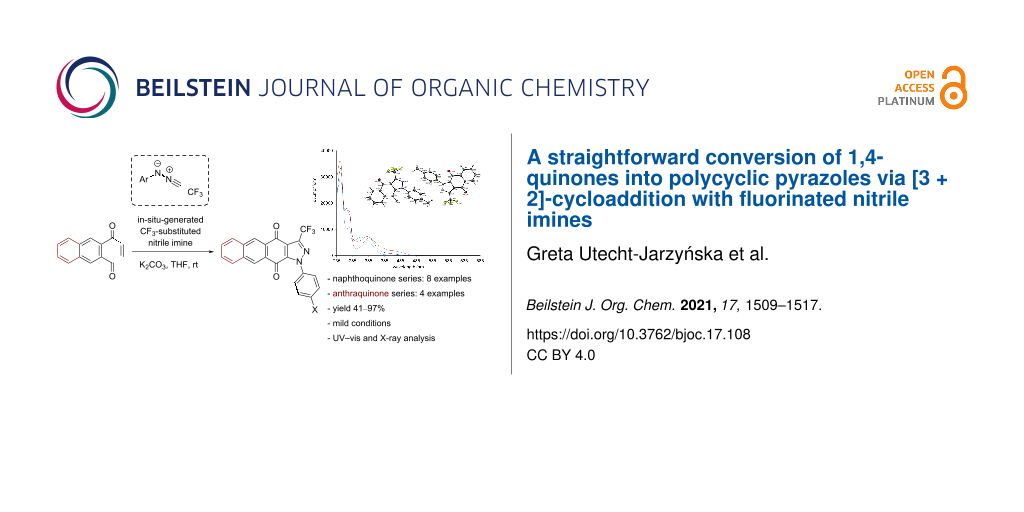
Abstract
In-situ-generated N-aryl nitrile imines derived from trifluoroacetonitrile efficiently react with polycyclic 1,4-quinones, yielding fused pyrazole derivatives as the exclusive products. The reactions proceed via the initially formed [3 + 2]-cycloadducts, which undergo spontaneous aerial oxidation to give aromatized heterocyclic products. Only for 2,3,5,6-tetramethyl-1,4-benzoquinone, the expected [3 + 2]-cycloadduct exhibited fair stability and could be isolated in moderate yield (53%). The presented method offers a straightforward access to hitherto little known trifluoromethylated polycyclic pyrazoles. All products were isolated as pale colored solids with medium-intensity absorption maxima in the range of 310–340 nm for naphthoquinone-derived products and low-intensity bands in the visible region (≈400 nm) for the anthraquinone series.
Graphical Abstract
Introduction
The 1,4-quinone scaffold belongs to the most important structural motifs present in naturally occurring compounds as well as synthetic drugs and other functionalized organic molecules of great practical importance, e.g., in materials chemistry (Figure 1) [1-4].
Figure 1: Structures of exemplary benzo- and heteroaromatic fused 1,4-quinone drugs and natural products.
Figure 1: Structures of exemplary benzo- and heteroaromatic fused 1,4-quinone drugs and natural products.
Selective functionalization of 1,4-quinones is a challenging task in current organic synthesis, and diverse transformations are known to create new C–C bonds and/or to extend the (poly)cyclic system. In this context, cycloadditions are of special importance, and Diels–Alder reactions have successfully been explored in conversions aimed at the construction of a new, six-membered ring [5]. However, [3 + 2]-cycloadditions leading to five-membered heterocycles are less often employed in spite of the high dipolarophilicity of the α,β-unsaturated diketone system [6-9]. Notably, in the already reported reactions of propargylic 1,3-dipoles, such as nitrile oxides or nitrile ylides, with 1,4-quinones, competitive reaction courses involving either ethylenic C=C or carbonyl C=O bonds were observed. For example, the more polar arylnitrile oxides and 1,4-benzoquinones reacted via addition to the C=C bond to give fused isoxazole derivatives [10-12] as well as with the C=O bond yielding spirocyclic 1,4,2-dioxazole derivatives [13,14]. Furthermore, for photochemically generated benzonitrile isopropanide, competitive C=O and C=C additions with 1,4-quinones were observed [15]. On the other hand, the slightly less polar benzonitrile benzylide underwent [3 + 2]-cycloaddition to the C=C bond exclusively [16]. Noteworthy, [3 + 2]-cycloadditions of nitrile imines to the C=O group of 1,4-quinones have not yet been reported.
In a historical work by Rolf Huisgen et al., the first [3 + 2]-cycloadditions of some 1,4-quinones, e.g., 1,4-naphthoquinone (1a), with C,N-diphenyl nitrile imine (2) were reported in the 1960s [17]. The latter 1,3-dipole was generated thermally from the respective tetrazole derivative 3, and the observed [3 + 2]-cycloadditions occurred chemoselectively to provide fused pyrazoles of the type 4 as exclusive products (Scheme 1).
Scheme 1: First [3 + 2]-cycloaddition of 1,4-naphthoquinone (1a) with intermediate nitrile imine 2, generated from tetrazole 3, reported by Huisgen et al. [17].
Scheme 1: First [3 + 2]-cycloaddition of 1,4-naphthoquinone (1a) with intermediate nitrile imine 2, generated...
This type of cycloaddition attracted considerable attention of other groups [18,19] and recently has been applied for the preparation of some π-extended pyrazole derivatives, which exhibited promising biological activity [20].
In a series of our recent publications, efficient syntheses of fluoromethylated five- and six-membered N,S-heterocycles, such as 5 and 6, available via [3 + 2]-cycloadditions [21-25] or [3 + 3]-annulations [26] of trifluoroacetonitrile imines 7, respectively, were reported (Scheme 2).
Scheme 2: Selected applications of trifluoroacetonitrile imines 7 in the synthesis of S-containing 5- and 6-membered heterocycles.
Scheme 2: Selected applications of trifluoroacetonitrile imines 7 in the synthesis of S-containing 5- and 6-m...
Noteworthy, the latter 1,3-dipoles are easily generated under mild conditions via base-mediated dehydrobromination of the respective hydrazonoyl bromides 8 and smoothly undergo [3 + 2]-cycloadditions with both electron-rich C=C dipolarophiles [27-29] and arynes [30], yielding the corresponding pyrazole derivatives. Unexpectedly, they reacted also with electron-deficient polyfluorinated thioamides to give the desired 1,3,4-thiadiazoles as the products of [3 + 2]-cycloaddition to the C=S bond [25].
Taking into account that fluorinated heterocycles [31-34], including pyrazoles [31,35,36], are of great significance for various medicinal and agricultural applications, the development of new methods for the construction of fluorine-containing organic molecules combined with the 1,4-quinone moiety can be considered as a challenging problem of current organic synthesis. Thus, the main goal of the present study was to check the course of [3 + 2]-cycloaddition reactions of electron-deficient CF3-substituted nitrile imines 7 with 1,4-naphthoquinone (1a) and 1,4-anthraquinone (1b), which were selected as model dipolarophiles. In addition, an important issue of the work was the examination of the chemoselectivity governing the formation of five-membered rings via competitive cycloaddition of the in-situ-generated 1,3-dipoles either onto the C=C or C=O bond. The present work should also be considered as an extension of our earlier studies focused on the exploration of 1,4-quinones in the [3 + 2]-cycloaddition and hetero-Diels–Alder reaction performed with thiocarbonyl S-methanides and thiochalcones, respectively [37,38].
Results and Discussion
In a preliminary experiment, the reaction of 1,4-naphthoquinone (1a) with N-phenyltrifluoroacetohydrazonoyl bromide (8a), used as an easy-to-handle precursor of nitrile imine 7a, was examined. The reaction was performed in selected aprotic organic solvents by using organic and inorganic bases, such as Et3N, K2CO3, and DBU. Along with the “traditional” reaction performed in solution, a mechanochemical approach using ball milling was also tested. The obtained results are collected and compared in Table 1. They show that the best results were achieved using dry THF as a solvent and K2CO3 as a base; under these conditions, the best conversion of the starting materials and the highest yield (93%) for the expected product 9a were observed. Typically, for cycloadducts obtained from 1,4-naphthoquinone [38], the initially formed [3 + 2]-cycloadduct 10a smoothly underwent air oxidation during the work-up to give pyrazole 9a as the final product.
Table 1: Results of the [3 + 2]-cycloaddition of quinone 1a with the fluorinated nitrile imine 7a generated from 8a.
|
|
|||
| base | solvent | reaction time | yield (%)a |
| Et3N | THF | 2 d | 93 |
| K2CO3 | THF | 2 d | 97 (93) |
| Cs2CO3 | THF | 2 d | 90 |
| DBU | THF | 2 d | —b |
| K2CO3 | DCM | 2 d | 96 |
| K2CO3 | toluene | 2 d | 22 |
| K2CO3 | —c | 3 h | 85 |
| Et3N | —c | 3 h | 79d |
aConversion of naphthoquinone (1a) into 9a estimated on the basis of 1H NMR spectra of the crude reaction mixture. Yield of isolated product given in parentheses. bPyrazole 9a was not found in the mixture. cSolvent-free ball mill reaction. dPartial decomposition of the starting bromide 8a.
It is worth mentioning that the solvent-free mechanochemical approach also led to the desired product, albeit a lower yield of 9a was noticed. In these reactions, a sticky material was obtained, which was difficult to grind and therefore, completion of these reactions was practically impossible. On the other hand, the ball mill approach provided the target material after a significantly shorter reaction time (3 h instead of 2 d).
Based on the optimized protocol, a series of cycloadditions with nitrile imines 7 was carried out using 1a (Scheme 3). Irrespective of the electronic properties of the substituent X located at the C(4) atom of the aryl ring in the precursor 8, generally, a high yield of the isolated products 9 was observed (78–97%). Only for derivatives bearing strongly electron-withdrawing groups X (i.e., CN, NO2), a longer reaction time (up to 6 d) was required to complete these experiments, and the yield of the isolated products dropped significantly (to 41 and 64%, respectively). In analogy to the tricyclic pyrazole derivative 9a, the expected products 9b–h formed after spontaneous aromatization of the initial [3 + 2]-cycloadducts by air oxidation were isolated exclusively. Additionally, the X-ray structure of 9d is shown in Figure 2.
Scheme 3: Synthesis of [3 + 2]-cycloadducts 9a–l derived from CF3-substituted nitrile imines 7a–h and 1,4-quinones 1a and 1b.
Scheme 3: Synthesis of [3 + 2]-cycloadducts 9a–l derived from CF3-substituted nitrile imines 7a–h and 1,4-qui...
![[1860-5397-17-108-2]](/bjoc/content/figures/1860-5397-17-108-2.png?scale=2.0&max-width=1024&background=FFFFFF)
Figure 2: X-ray structure of 3-trifluoromethylpyrazole derivative 9d. The labeling scheme of the asymmetric unit is shown. Anisotropic displacement parameters of nonhydrogen atoms are drawn as ellipsoids with a 50% probability level. N atoms in blue, O atoms in red, C atoms in grey, H atoms as small white spheres. Structure deposited under deposition number CCDC-2078498.
Figure 2: X-ray structure of 3-trifluoromethylpyrazole derivative 9d. The labeling scheme of the asymmetric u...
Similar results were obtained starting with 1,4-anthraquinone (1b) and selected hydrazonoyl bromides 8. In this series, fused pyrazoles 9i–l were obtained in high yield (63–92%, Scheme 3).
Finally, the experiment performed under the optimized conditions with the nonsymmetric menadione (1c, vitamin K3), bearing the Me group at C(2), with in-situ-generated nitrile imines 7c (X = OMe) and 7d (X = Me) led to complex mixtures of unidentified products (Scheme 4). These results differ from that reported by Huisgen [17], who in the reaction of nitrile imine 2 with the same dipolarophile obtained the expected [3 + 2]-cycloadduct to the C=C bond as a single regioisomer, which was isolated in 33% yield. The observed outcome suggests that the thermally generated nitrile imine 2 undergoes [3 + 2]-cycloaddition more efficiently than fluorinated nitrile imines 7 generated under basic conditions. It seems likely that in the presence of a base, sequential reactions of the conjugated C–H-acidic quinone 1c occur, which lead to a complex mixture of products. In contrast, the reaction of the same nitrile imine 7d with the structurally analogous 1,4-quinone 1d, bearing the OMe group at C(2), provided the known pyrazole 9d as the only product in the excellent yield of 96% after typical aqueous work-up, although the reaction required a longer reaction time (3 d, Scheme 4). Presumably, the initially formed [3 + 2]-cycloadduct 10b undergoes spontaneous elimination of MeOH, yielding the aromatized product 9d. A similar mechanistic scenario was observed and discussed previously for the reactions of trifluoroacetonitrile imines 7 with enol ethers used as dipolarophiles [27].
Scheme 4: Thermal elimination of MeOH from the initial [3 + 2]-cycloadduct of 1d and nitrile imine 7d generated from 8d.
Scheme 4: Thermal elimination of MeOH from the initial [3 + 2]-cycloadduct of 1d and nitrile imine 7d generat...
An additional experiment deserves a brief comment. The [3 + 2]-cycloaddition of 7d (X = Me), generated from 8d, with 2,3,5,6-tetramethyl-1,4-benzoquinone (1e), performed under the optimized conditions (2 d at rt), yielded the fairly stable nonaromatic [3 + 2]-cycloadduct 10c, which was isolated by chromatographic work-up in 53% yield (Scheme 5).
Scheme 5: Formation of the thermally stable, initially formed [3 + 2]-cycloadduct 10c obtained from 1e and nitrile imine 7d generated from 8d.
Scheme 5: Formation of the thermally stable, initially formed [3 + 2]-cycloadduct 10c obtained from 1e and ni...
The presence of ≈40% of unconsumed quinone 1e in the crude reaction mixture evidenced that the observed fully chemoselective cycloaddition reaction with the sterically congested C=C bond in 1e occurs less efficiently than in 2,3-unsubstituted 1,4-quinones, such as 1a and 1b. However, in that case, the subsequent elimination step leading to an aromatized product cannot take place.
All products of the type 9 are colored, typically pale yellow, both in the solid state and in solution. The UV–vis spectroscopic analysis of the naphthoquinone-derived series (compounds 9a–h) revealed less intense absorption in the visible range at ≈410 nm and medium-intensity absorption between 310–340 nm. In the latter region, a significant hypsochromic shift of the maxima with increasing electron-withdrawing character of the substituent X could be observed (Figure 3).
![[1860-5397-17-108-3]](/bjoc/content/figures/1860-5397-17-108-3.png?scale=2.0&max-width=1024&background=FFFFFF)
Figure 3: Electronic absorption spectra for selected a) naphthoquinone-derived (9c, 9d, 9g) and b) anthraquinone-derived (9i, 9k, 9l) trifluoromethylated pyrazoles in CH2Cl2.
Figure 3: Electronic absorption spectra for selected a) naphthoquinone-derived (9c, 9d, 9g) and b) anthraquin...
Remarkably, the 1,4-anthraquinone-derived products 9i, 9k, and 9l exhibit two overlapping absorption bands in the visible range with maxima at λmax ≈ 400 nm and ≈ 425 nm, respectively.
Conclusion
The presented study demonstrated that 1,4-quinones undergo efficient [3 + 2]-cycloadditions with in-situ-generated electron-deficient trifluoroacetonitrile imines to give polycyclic pyrazole derivatives as exclusive products formed via spontaneous air oxidation of the initial [3 + 2]-cycloadducts. In contrast to some arylnitrile oxides and benzonitrile ylides, no competitive cycloaddition to the C=O bonds of the quinone molecule has been observed, and all studied reactions proceeded chemoselectively with the C=C bond as the only dipolarophilic center. This result corresponds to other reported cases in which nonfluorinated nitrile imines were employed to form fused pyrazoles in reactions with 1,4-quinones. Interestingly, reactions of para-quinone methides with common nitrile imines proceeded with different chemoselectivity, and in these cases, the exocyclic C=C bond played the role of the exclusive dipolarophilic center, yielding corresponding spiropyrazoles [39,40].
This unusual selectivity in [3 + 2]-cycloadditions of less electron-deficient nitrile imines was studied computationally to rationalize the observed pathways [41]. Hence, it is worth mentioning that the fused pyrazoles, which are attractive compounds for diverse practical applications in medicinal and materials chemistry, are available not only by the aza-Nenitzescu reaction [42,43] but also via simple and highly efficient [3 + 2]-cycloadditions of nitrile imines with the C=C bond of 1,4-quinones. In general, the present study emphasizes the great importance of nitrile imines as versatile 1,3-dipoles useful for the preparation of N-heterocycles of potential importance for medicinal chemistry, agrochemistry, and materials chemistry [44,45].
Experimental
General information
If not stated otherwise, reactions were carried out under inert atmosphere (argon) in flame-dried flasks with addition of the reactants by using syringes; subsequent manipulations were conducted in air. Products were purified by standard column chromatography (CC) on silica gel (230–400 mesh; deactivated prior to use with 2% Et3N in petroleum ether) by using freshly distilled solvents as eluents (petroleum ether, CH2Cl2, AcOEt) or recrystallized from hot CHCl3. THF was dried over sodium and benzophenone and freshly distilled before use; anhydrous DMF was purchased and used as received. The NMR spectra were measured on a Bruker AVIII instrument (1H at 600 MHz, 13C at 151 MHz, and 19F at 565 MHz). Chemical shifts are reported relative to residual undeuterated solvent peaks (for CDCl3: 1H NMR δ = 7.26 ppm, 13C NMR δ = 77.0 ppm; for 1,1,2,2-tetrachloroethane-d2 (C2D2Cl4): 1H NMR δ = 6.0 ppm, 13C NMR δ = 73.8 ppm) or to CFCl3 (19F NMR δ = 0.00 ppm) used as external standard. Multiplicities of the signals in 13C NMR spectra were assigned based on supplementary 2D measurements (COSY, HMQC, and HMBC). The UV–vis spectra were measured on a PerkinElmer Lambda 45 spectrophotometer in spectroscopic grade CH2Cl2. MS (ESI) were performed with a Varian 500-MS LC Ion Trap. The IR spectra were measured neat with an Agilent Cary 630 FTIR spectrometer. Elemental analyses were obtained with a Vario EL III instrument (Elementar Analysensysteme GmbH). Melting points were determined in capillaries with a MEL-TEMP apparatus (Aldrich) or with a polarizing optical microscope (Opta-Tech) and are uncorrected.
Starting materials
The requisite hydrazonoyl bromides 8a–h were prepared by NBS-mediated bromination of the corresponding trifluoroacetaldehyde hydrazones in dry DMF at room temperature as described in an earlier publication [21]. The latter arylhydrazones were obtained according to a general literature protocol by condensation of aqueous fluoral hydrate (≈75% in H2O) with commercially available hydrazines in a closed ampoule at 75 °C in methanol in the presence of molecular sieves (4 Å) [46].
General procedure
To a stirred solution of the respective 1,4-quinone 1 (1.0 mmol) and K2CO3 in dry THF (10 mL), a hydrazonoyl bromide 8 (1.1 mmol) was added, and stirring was continued at room temperature until the starting material 1 was fully consumed (based on TLC monitoring, petroleum ether/dichloromethane 1:1). After the resulting precipitate and unconsumed carbonate were filtered off, the solvent was removed under reduced pressure. The crude mixtures were purified by CC using silica gel as the stationary phase and either petroleum ether (or hexanes)/dichloromethane or petroleum ether/AcOEt mixtures as eluent to give analytically pure products 9a–h and 10c.
1-Phenyl-3-(trifluoromethyl)-1H-benzo[f]indazole-4,9-dione (9a)
Reaction time 2 d; CC (SiO2, petroleum ether/CH2Cl2 2:1); 320 mg (93%); yellow solid; mp 196–197 °C; 1H NMR (CDCl3, 600 MHz) δ 7.56–7.62 (m, 5H, Ph), 7.79, 7.84 (2 t, J = 7.5 Hz, 1H each, C6H4), 8.17, 8.30 (2 d, J = 7.7 Hz, 1H each, C6H4); 13C NMR (CDCl3, 151 MHz) δ 119.9 (q, 1JC,F = 270.4 Hz, CF3), 120.8 (i-C), 125.7 (2CH), 127.3, 127.5 (CH each), 129.0 (2CH), 130.3 (CH), 132.9, 133.4 (2i-C), 134.2, 135.0 (CH each), 138.1, 139.0 (2i-C), 140.8 (q, 2JC,F = 40.8 Hz, C(3)), 174.5, 177.3 (2C=O); 19F NMR (CDCl3, 565 MHz) δ −62.82 ppm; UV–vis (CH2Cl2) λmax (log ε) 247 (4.45), 266 (4.24), 275 (4.25), 340 (3.72), 409 (2.61), 496 nm (1.70); IR (neat) νmax: 3082, 1677 (C=O), 1588, 1521, 1495, 1331, 1279, 1230, 1133, 1100, 921, 716 cm−1; ESIMS (m/z): 365.1 (100, [M + Na]+), 343.1 (12, [M + H]+); Anal. calcd for C18H9F3N2O2: C, 63.16; H, 2.65; N, 8.18; found: C, 63.34; H, 2.63; N, 8.24 (all values are given as percentages).
3a,5,6,7a-Tetramethyl-1-(p-tolyl)-3-(trifluoromethyl)-3a,7a-dihydro-1H-indazole-4,7-dione (10c)
Reaction time 2 d; CC (SiO2, petroleum ether/AcOEt 20:1); 193 mg (53%); yellow oil; 1H NMR (CDCl3, 600 MHz) δ 1.39 (s, 3H, Me), 1.52 (qbr, J = 0.5 Hz, 3H, Me), 2.05–2.07 (m, 6H, 2 Me), 2.32 (s, 3H, Me), 6.95–6.97, 7.10–7.12 (2 m, 2H each, Tol); 13C NMR (CDCl3, 151 MHz) δ 13.6, 13.9, 14.2, 14.6, 20.8 (5Me), 64.8, 80.1 (2i-C), 120.7 (q,1JC,F = 271.1 Hz, CF3), 120.7, 129.6 (2CH each), 134.8 (i-C), 140.0 (q, 2JC,F = 36.3 Hz, C(3)), 139.5, 146.9, 147.1 (3i-C), 192.6, 195.1 (2C=O) ppm; 19F NMR (CDCl3, 565 MHz) δ −61.45 ppm; IR (neat) νmax: 2930, 1677 (C=O), 1513, 1506, 1379, 1267, 1170, 1118, 1070, 1029, 954, 850, 816, 712 cm−1; ESIMS (m/z): 365.4 (100, [M + H]+); Anal. calcd for C19H19F3N2O2: C, 62.63; H, 5.26; N, 7.69; found: C, 62.76; H, 5.39; N, 7.95.
Supporting Information
| Supporting Information File 1: General information and experimental data of all isolated products, details of the crystal structure determination, and copies of 1H and 13C NMR spectra for all products. | ||
| Format: PDF | Size: 1.5 MB | Download |
References
-
El-Najjar, N.; Gali-Muhtasib, H.; Ketola, R. A.; Vuorela, P.; Urtti, A.; Vuorela, H. Phytochem. Rev. 2011, 10, 353–370. doi:10.1007/s11101-011-9209-1
Return to citation in text: [1] -
Wang, Y.; Zhu, S.; Zou, L.-H. Eur. J. Org. Chem. 2019, 2179–2201. doi:10.1002/ejoc.201900028
Return to citation in text: [1] -
Han, C.; Li, H.; Shi, R.; Zhang, T.; Tong, J.; Li, J.; Li, B. J. Mater. Chem. A 2019, 7, 23378–23415. doi:10.1039/c9ta05252f
Return to citation in text: [1] -
Patel, O. P. S.; Beteck, R. M.; Legoabe, L. J. Eur. J. Med. Chem. 2021, 210, 113084. doi:10.1016/j.ejmech.2020.113084
Return to citation in text: [1] -
Nawrat, C. C.; Moody, C. J. Angew. Chem., Int. Ed. 2014, 53, 2056–2077. doi:10.1002/anie.201305908
Angew. Chem. 2014, 126, 2086–2109. doi:10.1002/ange.201305908
Return to citation in text: [1] -
Tapia, R. A.; Carrasco, C.; Ojeda, S.; Salas, C.; Valderrama, J. A.; Morello, A.; Repetto, Y. J. Heterocycl. Chem. 2002, 39, 1093–1096. doi:10.1002/jhet.5570390540
Return to citation in text: [1] -
Wang, C.; Chen, X.-H.; Zhou, S.-M.; Gong, L.-Z. Chem. Commun. 2010, 46, 1275–1277. doi:10.1039/b917246g
Return to citation in text: [1] -
Berhe, S.; Slupe, A.; Luster, C.; Charlier, H. A., Jr.; Warner, D. L.; Zalkow, L. H.; Burgess, E. M.; Enwerem, N. M.; Bakare, O. Bioorg. Med. Chem. 2010, 18, 134–141. doi:10.1016/j.bmc.2009.11.011
Return to citation in text: [1] -
Huang, H.-M.; Gao, J.-R.; Ye, Q.; Yu, W.-B.; Sheng, W.-J.; Li, Y.-J. RSC Adv. 2014, 4, 15526–15533. doi:10.1039/c4ra01593b
Return to citation in text: [1] -
Morrocchi, S.; Quilico, A.; Ricca, A.; Selva, A. Gazz. Chim. Ital. 1968, 98, 891–906.
Return to citation in text: [1] -
Shiraishi, S.; Holla, B. S.; Imamura, K. Bull. Chem. Soc. Jpn. 1983, 56, 3457–3463. doi:10.1246/bcsj.56.3457
Return to citation in text: [1] -
Hamadi, N. B.; Msaddek, M. Heterocycl. Commun. 2006, 12, 457–462. doi:10.1515/hc.2006.12.6.457
Return to citation in text: [1] -
Shiraishi, S.; Ikeuchi, S.; Senō, M.; Asahara, T. Bull. Chem. Soc. Jpn. 1977, 50, 910–913. doi:10.1246/bcsj.50.910
Return to citation in text: [1] -
Shiraishi, S.; Ikeuchi, S.; Senō, M.; Asahara, T. Bull. Chem. Soc. Jpn. 1978, 51, 921–925. doi:10.1246/bcsj.51.921
Return to citation in text: [1] -
Stegmann, W.; Uebelhart, P.; Heimgartner, H. Helv. Chim. Acta 1983, 66, 2252–2268. doi:10.1002/hlca.19830660736
Return to citation in text: [1] -
Gilgen, P.; Jackson, B.; Hansen, H.-J.; Heimgartner, H.; Schmid, H. Helv. Chim. Acta 1974, 57, 2634–2643. doi:10.1002/hlca.19740570842
Return to citation in text: [1] -
Huisgen, R.; Seidel, M.; Wallbillich, G.; Knupfer, H. Tetrahedron 1962, 17, 3–29. doi:10.1016/s0040-4020(01)99001-5
Return to citation in text: [1] [2] [3] -
Argyropoulos, N. G.; Mentzafos, D.; Terzis, A. J. Heterocycl. Chem. 1990, 27, 1983–1988. doi:10.1002/jhet.5570270725
Return to citation in text: [1] -
Ortiz‐Rojano, L.; Rojas‐Martín, J.; Rodríguez‐Diaz, C.; Carreño, M. C.; Ribagorda, M. Chem. – Eur. J. 2019, 25, 15050–15054. doi:10.1002/chem.201904138
Return to citation in text: [1] -
Bertuzzi, G.; Crotti, S.; Calandro, P.; Bonini, B. F.; Monaco, I.; Locatelli, E.; Fochi, M.; Zani, P.; Strocchi, E.; Mazzanti, A.; Chiariello, M.; Franchini, M. C. ChemMedChem 2018, 13, 1744–1750. doi:10.1002/cmdc.201800251
Return to citation in text: [1] -
Mlostoń, G.; Urbaniak, K.; Utecht, G.; Lentz, D.; Jasiński, M. J. Fluorine Chem. 2016, 192, 147–154. doi:10.1016/j.jfluchem.2016.10.018
Return to citation in text: [1] [2] -
Utecht, G.; Sioma, J.; Jasiński, M.; Mlostoń, G. J. Fluorine Chem. 2017, 201, 68–75. doi:10.1016/j.jfluchem.2017.07.014
Return to citation in text: [1] -
Grzelak, P.; Utecht, G.; Jasiński, M.; Mlostoń, G. Synthesis 2017, 49, 2129–2137. doi:10.1055/s-0036-1588774
Return to citation in text: [1] -
Utecht-Jarzyńska, G.; Jasiński, M.; Świątek, K.; Mlostoń, G.; Heimgartner, H. Heterocycles 2020, 101, 251–262. doi:10.3987/com-19-s(f)20
Return to citation in text: [1] -
Utecht-Jarzyńska, G.; Mykhaylychenko, S. S.; Rusanov, E. B.; Shermolovich, Y. G.; Jasiński, M.; Mlostoń, G. J. Fluorine Chem. 2021, 242, 109702. doi:10.1016/j.jfluchem.2020.109702
Return to citation in text: [1] [2] -
Utecht-Jarzyńska, G.; Michalak, A.; Banaś, J.; Mlostoń, G.; Jasiński, M. J. Fluorine Chem. 2019, 222–223, 8–14. doi:10.1016/j.jfluchem.2019.04.012
Return to citation in text: [1] -
Utecht, G.; Fruziński, A.; Jasiński, M. Org. Biomol. Chem. 2018, 16, 1252–1257. doi:10.1039/c7ob03126b
Return to citation in text: [1] [2] -
Utecht, G.; Mlostoń, G.; Jasiński, M. Synlett 2018, 29, 1753–1758. doi:10.1055/s-0037-1610454
Return to citation in text: [1] -
Tian, Y.-C.; Li, J.-K.; Zhang, F.-G.; Ma, J.-A. Adv. Synth. Catal. 2021, 363, 2093–2097. doi:10.1002/adsc.202100091
Return to citation in text: [1] -
Kowalczyk, A.; Utecht-Jarzyńska, G.; Mlostoń, G.; Jasiński, M. J. Fluorine Chem. 2021, 241, 109691. doi:10.1016/j.jfluchem.2020.109691
Return to citation in text: [1] -
Fustero, S.; Sánchez-Roselló, M.; Barrio, P.; Simón-Fuentes, A. Chem. Rev. 2011, 111, 6984–7034. doi:10.1021/cr2000459
Return to citation in text: [1] [2] -
Kaur, K.; Kumar, V.; Gupta, G. K. J. Fluorine Chem. 2015, 178, 306–326. doi:10.1016/j.jfluchem.2015.08.015
Return to citation in text: [1] -
Zhou, Y.; Wang, J.; Gu, Z.; Wang, S.; Zhu, W.; Aceña, J. L.; Soloshonok, V. A.; Izawa, K.; Liu, H. Chem. Rev. 2016, 116, 422–518. doi:10.1021/acs.chemrev.5b00392
Return to citation in text: [1] -
Hu, X.-G.; Hunter, L. Beilstein J. Org. Chem. 2013, 9, 2696–2708. doi:10.3762/bjoc.9.306
Return to citation in text: [1] -
Mykhailiuk, P. K. Chem. Rev. 2021, 121, 1670–1715. doi:10.1021/acs.chemrev.0c01015
Return to citation in text: [1] -
Lipunova, G. N.; Nosova, E. V.; Charushin, V. N.; Chupakhin, O. N. J. Fluorine Chem. 2015, 175, 84–109. doi:10.1016/j.jfluchem.2015.03.011
Return to citation in text: [1] -
Mlostoń, G.; Urbaniak, K.; Urbaniak, P.; Marko, A.; Linden, A.; Heimgartner, H. Beilstein J. Org. Chem. 2018, 14, 1834–1839. doi:10.3762/bjoc.14.156
Return to citation in text: [1] -
Mlostoń, G.; Celeda, M.; Heimgartner, H. Heterocycles 2003, 59, 767–777. doi:10.3987/com-02-s61
Return to citation in text: [1] [2] -
Woolhouse, A. D. Aust. J. Chem. 1977, 30, 1145–1152. doi:10.1071/ch9771145
Return to citation in text: [1] -
Su, Y.; Zhao, Y.; Chang, B.; Zhao, X.; Zhang, R.; Liu, X.; Huang, D.; Wang, K.-H.; Huo, C.; Hu, Y. J. Org. Chem. 2019, 84, 6719–6728. doi:10.1021/acs.joc.9b00434
Return to citation in text: [1] -
Soleymani, M.; Jahanparvar, S. Monatsh. Chem. 2020, 151, 51–61. doi:10.1007/s00706-019-02531-2
Return to citation in text: [1] -
Lyubchanskaya, V. M.; Alekseeva, L. M.; Granik, V. G. Chem. Heterocycl. Compd. 1999, 35, 570–574. doi:10.1007/bf02324640
Return to citation in text: [1] -
Janardhanan, J. C.; Mishra, R. K.; Das, G.; Sini, S.; Jayamurthy, P.; Suresh, C. H.; Praveen, V. K.; Manoj, N.; Babu, B. P. Asian J. Org. Chem. 2018, 7, 2094–2104. doi:10.1002/ajoc.201800413
Return to citation in text: [1] -
Quadrelli, P., Ed. Modern Applications of Cycloaddition Chemistry; Elsevier: Amsterdam, Netherlands, 2019. doi:10.1016/c2017-0-03259-7
Return to citation in text: [1] -
Jamieson, C.; Livingstone, K., Eds. The Nitrile Imine 1,3-Dipoles – Properties, Reactivity and Applications; Springer: Cham, Switzerland, 2020. doi:10.1007/978-3-030-43481-6
Return to citation in text: [1] -
Wojciechowska, A.; Jasiński, M.; Kaszyński, P. Tetrahedron 2015, 71, 2349–2356. doi:10.1016/j.tet.2015.03.015
Return to citation in text: [1]
| 46. | Wojciechowska, A.; Jasiński, M.; Kaszyński, P. Tetrahedron 2015, 71, 2349–2356. doi:10.1016/j.tet.2015.03.015 |
| 1. | El-Najjar, N.; Gali-Muhtasib, H.; Ketola, R. A.; Vuorela, P.; Urtti, A.; Vuorela, H. Phytochem. Rev. 2011, 10, 353–370. doi:10.1007/s11101-011-9209-1 |
| 2. | Wang, Y.; Zhu, S.; Zou, L.-H. Eur. J. Org. Chem. 2019, 2179–2201. doi:10.1002/ejoc.201900028 |
| 3. | Han, C.; Li, H.; Shi, R.; Zhang, T.; Tong, J.; Li, J.; Li, B. J. Mater. Chem. A 2019, 7, 23378–23415. doi:10.1039/c9ta05252f |
| 4. | Patel, O. P. S.; Beteck, R. M.; Legoabe, L. J. Eur. J. Med. Chem. 2021, 210, 113084. doi:10.1016/j.ejmech.2020.113084 |
| 13. | Shiraishi, S.; Ikeuchi, S.; Senō, M.; Asahara, T. Bull. Chem. Soc. Jpn. 1977, 50, 910–913. doi:10.1246/bcsj.50.910 |
| 14. | Shiraishi, S.; Ikeuchi, S.; Senō, M.; Asahara, T. Bull. Chem. Soc. Jpn. 1978, 51, 921–925. doi:10.1246/bcsj.51.921 |
| 30. | Kowalczyk, A.; Utecht-Jarzyńska, G.; Mlostoń, G.; Jasiński, M. J. Fluorine Chem. 2021, 241, 109691. doi:10.1016/j.jfluchem.2020.109691 |
| 10. | Morrocchi, S.; Quilico, A.; Ricca, A.; Selva, A. Gazz. Chim. Ital. 1968, 98, 891–906. |
| 11. | Shiraishi, S.; Holla, B. S.; Imamura, K. Bull. Chem. Soc. Jpn. 1983, 56, 3457–3463. doi:10.1246/bcsj.56.3457 |
| 12. | Hamadi, N. B.; Msaddek, M. Heterocycl. Commun. 2006, 12, 457–462. doi:10.1515/hc.2006.12.6.457 |
| 25. | Utecht-Jarzyńska, G.; Mykhaylychenko, S. S.; Rusanov, E. B.; Shermolovich, Y. G.; Jasiński, M.; Mlostoń, G. J. Fluorine Chem. 2021, 242, 109702. doi:10.1016/j.jfluchem.2020.109702 |
| 6. | Tapia, R. A.; Carrasco, C.; Ojeda, S.; Salas, C.; Valderrama, J. A.; Morello, A.; Repetto, Y. J. Heterocycl. Chem. 2002, 39, 1093–1096. doi:10.1002/jhet.5570390540 |
| 7. | Wang, C.; Chen, X.-H.; Zhou, S.-M.; Gong, L.-Z. Chem. Commun. 2010, 46, 1275–1277. doi:10.1039/b917246g |
| 8. | Berhe, S.; Slupe, A.; Luster, C.; Charlier, H. A., Jr.; Warner, D. L.; Zalkow, L. H.; Burgess, E. M.; Enwerem, N. M.; Bakare, O. Bioorg. Med. Chem. 2010, 18, 134–141. doi:10.1016/j.bmc.2009.11.011 |
| 9. | Huang, H.-M.; Gao, J.-R.; Ye, Q.; Yu, W.-B.; Sheng, W.-J.; Li, Y.-J. RSC Adv. 2014, 4, 15526–15533. doi:10.1039/c4ra01593b |
| 26. | Utecht-Jarzyńska, G.; Michalak, A.; Banaś, J.; Mlostoń, G.; Jasiński, M. J. Fluorine Chem. 2019, 222–223, 8–14. doi:10.1016/j.jfluchem.2019.04.012 |
| 5. |
Nawrat, C. C.; Moody, C. J. Angew. Chem., Int. Ed. 2014, 53, 2056–2077. doi:10.1002/anie.201305908
Angew. Chem. 2014, 126, 2086–2109. doi:10.1002/ange.201305908 |
| 27. | Utecht, G.; Fruziński, A.; Jasiński, M. Org. Biomol. Chem. 2018, 16, 1252–1257. doi:10.1039/c7ob03126b |
| 28. | Utecht, G.; Mlostoń, G.; Jasiński, M. Synlett 2018, 29, 1753–1758. doi:10.1055/s-0037-1610454 |
| 29. | Tian, Y.-C.; Li, J.-K.; Zhang, F.-G.; Ma, J.-A. Adv. Synth. Catal. 2021, 363, 2093–2097. doi:10.1002/adsc.202100091 |
| 17. | Huisgen, R.; Seidel, M.; Wallbillich, G.; Knupfer, H. Tetrahedron 1962, 17, 3–29. doi:10.1016/s0040-4020(01)99001-5 |
| 20. | Bertuzzi, G.; Crotti, S.; Calandro, P.; Bonini, B. F.; Monaco, I.; Locatelli, E.; Fochi, M.; Zani, P.; Strocchi, E.; Mazzanti, A.; Chiariello, M.; Franchini, M. C. ChemMedChem 2018, 13, 1744–1750. doi:10.1002/cmdc.201800251 |
| 17. | Huisgen, R.; Seidel, M.; Wallbillich, G.; Knupfer, H. Tetrahedron 1962, 17, 3–29. doi:10.1016/s0040-4020(01)99001-5 |
| 21. | Mlostoń, G.; Urbaniak, K.; Utecht, G.; Lentz, D.; Jasiński, M. J. Fluorine Chem. 2016, 192, 147–154. doi:10.1016/j.jfluchem.2016.10.018 |
| 22. | Utecht, G.; Sioma, J.; Jasiński, M.; Mlostoń, G. J. Fluorine Chem. 2017, 201, 68–75. doi:10.1016/j.jfluchem.2017.07.014 |
| 23. | Grzelak, P.; Utecht, G.; Jasiński, M.; Mlostoń, G. Synthesis 2017, 49, 2129–2137. doi:10.1055/s-0036-1588774 |
| 24. | Utecht-Jarzyńska, G.; Jasiński, M.; Świątek, K.; Mlostoń, G.; Heimgartner, H. Heterocycles 2020, 101, 251–262. doi:10.3987/com-19-s(f)20 |
| 25. | Utecht-Jarzyńska, G.; Mykhaylychenko, S. S.; Rusanov, E. B.; Shermolovich, Y. G.; Jasiński, M.; Mlostoń, G. J. Fluorine Chem. 2021, 242, 109702. doi:10.1016/j.jfluchem.2020.109702 |
| 16. | Gilgen, P.; Jackson, B.; Hansen, H.-J.; Heimgartner, H.; Schmid, H. Helv. Chim. Acta 1974, 57, 2634–2643. doi:10.1002/hlca.19740570842 |
| 15. | Stegmann, W.; Uebelhart, P.; Heimgartner, H. Helv. Chim. Acta 1983, 66, 2252–2268. doi:10.1002/hlca.19830660736 |
| 18. | Argyropoulos, N. G.; Mentzafos, D.; Terzis, A. J. Heterocycl. Chem. 1990, 27, 1983–1988. doi:10.1002/jhet.5570270725 |
| 19. | Ortiz‐Rojano, L.; Rojas‐Martín, J.; Rodríguez‐Diaz, C.; Carreño, M. C.; Ribagorda, M. Chem. – Eur. J. 2019, 25, 15050–15054. doi:10.1002/chem.201904138 |
| 37. | Mlostoń, G.; Urbaniak, K.; Urbaniak, P.; Marko, A.; Linden, A.; Heimgartner, H. Beilstein J. Org. Chem. 2018, 14, 1834–1839. doi:10.3762/bjoc.14.156 |
| 38. | Mlostoń, G.; Celeda, M.; Heimgartner, H. Heterocycles 2003, 59, 767–777. doi:10.3987/com-02-s61 |
| 31. | Fustero, S.; Sánchez-Roselló, M.; Barrio, P.; Simón-Fuentes, A. Chem. Rev. 2011, 111, 6984–7034. doi:10.1021/cr2000459 |
| 32. | Kaur, K.; Kumar, V.; Gupta, G. K. J. Fluorine Chem. 2015, 178, 306–326. doi:10.1016/j.jfluchem.2015.08.015 |
| 33. | Zhou, Y.; Wang, J.; Gu, Z.; Wang, S.; Zhu, W.; Aceña, J. L.; Soloshonok, V. A.; Izawa, K.; Liu, H. Chem. Rev. 2016, 116, 422–518. doi:10.1021/acs.chemrev.5b00392 |
| 34. | Hu, X.-G.; Hunter, L. Beilstein J. Org. Chem. 2013, 9, 2696–2708. doi:10.3762/bjoc.9.306 |
| 31. | Fustero, S.; Sánchez-Roselló, M.; Barrio, P.; Simón-Fuentes, A. Chem. Rev. 2011, 111, 6984–7034. doi:10.1021/cr2000459 |
| 35. | Mykhailiuk, P. K. Chem. Rev. 2021, 121, 1670–1715. doi:10.1021/acs.chemrev.0c01015 |
| 36. | Lipunova, G. N.; Nosova, E. V.; Charushin, V. N.; Chupakhin, O. N. J. Fluorine Chem. 2015, 175, 84–109. doi:10.1016/j.jfluchem.2015.03.011 |
| 44. | Quadrelli, P., Ed. Modern Applications of Cycloaddition Chemistry; Elsevier: Amsterdam, Netherlands, 2019. doi:10.1016/c2017-0-03259-7 |
| 45. | Jamieson, C.; Livingstone, K., Eds. The Nitrile Imine 1,3-Dipoles – Properties, Reactivity and Applications; Springer: Cham, Switzerland, 2020. doi:10.1007/978-3-030-43481-6 |
| 21. | Mlostoń, G.; Urbaniak, K.; Utecht, G.; Lentz, D.; Jasiński, M. J. Fluorine Chem. 2016, 192, 147–154. doi:10.1016/j.jfluchem.2016.10.018 |
| 41. | Soleymani, M.; Jahanparvar, S. Monatsh. Chem. 2020, 151, 51–61. doi:10.1007/s00706-019-02531-2 |
| 42. | Lyubchanskaya, V. M.; Alekseeva, L. M.; Granik, V. G. Chem. Heterocycl. Compd. 1999, 35, 570–574. doi:10.1007/bf02324640 |
| 43. | Janardhanan, J. C.; Mishra, R. K.; Das, G.; Sini, S.; Jayamurthy, P.; Suresh, C. H.; Praveen, V. K.; Manoj, N.; Babu, B. P. Asian J. Org. Chem. 2018, 7, 2094–2104. doi:10.1002/ajoc.201800413 |
| 27. | Utecht, G.; Fruziński, A.; Jasiński, M. Org. Biomol. Chem. 2018, 16, 1252–1257. doi:10.1039/c7ob03126b |
| 39. | Woolhouse, A. D. Aust. J. Chem. 1977, 30, 1145–1152. doi:10.1071/ch9771145 |
| 40. | Su, Y.; Zhao, Y.; Chang, B.; Zhao, X.; Zhang, R.; Liu, X.; Huang, D.; Wang, K.-H.; Huo, C.; Hu, Y. J. Org. Chem. 2019, 84, 6719–6728. doi:10.1021/acs.joc.9b00434 |
| 38. | Mlostoń, G.; Celeda, M.; Heimgartner, H. Heterocycles 2003, 59, 767–777. doi:10.3987/com-02-s61 |
| 17. | Huisgen, R.; Seidel, M.; Wallbillich, G.; Knupfer, H. Tetrahedron 1962, 17, 3–29. doi:10.1016/s0040-4020(01)99001-5 |
© 2021 Utecht-Jarzyńska et al.; licensee Beilstein-Institut.
This is an Open Access article under the terms of the Creative Commons Attribution License (https://creativecommons.org/licenses/by/4.0). Please note that the reuse, redistribution and reproduction in particular requires that the author(s) and source are credited and that individual graphics may be subject to special legal provisions.
The license is subject to the Beilstein Journal of Organic Chemistry terms and conditions: (https://www.beilstein-journals.org/bjoc/terms)