V. M.; Jayaramulu, K.; Hazra, A.; Maji, T. K. Nat. Commun. 2018, 9, No. 3587. doi:10.1038/s41467-018-05818-w
Return to citation in text: [1] -
Sano, M.; Kamino, A.; Okamura, J.; Shinkai, S. Nano Lett. 2002, 2, 531–533. doi:10.1021/nl025525z
Return to citation in text: [1] -
Patra, N.; Song, Y.; Král, P. ACS Nano 2011, 5, 1798–1804. doi:10.1021/nn102531h
Return to citation in text: [1] -
Poonia, M.; Gupta, R. K.; Manjuladevi, V.; Gupta, S. K.; Akhtar, J. J. Nanopart. Res. 2014, 16, 2572. doi:10.1007/s11051-014-2572-2
Return to citation in text: [1] -
Derenskyi, V.; Gomulya, W.; Talsma, W.; Salazar-Rios, J. M.; Fritsch, M.; Nirmalraj, P.; Riel, H.; Allard, S.; Scherf, U.; Loi, M. A. Adv. Mater. (Weinheim, Ger.) 2017, 29, 1606757. doi:10.1002/adma.201606757
Return to citation in text: [1] -
Bastings, M. M. C.; Koudstaal, S.; Kieltyka, R. E.; Nakano, Y.; Pape, A. C. H.; Feyen, D. A. M.; van Slochteren, F. J.; Doevendans, P. A.; Sluijter, J. P. G.; Meijer, E. W.; Chamuleau, S. A. J.; Dankers, P. Y. W. Adv. Healthcare Mater. 2014, 3, 70–78. doi:10.1002/adhm.201300076
Return to citation in text: [1] -
Kakuta, T.; Takashima, Y.; Nakahata, M.; Otsubo, M.; Yamaguchi, H.; Harada, A. Adv. Mater. (Weinheim, Ger.) 2013, 25, 2849–2853. doi:10.1002/adma.201205321
Return to citation in text: [1] -
Lu, H. D.; Charati, M. B.; Kim, I. L.; Burdick, J. A. Biomaterials 2012, 33, 2145–2153. doi:10.1016/j.biomaterials.2011.11.076
Return to citation in text: [1] -
Bode, S.; Zedler, L.; Schacher, F. H.; Dietzek, B.; Schmitt, M.; Popp, J.; Hager, M. D.; Schubert, U. S. Adv. Mater. (Weinheim, Ger.) 2013, 25, 1634–1638. doi:10.1002/adma.201203865
Return to citation in text: [1] -
Chen, H.; Ma, X.; Wu, S.; Tian, H. Angew. Chem., Int. Ed. 2014, 53, 14149–14152. doi:10.1002/anie.201407402
Return to citation in text: [1] -
Zhang, M.; Xu, D.; Yan, X.; Chen, J.; Dong, S.; Zheng, B.; Huang, F. Angew. Chem., Int. Ed. 2012, 51, 7011–7015. doi:10.1002/anie.201203063
Return to citation in text: [1] -
Prathap, A.; Sureshan, K. M. Angew. Chem., Int. Ed. 2017, 56, 9405–9409. doi:10.1002/anie.201704699
Return to citation in text: [1] -
Molji, C.; Aashish, A.; Neethu, K. S.; Devaki, S. J. J. Mater. Chem. A 2017, 5, 16636–16645. doi:10.1039/c7ta05215d
Return to citation in text: [1] -
Dell'Elce, S.; Liscio, F.; Kovtun, A.; Allegri, S.; Roscioni, O. M.; Albonetti, C.; De Luca, G.; Amenitsch, H. W.; Demitri, N.; Giorgini, L.; Morandi, V.; Stellacci, F.; Liscio, A.; Palermo, V. Nanoscale 2018, 10, 23018–23026. doi:10.1039/c8nr07109h
Return to citation in text: [1] -
Bera, S.; Haldar, D. J. Mater. Chem. A 2016, 4, 6933–6939. doi:10.1039/c5ta08010j
Return to citation in text: [1] -
Xie, S.; Wu, S.; Bao, S.; Wang, Y.; Zheng, Y.; Deng, D.; Huang, L.; Zhang, L.; Lee, M.; Huang, Z. Adv. Mater. (Weinheim, Ger.) 2018, 30, 1800683. doi:10.1002/adma.201800683
Return to citation in text: [1] -
Cheng, H.-B.; Li, Z.; Huang, Y.-D.; Liu, L.; Wu, H.-C. ACS Appl. Mater. Interfaces 2017, 9, 11889–11894. doi:10.1021/acsami.7b00363
Return to citation in text: [1] -
Zheng, J.; Zhu, G.; Li, Y.; Li, C.; You, M.; Chen, T.; Song, E.; Yang, R.; Tan, W. ACS Nano 2013, 7, 6545–6554. doi:10.1021/nn402344v
Return to citation in text: [1] -
Molino, N. M.; Wang, S.-W. Curr. Opin. Biotechnol. 2014, 28, 75–82. doi:10.1016/j.copbio.2013.12.007
Return to citation in text: [1] -
Liang, H.; Zhang, X.-B.; Lv, Y.; Gong, L.; Wang, R.; Zhu, X.; Yang, R.; Tan, W. Acc. Chem. Res. 2014, 47, 1891–1901. doi:10.1021/ar500078f
Return to citation in text: [1] -
Albelda, M. T.; Frías, J. C.; García-España, E.; Schneider, H.-J. Chem. Soc. Rev. 2012, 41, 3859–3877. doi:10.1039/c2cs35008d
Return to citation in text: [1] -
Biros, S. M.; Rebek, J., Jr.. Chem. Soc. Rev. 2007, 36, 93–104. doi:10.1039/b508530f
Return to citation in text: [1] -
Kataev, E. A.; Müller, C. Tetrahedron 2014, 70, 137–167. doi:10.1016/j.tet.2013.11.010
Return to citation in text: [1] -
Oshovsky, G. V.; Reinhoudt, D. N.; Verboom, W. Angew. Chem., Int. Ed. 2007, 46, 2366–2393. doi:10.1002/anie.200602815
Return to citation in text: [1] -
Percec, V.; Johansson, G.; Ungar, G.; Zhou, J. J. Am. Chem. Soc. 1996, 118, 9855–9866. doi:10.1021/ja9615738
Return to citation in text: [1] -
Engelkamp, H.; Middelbeek, S.; Nolte, R. J. M. Science 1999, 284, 785–788. doi:10.1126/science.284.5415.785
Return to citation in text: [1] -
He, L.; Liu, X.; Liang, J.; Cong, Y.; Weng, Z.; Bu, W. Chem. Commun. 2015, 51, 7148–7151. doi:10.1039/c5cc00934k
Return to citation in text: [1] -
Dong, S.; Zheng, B.; Xu, D.; Yan, X.; Zhang, M.; Huang, F. Adv. Mater. (Weinheim, Ger.) 2012, 24, 3191–3195. doi:10.1002/adma.201200837
Return to citation in text: [1] -
Li, H.; Fan, X.; Qi, M.; Yang, Z.; Zhang, H.; Tian, W. Chem. – Eur. J. 2016, 22, 101–105. doi:10.1002/chem.201504012
Return to citation in text: [1] -
Chen, D.; Zhan, J.; Zhang, M.; Zhang, J.; Tao, J.; Tang, D.; Shen, A.; Qiu, H.; Yin, S. Polym. Chem. 2015, 6, 25–29. doi:10.1039/c4py01206b
Return to citation in text: [1] -
Hu, X.-Y.; Xiao, T.; Lin, C.; Huang, F.; Wang, L. Acc. Chem. Res. 2014, 47, 2041–2051. doi:10.1021/ar5000709
Return to citation in text: [1] -
Ding, Y.; Wang, P.; Tian, Y.-K.; Tian, Y.-J.; Wang, F. Chem. Commun. 2013, 49, 5951–5953. doi:10.1039/c3cc42511h
Return to citation in text: [1] -
Xiao, T.; Feng, X.; Wang, Q.; Lin, C.; Wang, L.; Pan, Y. Chem. Commun. 2013, 49, 8329–8331. doi:10.1039/c3cc44525a
Return to citation in text: [1] -
Zhan, J.; Li, Q.; Hu, Q.; Wu, Q.; Li, C.; Qiu, H.; Zhang, M.; Yin, S. Chem. Commun. 2014, 50, 722–724. doi:10.1039/c3cc47468b
Return to citation in text: [1] -
Wang, W.; Zhang, Y.; Sun, B.; Chen, L.-J.; Xu, X.-D.; Wang, M.; Li, X.; Yu, Y.; Jiang, W.; Yang, H.-B. Chem. Sci. 2014, 5, 4554–4560. doi:10.1039/c4sc01550a
Return to citation in text: [1] -
Ji, X.; Chen, J.; Xue, M. Macromol. Chem. Phys. 2014, 215, 536–543. doi:10.1002/macp.201300691
Return to citation in text: [1] -
Chen, L.; Tian, Y.-K.; Ding, Y.; Tian, Y.-J.; Wang, F. Macromolecules 2012, 45, 8412–8419. doi:10.1021/ma3016486
Return to citation in text: [1] -
Li, S.; Weng, G.-H.; Lin, W.; Sun, Z.-B.; Zhou, M.; Zhu, B.; Ye, Y.; Wu, J. Polym. Chem. 2014, 5, 3994–4001. doi:10.1039/c4py00409d
Return to citation in text: [1] -
Park, J.; Kim, K. Y.; Kim, C.; Lee, J. H.; Kim, J. H.; Lee, S. S.; Choi, Y.; Jung, J. H. Polym. Chem. 2018, 9, 3900–3907. doi:10.1039/c8py00644j
Return to citation in text: [1] -
Xu, L.; Chen, D.; Zhang, Q.; He, T.; Lu, C.; Shen, X.; Tang, D.; Qiu, H.; Zhang, M.; Yin, S. Polym. Chem. 2018, 9, 399–403. doi:10.1039/c7py01788j
Return to citation in text: [1] -
Huang, G.; Jiang, W. Prog. Chem. 2015, 27, 744–754.
Return to citation in text: [1] -
Zheng, B.; Wang, F.; Dong, S.; Huang, F. Chem. Soc. Rev. 2012, 41, 1621–1636. doi:10.1039/c1cs15220c
Return to citation in text: [1] -
Dong, S.; Wang, L.; Wu, J.; Jin, L.; Ge, Y.; Qi, Z.; Wu, C. Langmuir 2017, 33, 13861–13866. doi:10.1021/acs.langmuir.7b03431
Return to citation in text: [1] -
Qi, Z.; Chiappisi, L.; Gong, H.; Pan, R.; Cui, N.; Ge, Y.; Böttcher, C.; Dong, S. Chem. – Eur. J. 2018, 24, 3854–3861. doi:10.1002/chem.201705838
Return to citation in text: [1] -
Dong, S.; Leng, J.; Feng, Y.; Liu, M.; Stackhouse, C. J.; Schönhals, A.; Chiappisi, L.; Gao, L.; Chen, W.; Shang, J.; Jin, L.; Qi, Z.; Schalley, C. A. Sci. Adv. 2017, 3, eaao0900. doi:10.1126/sciadv.aao0900
Return to citation in text: [1] -
Singh, P.; Mittal, L. S.; Kaur, S.; Kaur, S.; Bhargava, G.; Kumar, S. Sens. Actuators, B 2018, 255, 478–489. doi:10.1016/j.snb.2017.08.072
Return to citation in text: [1] -
Liu, K.; Xu, Z.; Yin, M. Prog. Polym. Sci. 2015, 46, 25–54. doi:10.1016/j.progpolymsci.2014.11.005
Return to citation in text: [1] -
Dimitrakopoulos, C. D.; Malenfant, P. R. L. Adv. Mater. (Weinheim, Ger.) 2002, 14, 99–117. doi:10.1002/1521-4095(20020116)14:2<99::aid-adma99>3.0.co;2-9
Return to citation in text: [1] -
Qu, J.; Mu, Z.; Lai, H.; Xie, M.; Liu, L.; Lu, W.; Chen, W.; He, F. ACS Appl. Energy Mater. 2018, 1, 833–840. doi:10.1021/acsaem.7b00277
Return to citation in text: [1] -
Hadmojo, W. T.; Yim, D.; Aqoma, H.; Ryu, D. Y.; Shin, T. J.; Kim, H. W.; Hwang, E.; Jang, W.-D.; Jung, I. H.; Jang, S.-Y. Chem. Sci. 2017, 8, 5095–5100. doi:10.1039/c7sc01275f
Return to citation in text: [1] -
Zhao, Y.; Wang, H.; Xia, S.; Zhou, F.; Luo, Z.; Luo, J.; He, F.; Yang, C. Chem. – Eur. J. 2018, 24, 4149–4156. doi:10.1002/chem.201705480
Return to citation in text: [1] -
Sung, J.; Kim, P.; Fimmel, B.; Würthner, F.; Kim, D. Nat. Commun. 2015, 6, 8646. doi:10.1038/ncomms9646
Return to citation in text: [1] -
Würthner, F. Chem. Commun. 2004, 1564–1579. doi:10.1039/b401630k
Return to citation in text: [1] -
Li, X.-Q.; Zhang, X.; Ghosh, S.; Würthner, F. Chem. – Eur. J. 2008, 14, 8074–8078. doi:10.1002/chem.200800915
Return to citation in text: [1] -
Gershberg, J.; Fennel, F.; Rehm, T. H.; Lochbrunner, S.; Würthner, F. Chem. Sci. 2016, 7, 1729–1737. doi:10.1039/c5sc03759j
Return to citation in text: [1] [2] [3] -
Görl, D.; Zhang, X.; Stepanenko, V.; Würthner, F. Nat. Commun. 2015, 6, 7009. doi:10.1038/ncomms8009
Return to citation in text: [1] [2] -
Sung, J.; Nowak-Król, A.; Schlosser, F.; Fimmel, B.; Kim, W.; Kim, D.; Würthner, F. J. Am. Chem. Soc. 2016, 138, 9029–9032. doi:10.1021/jacs.6b04591
Return to citation in text: [1] -
Wu, Y.; Young, R. M.; Frasconi, M.; Schneebeli, S. T.; Spenst, P.; Gardner, D. M.; Brown, K. E.; Würthner, F.; Stoddart, J. F.; Wasielewski, M. R. J. Am. Chem. Soc. 2015, 137, 13236–13239. doi:10.1021/jacs.5b08386
Return to citation in text: [1] -
Chen, Z.; Lohr, A.; Saha-Möller, C. R.; Würthner, F. Chem. Soc. Rev. 2009, 38, 564–584. doi:10.1039/b809359h
Return to citation in text: [1] -
Brixner, T.; Hildner, R.; Köhler, J.; Lambert, C.; Würthner, F. Adv. Energy Mater. 2017, 7, 1700236. doi:10.1002/aenm.201700236
Return to citation in text: [1] -
Pochas, C. M.; Kistler, K. A.; Yamagata, H.; Matsika, S.; Spano, F. C. J. Am. Chem. Soc. 2013, 135, 3056–3066. doi:10.1021/ja3087449
Return to citation in text: [1] -
Zhang, B.; Soleimaninejad, H.; Jones, D. J.; White, J. M.; Ghiggino, K. P.; Smith, T. A.; Wong, W. W. H. Chem. Mater. 2017, 29, 8395–8403. doi:10.1021/acs.chemmater.7b02968
Return to citation in text: [1] -
Krieg, E.; Rybtchinski, B. Chem. – Eur. J. 2011, 17, 9016–9026. doi:10.1002/chem.201100809
Return to citation in text: [1] -
Cohen, E.; Weissman, H.; Pinkas, I.; Shimoni, E.; Rehak, P.; Král, P.; Rybtchinski, B. ACS Nano 2018, 12, 317–326. doi:10.1021/acsnano.7b06376
Return to citation in text: [1] -
Tidhar, Y.; Weissman, H.; Wolf, S. G.; Gulino, A.; Rybtchinski, B. Chem. – Eur. J. 2011, 17, 6068–6075. doi:10.1002/chem.201003419
Return to citation in text: [1] [2] -
Zhang, X.; Rehm, S.; Safont-Sempere, M. M.; Würthner, F. Nat. Chem. 2009, 1, 623–629. doi:10.1038/nchem.368
Return to citation in text: [1] -
Zhang, X.; Chen, Z.; Würthner, F. J. Am. Chem. Soc. 2007, 129, 4886–4887. doi:10.1021/ja070994u
Return to citation in text: [1] [2] -
Ustinov, A.; Weissman, H.; Shirman, E.; Pinkas, I.; Zuo, X.; Rybtchinski, B. J. Am. Chem. Soc. 2011, 133, 16201–16211. doi:10.1021/ja2066225
Return to citation in text: [1] -
Krieg, E.; Shirman, E.; Weissman, H.; Shimoni, E.; Wolf, S. G.; Pinkas, I.; Rybtchinski, B. J. Am. Chem. Soc. 2009, 131, 14365–14373. doi:10.1021/ja903938g
Return to citation in text: [1] -
Langhals, H.; Jona, W.; Einsiedl, F.; Wohnlich, S. Adv. Mater. (Weinheim, Ger.) 1998, 10, 1022–1024. doi:10.1002/(sici)1521-4095(199809)10:13<1022::aid-adma1022>3.0.co;2-o
Return to citation in text: [1] -
Licchelli, M.; Orbelli Biroli, A.; Poggi, A. Org. Lett. 2006, 8, 915–918. doi:10.1021/ol053084v
Return to citation in text: [1] -
Ma, Y.; Marszalek, T.; Yuan, Z.; Stangenberg, R.; Pisula, W.; Chen, L.; Müllen, K. Chem. – Asian J. 2015, 10, 139–143. doi:10.1002/asia.201403037
Return to citation in text: [1] [2] -
Weißenstein, A.; Würthner, F. Chem. Commun. 2015, 51, 3415–3418. doi:10.1039/c4cc09443c
Return to citation in text: [1] -
Qi, Z.; Malo de Molina, P.; Jiang, W.; Wang, Q.; Nowosinski, K.; Schulz, A.; Gradzielski, M.; Schalley, C. A. Chem. Sci. 2012, 3, 2073–2082. doi:10.1039/c2sc01018f
Return to citation in text: [1] -
Jiang, W.; Schalley, C. A. Beilstein J. Org. Chem. 2010, 6, No. 14. doi:10.3762/bjoc.6.14
Return to citation in text: [1] -
Qi, Z.; Traulsen, N. L.; Malo de Molina, P.; Schlaich, C.; Gradzielski, M.; Schalley, C. A. Org. Biomol. Chem. 2014, 12, 503–510. doi:10.1039/c3ob41523f
Return to citation in text: [1] -
Qi, Z.; Wu, C.; Malo de Molina, P.; Sun, H.; Schulz, A.; Griesinger, C.; Gradzielski, M.; Haag, R.; Ansorge-Schumacher, M. B.; Schalley, C. A. Chem. – Eur. J. 2013, 19, 10150–10159. doi:10.1002/chem.201300193
Return to citation in text: [1] -
Huang, D.; Zhang, Q.; Deng, Y.; Luo, Z.; Li, B.; Shen, X.; Qi, Z.; Dong, S.; Ge, Y.; Chen, W. Polym. Chem. 2018, 9, 2574–2579. doi:10.1039/c8py00412a
Return to citation in text: [1] -
Qi, Z.; Schlaich, C.; Schalley, C. A. Chem. – Eur. J. 2013, 19, 14867–14875. doi:10.1002/chem.201301951
Return to citation in text: [1] -
Ge, Y.; Gong, H.; Shang, J.; Jin, L.; Pan, T.; Zhang, Q.; Dong, S.; Wang, Y.; Qi, Z. Macromol. Rapid Commun. 2019, 1800731. doi:10.1002/marc.201800731
Return to citation in text: [1] -
Li, A. D. Q.; Wang, W.; Wang, L.-Q. Chem. – Eur. J. 2003, 9, 4594–4601. doi:10.1002/chem.200305025
Return to citation in text: [1] -
Dehm, V.; Büchner, M.; Seibt, J.; Engel, V.; Würthner, F. Chem. Sci. 2011, 2, 2094–2100. doi:10.1039/c1sc00435b
Return to citation in text: [1] -
Fennel, F.; Gershberg, J.; Stolte, M.; Würthner, F. Phys. Chem. Chem. Phys. 2018, 20, 7612–7620. doi:10.1039/c7cp07778e
Return to citation in text: [1] -
Beckers, E. H. A.; Meskers, S. C. J.; Schenning, A. P. H. J.; Chen, Z.; Würthner, F.; Janssen, R. A. J. J. Phys. Chem. A 2004, 108, 6933–6937. doi:10.1021/jp048980n
Return to citation in text: [1] -
Würthner, F.; Thalacker, C.; Diele, S.; Tschierske, C. Chem. – Eur. J. 2001, 7, 2245–2253. doi:10.1002/1521-3765(20010518)7:10<2245::aid-chem2245>3.0.co;2-w
Return to citation in text: [1] -
Li, Z.-Y.; Zhang, Y.; Zhang, C.-W.; Chen, L.-J.; Wang, C.; Tan, H.; Yu, Y.; Li, X.; Yang, H.-B. J. Am. Chem. Soc. 2014, 136, 8577–8589. doi:10.1021/ja413047r
Return to citation in text: [1] -
Chen, L.-J.; Zhao, G.-Z.; Jiang, B.; Sun, B.; Wang, M.; Xu, L.; He, J.; Abliz, Z.; Tan, H.; Li, X.; Yang, H.-B. J. Am. Chem. Soc. 2014, 136, 5993–6001. doi:10.1021/ja500152a
Return to citation in text: [1]
| 84. | Ma, Y.; Marszalek, T.; Yuan, Z.; Stangenberg, R.; Pisula, W.; Chen, L.; Müllen, K. Chem. – Asian J. 2015, 10, 139–143. doi:10.1002/asia.201403037 |
| 67. | Gershberg, J.; Fennel, F.; Rehm, T. H.; Lochbrunner, S.; Würthner, F. Chem. Sci. 2016, 7, 1729–1737. doi:10.1039/c5sc03759j |
| 95. | Fennel, F.; Gershberg, J.; Stolte, M.; Würthner, F. Phys. Chem. Chem. Phys. 2018, 20, 7612–7620. doi:10.1039/c7cp07778e |
| 96. | Beckers, E. H. A.; Meskers, S. C. J.; Schenning, A. P. H. J.; Chen, Z.; Würthner, F.; Janssen, R. A. J. J. Phys. Chem. A 2004, 108, 6933–6937. doi:10.1021/jp048980n |
| 97. | Würthner, F.; Thalacker, C.; Diele, S.; Tschierske, C. Chem. – Eur. J. 2001, 7, 2245–2253. doi:10.1002/1521-3765(20010518)7:10<2245::aid-chem2245>3.0.co;2-w |
| 1. | Ariga, K.; Hill, J. P.; Lee, M. V.; Vinu, A.; Charvet, R.; Acharya, S. Adv. Mater. (Weinheim, Ger.) 2009, 9, 014109. doi:10.1088/1468-6996/9/1/014109 |
| 2. | Abbas, M.; Zou, Q.; Li, S.; Yan, X. Adv. Mater. (Weinheim, Ger.) 2017, 29, 1605021. doi:10.1002/adma.201605021 |
| 3. | Schacher, F. H.; Rupar, P. A.; Manners, I. Angew. Chem., Int. Ed. 2012, 51, 7898–7921. doi:10.1002/anie.201200310 |
| 4. | Li, X.; Iocozzia, J.; Chen, Y.; Zhao, S.; Cui, X.; Wang, W.; Yu, H.; Lin, S.; Lin, Z. Angew. Chem., Int. Ed. 2018, 57, 2046–2070. doi:10.1002/anie.201705019 |
| 5. | Chen, L.-J.; Yang, H.-B. Acc. Chem. Res. 2018, 51, 2699–2710. doi:10.1021/acs.accounts.8b00317 |
| 6. | Li, B.; Wang, B.; Huang, X.; Dai, L.; Cui, L.; Li, J.; Jia, X.; Li, C. Angew. Chem., Int. Ed. 2019, 58, 3885–3889. doi:10.1002/anie.201813972 |
| 14. | Sano, M.; Kamino, A.; Okamura, J.; Shinkai, S. Nano Lett. 2002, 2, 531–533. doi:10.1021/nl025525z |
| 15. | Patra, N.; Song, Y.; Král, P. ACS Nano 2011, 5, 1798–1804. doi:10.1021/nn102531h |
| 16. | Poonia, M.; Gupta, R. K.; Manjuladevi, V.; Gupta, S. K.; Akhtar, J. J. Nanopart. Res. 2014, 16, 2572. doi:10.1007/s11051-014-2572-2 |
| 17. | Derenskyi, V.; Gomulya, W.; Talsma, W.; Salazar-Rios, J. M.; Fritsch, M.; Nirmalraj, P.; Riel, H.; Allard, S.; Scherf, U.; Loi, M. A. Adv. Mater. (Weinheim, Ger.) 2017, 29, 1606757. doi:10.1002/adma.201606757 |
| 56. | Qi, Z.; Chiappisi, L.; Gong, H.; Pan, R.; Cui, N.; Ge, Y.; Böttcher, C.; Dong, S. Chem. – Eur. J. 2018, 24, 3854–3861. doi:10.1002/chem.201705838 |
| 11. | Xu, Y.; Sheng, K.; Li, C.; Shi, G. ACS Nano 2010, 4, 4324–4330. doi:10.1021/nn101187z |
| 12. | Hauser, C. A. E.; Deng, R.; Mishra, A.; Loo, Y.; Khoe, U.; Zhuang, F.; Cheong, D. W.; Accardo, A.; Sullivan, M. B.; Riekel, C.; Ying, J. Y.; Hauser, U. A. Proc. Natl. Acad. Sci. U. S. A. 2011, 108, 1361–1366. doi:10.1073/pnas.1014796108 |
| 13. | Sutar, P.; Suresh, V. M.; Jayaramulu, K.; Hazra, A.; Maji, T. K. Nat. Commun. 2018, 9, No. 3587. doi:10.1038/s41467-018-05818-w |
| 57. | Dong, S.; Leng, J.; Feng, Y.; Liu, M.; Stackhouse, C. J.; Schönhals, A.; Chiappisi, L.; Gao, L.; Chen, W.; Shang, J.; Jin, L.; Qi, Z.; Schalley, C. A. Sci. Adv. 2017, 3, eaao0900. doi:10.1126/sciadv.aao0900 |
| 8. | Pandya, M. J.; Spooner, G. M.; Sunde, M.; Thorpe, J. R.; Rodger, A.; Woolfson, D. N. Biochemistry 2000, 39, 8728–8734. doi:10.1021/bi000246g |
| 9. | Mulyasasmita, W.; Lee, J. S.; Heilshorn, S. C. Biomacromolecules 2011, 12, 3406–3411. doi:10.1021/bm200959e |
| 10. | Luo, Q.; Hou, C.; Bai, Y.; Wang, R.; Liu, J. Chem. Rev. 2016, 116, 13571–13632. doi:10.1021/acs.chemrev.6b00228 |
| 54. | Zheng, B.; Wang, F.; Dong, S.; Huang, F. Chem. Soc. Rev. 2012, 41, 1621–1636. doi:10.1039/c1cs15220c |
| 7. | Lehn, J.-M. Angew. Chem., Int. Ed. Engl. 1988, 27, 89–112. doi:10.1002/anie.198800891 |
| 55. | Dong, S.; Wang, L.; Wu, J.; Jin, L.; Ge, Y.; Qi, Z.; Wu, C. Langmuir 2017, 33, 13861–13866. doi:10.1021/acs.langmuir.7b03431 |
| 28. | Xie, S.; Wu, S.; Bao, S.; Wang, Y.; Zheng, Y.; Deng, D.; Huang, L.; Zhang, L.; Lee, M.; Huang, Z. Adv. Mater. (Weinheim, Ger.) 2018, 30, 1800683. doi:10.1002/adma.201800683 |
| 29. | Cheng, H.-B.; Li, Z.; Huang, Y.-D.; Liu, L.; Wu, H.-C. ACS Appl. Mater. Interfaces 2017, 9, 11889–11894. doi:10.1021/acsami.7b00363 |
| 33. | Albelda, M. T.; Frías, J. C.; García-España, E.; Schneider, H.-J. Chem. Soc. Rev. 2012, 41, 3859–3877. doi:10.1039/c2cs35008d |
| 34. | Biros, S. M.; Rebek, J., Jr.. Chem. Soc. Rev. 2007, 36, 93–104. doi:10.1039/b508530f |
| 35. | Kataev, E. A.; Müller, C. Tetrahedron 2014, 70, 137–167. doi:10.1016/j.tet.2013.11.010 |
| 36. | Oshovsky, G. V.; Reinhoudt, D. N.; Verboom, W. Angew. Chem., Int. Ed. 2007, 46, 2366–2393. doi:10.1002/anie.200602815 |
| 98. | Li, Z.-Y.; Zhang, Y.; Zhang, C.-W.; Chen, L.-J.; Wang, C.; Tan, H.; Yu, Y.; Li, X.; Yang, H.-B. J. Am. Chem. Soc. 2014, 136, 8577–8589. doi:10.1021/ja413047r |
| 99. | Chen, L.-J.; Zhao, G.-Z.; Jiang, B.; Sun, B.; Wang, M.; Xu, L.; He, J.; Abliz, Z.; Tan, H.; Li, X.; Yang, H.-B. J. Am. Chem. Soc. 2014, 136, 5993–6001. doi:10.1021/ja500152a |
| 27. | Bera, S.; Haldar, D. J. Mater. Chem. A 2016, 4, 6933–6939. doi:10.1039/c5ta08010j |
| 37. | Percec, V.; Johansson, G.; Ungar, G.; Zhou, J. J. Am. Chem. Soc. 1996, 118, 9855–9866. doi:10.1021/ja9615738 |
| 38. | Engelkamp, H.; Middelbeek, S.; Nolte, R. J. M. Science 1999, 284, 785–788. doi:10.1126/science.284.5415.785 |
| 39. | He, L.; Liu, X.; Liang, J.; Cong, Y.; Weng, Z.; Bu, W. Chem. Commun. 2015, 51, 7148–7151. doi:10.1039/c5cc00934k |
| 40. | Dong, S.; Zheng, B.; Xu, D.; Yan, X.; Zhang, M.; Huang, F. Adv. Mater. (Weinheim, Ger.) 2012, 24, 3191–3195. doi:10.1002/adma.201200837 |
| 41. | Li, H.; Fan, X.; Qi, M.; Yang, Z.; Zhang, H.; Tian, W. Chem. – Eur. J. 2016, 22, 101–105. doi:10.1002/chem.201504012 |
| 42. | Chen, D.; Zhan, J.; Zhang, M.; Zhang, J.; Tao, J.; Tang, D.; Shen, A.; Qiu, H.; Yin, S. Polym. Chem. 2015, 6, 25–29. doi:10.1039/c4py01206b |
| 43. | Hu, X.-Y.; Xiao, T.; Lin, C.; Huang, F.; Wang, L. Acc. Chem. Res. 2014, 47, 2041–2051. doi:10.1021/ar5000709 |
| 44. | Ding, Y.; Wang, P.; Tian, Y.-K.; Tian, Y.-J.; Wang, F. Chem. Commun. 2013, 49, 5951–5953. doi:10.1039/c3cc42511h |
| 45. | Xiao, T.; Feng, X.; Wang, Q.; Lin, C.; Wang, L.; Pan, Y. Chem. Commun. 2013, 49, 8329–8331. doi:10.1039/c3cc44525a |
| 46. | Zhan, J.; Li, Q.; Hu, Q.; Wu, Q.; Li, C.; Qiu, H.; Zhang, M.; Yin, S. Chem. Commun. 2014, 50, 722–724. doi:10.1039/c3cc47468b |
| 47. | Wang, W.; Zhang, Y.; Sun, B.; Chen, L.-J.; Xu, X.-D.; Wang, M.; Li, X.; Yu, Y.; Jiang, W.; Yang, H.-B. Chem. Sci. 2014, 5, 4554–4560. doi:10.1039/c4sc01550a |
| 48. | Ji, X.; Chen, J.; Xue, M. Macromol. Chem. Phys. 2014, 215, 536–543. doi:10.1002/macp.201300691 |
| 49. | Chen, L.; Tian, Y.-K.; Ding, Y.; Tian, Y.-J.; Wang, F. Macromolecules 2012, 45, 8412–8419. doi:10.1021/ma3016486 |
| 50. | Li, S.; Weng, G.-H.; Lin, W.; Sun, Z.-B.; Zhou, M.; Zhu, B.; Ye, Y.; Wu, J. Polym. Chem. 2014, 5, 3994–4001. doi:10.1039/c4py00409d |
| 51. | Park, J.; Kim, K. Y.; Kim, C.; Lee, J. H.; Kim, J. H.; Lee, S. S.; Choi, Y.; Jung, J. H. Polym. Chem. 2018, 9, 3900–3907. doi:10.1039/c8py00644j |
| 52. | Xu, L.; Chen, D.; Zhang, Q.; He, T.; Lu, C.; Shen, X.; Tang, D.; Qiu, H.; Zhang, M.; Yin, S. Polym. Chem. 2018, 9, 399–403. doi:10.1039/c7py01788j |
| 53. | Huang, G.; Jiang, W. Prog. Chem. 2015, 27, 744–754. |
| 77. | Tidhar, Y.; Weissman, H.; Wolf, S. G.; Gulino, A.; Rybtchinski, B. Chem. – Eur. J. 2011, 17, 6068–6075. doi:10.1002/chem.201003419 |
| 24. | Prathap, A.; Sureshan, K. M. Angew. Chem., Int. Ed. 2017, 56, 9405–9409. doi:10.1002/anie.201704699 |
| 25. | Molji, C.; Aashish, A.; Neethu, K. S.; Devaki, S. J. J. Mater. Chem. A 2017, 5, 16636–16645. doi:10.1039/c7ta05215d |
| 26. | Dell'Elce, S.; Liscio, F.; Kovtun, A.; Allegri, S.; Roscioni, O. M.; Albonetti, C.; De Luca, G.; Amenitsch, H. W.; Demitri, N.; Giorgini, L.; Morandi, V.; Stellacci, F.; Liscio, A.; Palermo, V. Nanoscale 2018, 10, 23018–23026. doi:10.1039/c8nr07109h |
| 68. | Görl, D.; Zhang, X.; Stepanenko, V.; Würthner, F. Nat. Commun. 2015, 6, 7009. doi:10.1038/ncomms8009 |
| 18. | Bastings, M. M. C.; Koudstaal, S.; Kieltyka, R. E.; Nakano, Y.; Pape, A. C. H.; Feyen, D. A. M.; van Slochteren, F. J.; Doevendans, P. A.; Sluijter, J. P. G.; Meijer, E. W.; Chamuleau, S. A. J.; Dankers, P. Y. W. Adv. Healthcare Mater. 2014, 3, 70–78. doi:10.1002/adhm.201300076 |
| 19. | Kakuta, T.; Takashima, Y.; Nakahata, M.; Otsubo, M.; Yamaguchi, H.; Harada, A. Adv. Mater. (Weinheim, Ger.) 2013, 25, 2849–2853. doi:10.1002/adma.201205321 |
| 20. | Lu, H. D.; Charati, M. B.; Kim, I. L.; Burdick, J. A. Biomaterials 2012, 33, 2145–2153. doi:10.1016/j.biomaterials.2011.11.076 |
| 21. | Bode, S.; Zedler, L.; Schacher, F. H.; Dietzek, B.; Schmitt, M.; Popp, J.; Hager, M. D.; Schubert, U. S. Adv. Mater. (Weinheim, Ger.) 2013, 25, 1634–1638. doi:10.1002/adma.201203865 |
| 22. | Chen, H.; Ma, X.; Wu, S.; Tian, H. Angew. Chem., Int. Ed. 2014, 53, 14149–14152. doi:10.1002/anie.201407402 |
| 23. | Zhang, M.; Xu, D.; Yan, X.; Chen, J.; Dong, S.; Zheng, B.; Huang, F. Angew. Chem., Int. Ed. 2012, 51, 7011–7015. doi:10.1002/anie.201203063 |
| 30. | Zheng, J.; Zhu, G.; Li, Y.; Li, C.; You, M.; Chen, T.; Song, E.; Yang, R.; Tan, W. ACS Nano 2013, 7, 6545–6554. doi:10.1021/nn402344v |
| 31. | Molino, N. M.; Wang, S.-W. Curr. Opin. Biotechnol. 2014, 28, 75–82. doi:10.1016/j.copbio.2013.12.007 |
| 32. | Liang, H.; Zhang, X.-B.; Lv, Y.; Gong, L.; Wang, R.; Zhu, X.; Yang, R.; Tan, W. Acc. Chem. Res. 2014, 47, 1891–1901. doi:10.1021/ar500078f |
| 79. | Zhang, X.; Chen, Z.; Würthner, F. J. Am. Chem. Soc. 2007, 129, 4886–4887. doi:10.1021/ja070994u |
| 62. | Hadmojo, W. T.; Yim, D.; Aqoma, H.; Ryu, D. Y.; Shin, T. J.; Kim, H. W.; Hwang, E.; Jang, W.-D.; Jung, I. H.; Jang, S.-Y. Chem. Sci. 2017, 8, 5095–5100. doi:10.1039/c7sc01275f |
| 63. | Zhao, Y.; Wang, H.; Xia, S.; Zhou, F.; Luo, Z.; Luo, J.; He, F.; Yang, C. Chem. – Eur. J. 2018, 24, 4149–4156. doi:10.1002/chem.201705480 |
| 64. | Sung, J.; Kim, P.; Fimmel, B.; Würthner, F.; Kim, D. Nat. Commun. 2015, 6, 8646. doi:10.1038/ncomms9646 |
| 65. | Würthner, F. Chem. Commun. 2004, 1564–1579. doi:10.1039/b401630k |
| 58. | Singh, P.; Mittal, L. S.; Kaur, S.; Kaur, S.; Bhargava, G.; Kumar, S. Sens. Actuators, B 2018, 255, 478–489. doi:10.1016/j.snb.2017.08.072 |
| 59. | Liu, K.; Xu, Z.; Yin, M. Prog. Polym. Sci. 2015, 46, 25–54. doi:10.1016/j.progpolymsci.2014.11.005 |
| 60. | Dimitrakopoulos, C. D.; Malenfant, P. R. L. Adv. Mater. (Weinheim, Ger.) 2002, 14, 99–117. doi:10.1002/1521-4095(20020116)14:2<99::aid-adma99>3.0.co;2-9 |
| 61. | Qu, J.; Mu, Z.; Lai, H.; Xie, M.; Liu, L.; Lu, W.; Chen, W.; He, F. ACS Appl. Energy Mater. 2018, 1, 833–840. doi:10.1021/acsaem.7b00277 |
| 67. | Gershberg, J.; Fennel, F.; Rehm, T. H.; Lochbrunner, S.; Würthner, F. Chem. Sci. 2016, 7, 1729–1737. doi:10.1039/c5sc03759j |
| 93. | Li, A. D. Q.; Wang, W.; Wang, L.-Q. Chem. – Eur. J. 2003, 9, 4594–4601. doi:10.1002/chem.200305025 |
| 94. | Dehm, V.; Büchner, M.; Seibt, J.; Engel, V.; Würthner, F. Chem. Sci. 2011, 2, 2094–2100. doi:10.1039/c1sc00435b |
| 86. | Qi, Z.; Malo de Molina, P.; Jiang, W.; Wang, Q.; Nowosinski, K.; Schulz, A.; Gradzielski, M.; Schalley, C. A. Chem. Sci. 2012, 3, 2073–2082. doi:10.1039/c2sc01018f |
| 87. | Jiang, W.; Schalley, C. A. Beilstein J. Org. Chem. 2010, 6, No. 14. doi:10.3762/bjoc.6.14 |
| 88. | Qi, Z.; Traulsen, N. L.; Malo de Molina, P.; Schlaich, C.; Gradzielski, M.; Schalley, C. A. Org. Biomol. Chem. 2014, 12, 503–510. doi:10.1039/c3ob41523f |
| 89. | Qi, Z.; Wu, C.; Malo de Molina, P.; Sun, H.; Schulz, A.; Griesinger, C.; Gradzielski, M.; Haag, R.; Ansorge-Schumacher, M. B.; Schalley, C. A. Chem. – Eur. J. 2013, 19, 10150–10159. doi:10.1002/chem.201300193 |
| 90. | Huang, D.; Zhang, Q.; Deng, Y.; Luo, Z.; Li, B.; Shen, X.; Qi, Z.; Dong, S.; Ge, Y.; Chen, W. Polym. Chem. 2018, 9, 2574–2579. doi:10.1039/c8py00412a |
| 91. | Qi, Z.; Schlaich, C.; Schalley, C. A. Chem. – Eur. J. 2013, 19, 14867–14875. doi:10.1002/chem.201301951 |
| 92. | Ge, Y.; Gong, H.; Shang, J.; Jin, L.; Pan, T.; Zhang, Q.; Dong, S.; Wang, Y.; Qi, Z. Macromol. Rapid Commun. 2019, 1800731. doi:10.1002/marc.201800731 |
| 75. | Krieg, E.; Rybtchinski, B. Chem. – Eur. J. 2011, 17, 9016–9026. doi:10.1002/chem.201100809 |
| 76. | Cohen, E.; Weissman, H.; Pinkas, I.; Shimoni, E.; Rehak, P.; Král, P.; Rybtchinski, B. ACS Nano 2018, 12, 317–326. doi:10.1021/acsnano.7b06376 |
| 77. | Tidhar, Y.; Weissman, H.; Wolf, S. G.; Gulino, A.; Rybtchinski, B. Chem. – Eur. J. 2011, 17, 6068–6075. doi:10.1002/chem.201003419 |
| 78. | Zhang, X.; Rehm, S.; Safont-Sempere, M. M.; Würthner, F. Nat. Chem. 2009, 1, 623–629. doi:10.1038/nchem.368 |
| 79. | Zhang, X.; Chen, Z.; Würthner, F. J. Am. Chem. Soc. 2007, 129, 4886–4887. doi:10.1021/ja070994u |
| 80. | Ustinov, A.; Weissman, H.; Shirman, E.; Pinkas, I.; Zuo, X.; Rybtchinski, B. J. Am. Chem. Soc. 2011, 133, 16201–16211. doi:10.1021/ja2066225 |
| 81. | Krieg, E.; Shirman, E.; Weissman, H.; Shimoni, E.; Wolf, S. G.; Pinkas, I.; Rybtchinski, B. J. Am. Chem. Soc. 2009, 131, 14365–14373. doi:10.1021/ja903938g |
| 82. | Langhals, H.; Jona, W.; Einsiedl, F.; Wohnlich, S. Adv. Mater. (Weinheim, Ger.) 1998, 10, 1022–1024. doi:10.1002/(sici)1521-4095(199809)10:13<1022::aid-adma1022>3.0.co;2-o |
| 83. | Licchelli, M.; Orbelli Biroli, A.; Poggi, A. Org. Lett. 2006, 8, 915–918. doi:10.1021/ol053084v |
| 84. | Ma, Y.; Marszalek, T.; Yuan, Z.; Stangenberg, R.; Pisula, W.; Chen, L.; Müllen, K. Chem. – Asian J. 2015, 10, 139–143. doi:10.1002/asia.201403037 |
| 85. | Weißenstein, A.; Würthner, F. Chem. Commun. 2015, 51, 3415–3418. doi:10.1039/c4cc09443c |
| 66. | Li, X.-Q.; Zhang, X.; Ghosh, S.; Würthner, F. Chem. – Eur. J. 2008, 14, 8074–8078. doi:10.1002/chem.200800915 |
| 67. | Gershberg, J.; Fennel, F.; Rehm, T. H.; Lochbrunner, S.; Würthner, F. Chem. Sci. 2016, 7, 1729–1737. doi:10.1039/c5sc03759j |
| 68. | Görl, D.; Zhang, X.; Stepanenko, V.; Würthner, F. Nat. Commun. 2015, 6, 7009. doi:10.1038/ncomms8009 |
| 69. | Sung, J.; Nowak-Król, A.; Schlosser, F.; Fimmel, B.; Kim, W.; Kim, D.; Würthner, F. J. Am. Chem. Soc. 2016, 138, 9029–9032. doi:10.1021/jacs.6b04591 |
| 70. | Wu, Y.; Young, R. M.; Frasconi, M.; Schneebeli, S. T.; Spenst, P.; Gardner, D. M.; Brown, K. E.; Würthner, F.; Stoddart, J. F.; Wasielewski, M. R. J. Am. Chem. Soc. 2015, 137, 13236–13239. doi:10.1021/jacs.5b08386 |
| 71. | Chen, Z.; Lohr, A.; Saha-Möller, C. R.; Würthner, F. Chem. Soc. Rev. 2009, 38, 564–584. doi:10.1039/b809359h |
| 72. | Brixner, T.; Hildner, R.; Köhler, J.; Lambert, C.; Würthner, F. Adv. Energy Mater. 2017, 7, 1700236. doi:10.1002/aenm.201700236 |
| 73. | Pochas, C. M.; Kistler, K. A.; Yamagata, H.; Matsika, S.; Spano, F. C. J. Am. Chem. Soc. 2013, 135, 3056–3066. doi:10.1021/ja3087449 |
| 74. | Zhang, B.; Soleimaninejad, H.; Jones, D. J.; White, J. M.; Ghiggino, K. P.; Smith, T. A.; Wong, W. W. H. Chem. Mater. 2017, 29, 8395–8403. doi:10.1021/acs.chemmater.7b02968 |
© 2019 Shen et al.; licensee Beilstein-Institut.
This is an Open Access article under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/4.0). Please note that the reuse, redistribution and reproduction in particular requires that the authors and source are credited.
The license is subject to the Beilstein Journal of Organic Chemistry terms and conditions: (https://www.beilstein-journals.org/bjoc)