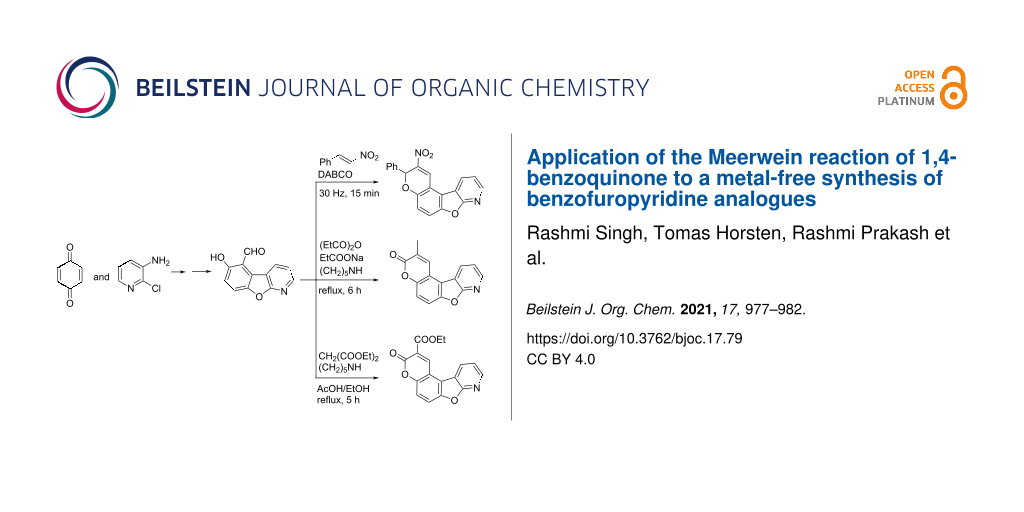
Abstract
Several new heterocyclic systems based on a hydroxybenzofuro[2,3-b]pyridine building block were prepared. This benzofuropyridine is easily available from the Meerwein reaction of benzoquinone and a heterocyclic diazonium salt, followed by reduction and cyclization. Electrophilic substitution and further condensations give polycyclic systems, including oxazolo- and chromeno-fused analogues.
Graphical Abstract
Introduction
Dibenzofurans are important oxygen-containing heterocycles present in multiple natural products [1,2] and have broad applications in areas ranging from medicinal chemistry [1-10] to materials science [11]. Figure 1 presents a few examples of dibenzofuran-containing molecules. Benzofurocoumarin analogues of 1 have antiproliferative effects on human cancer cell lines [12,13]. Fluoroquinophenoxazines 2 have been used as telomerase inhibitors in anticancer research [14]. Furthermore, benzofuroisoindoles 3 were part of a kinase inhibitor study [15]. Photobiologically active psoralene (linear furocoumarin) dibenzofurans 1 and angular furanocoumarin dibenzofurans 4 were also explored for the treatment of various skin diseases [16]. Furthermore, 2-substituted benzofurobenzofurans 5 with antitubercular activity have been reported [17]. Prado et al. reported the antimycobacterial activity of furo[3,2-f]chromene analogue 6 [18].
Figure 1: Biologically relevant 2-oxydibenzofuran-containing structures 1–6.
Figure 1: Biologically relevant 2-oxydibenzofuran-containing structures 1–6.
The aza analogues of dibenzofuran have been less explored, although they may have significant bioactivity. Introducing nitrogen to the dibenzofuran system is expected to increase the water solubility and potential bioavailability due to enhanced hydrogen bonding. Figure 2 presents a few examples of azadibenzofuran molecules. One example of a biologically active benzofuropyridine is revamilast (7), which has been used in Phase II clinical trials, studying the treatment of asthma and rheumatoid arthritis [19]. Other examples are the hydroxybenzofuro[2,3-b]pyridines 8 with efflux pump inhibitory activity useful in chemotherapy [20,21]. These compounds are presumably multitargeting drugs because of the diverse applications as insulin-like growth factor 1 receptor (IGF-1R) inhibitors [22], selective GSK-3β inhibitors important in Alzheimer's disease [23,24], and cyclin-dependent kinase (CDK) inhibitors [25-27]. Lastly, the aza analogue 9 of previously reported furo[3,2-f]chromene 6 was examined for antimycobacterial activity [28].
Figure 2: Representative bioactive structures containing benzofuro-fused pyridine analogues 7–9.
Figure 2: Representative bioactive structures containing benzofuro-fused pyridine analogues 7–9.
This evidence encouraged us to investigate new methodologies for the synthesis of aza analogues of dibenzofuran from commercially available aminochloropyridines. Furthermore, a diverse set of polycyclic derivatives was designed. The procedures towards such polycyclic building blocks include C–H-arylation strategies. In the classical Meerwein reaction, aryldiazonium salts are used as the reagents to couple aryl groups to electron-poor alkenes, and this process is assumed to proceed via a free-radical mechanism [29]. Similar reaction intermediates can be prepared using precursors such as organoboron reagents [30]. However, due to the accessibility, aryldiazonium salts are the reagents of choice. They can be prepared starting from the commercially available corresponding anilines. The present work focuses on a metal-free approach for the synthesis of benzofuropyridine analogues.
Results and Discussion
The synthesis of target compound 13 involved three steps (Scheme 1). C–H Arylation, as needed in the first step, is usually carried out using transition metal catalysis [31]. Furthermore, various metal-based approaches for arylation of quinone involving electrochemical [32], oxidative [33], and photochemical methods [34-36] are also available in the literature [37]. Langer and co-workers reported the synthesis of benzofuropyridines based on a domino reaction of 3-chlorochromones with aminoheterocycles [38]. Alternatively, the classical Meerwein reaction can be applied starting from 3-amino-2-chloropyridine (10), which was transformed into the diazonium salt and coupled in situ with 1,4-benzoquinone, forming arylated quinone 11 without an additional reducing agent [39]. The quinone was reduced to hydroquinone 12 with N,N-diethylhydroxylamine (N,N-DEHA) and cyclized via intramolecular nucleophilic aromatic substitution to isolate 6-hydroxybenzofuro[2,3-b]pyridine (13) with 82% yield. Conveniently, the synthesis of 13 was achieved in a one-pot reaction from 11 with no significant differences in the yield. To the best of our knowledge, this is the first procedure toward compound 13, without additional substituents on the pyridine ring. Furthermore, this method is complementary to the most common routes towards the biologically active 1-aza-9-oxafluorenes [20-26].
Scheme 1: Strategy for metal-free access to benzofuropyridine 13.
Scheme 1: Strategy for metal-free access to benzofuropyridine 13.
To expand the library of derivatives containing core structure 13, electrophilic aromatic substitution of this compound was explored (Scheme 2). Nitration of 13 using 70% nitric acid in glacial acetic acid gave the corresponding regioisomers 14 and 15 in 53% and 41% isolated yield, respectively. The 1H NMR spectrum of 15 showed two apparent singlets separately at δH 7.86 and δH 8.35, which confirmed that the hydrogen atoms of the phenol ring had a para-relationship. The 1H NMR spectrum of compound 14 was similar to that of compound 15 concerning the pyridine part. However, two separate doublet signals appeared at δH 7.98 (d, J = 9.1 Hz, 1H) and δH 7.35 (d, J = 9.0 Hz, 1H) indicating an ortho-relationship between the hydrogen atoms positioned on the phenolic ring.
Scheme 2: Electrophilic aromatic substitution of 6-hydroxybenzofuro[2,3-b]pyridine (13).
Scheme 2: Electrophilic aromatic substitution of 6-hydroxybenzofuro[2,3-b]pyridine (13).
Several methods of formylation of 13 were attempted, e.g., Vilsmeier formylation, where only the unstable formate ester was formed. Following the Duff formylation procedure, only traces of aldehyde 16 were detected. Rieche formylation with either SnCl4 or TiCl4 resulted in a low conversion of the starting material and only traces of 16 due to the limited solubility of 13 in DCM, DCE, or chloroform. Furthermore, 16 was isolated after a Reimer–Tiemann formylation; however, only in 13% yield. Finally, Casnati–Skattebøl formylation of 13 using MgCl2, iPr2EtN, and paraformaldehyde, i.e., (CH2O)n, has been successful in regioselectively affording aldehyde 16 in a reasonable yield of 39%. The regioselectivity of compound 16 was confirmed by 1H NMR spectroscopy, which indicated the presence of two doublets at δH 7.88 (d, J = 9 Hz, 1H) and δH 7.19 (d, J = 8.9 Hz, 1H), corresponding to the two adjacent hydrogen atoms of the phenolic ring. One singlet was observed at δH 10.58, evidencing the aldehyde proton. This access to previously unknown compounds 14–16 opens many synthetic possibilities for the preparation of novel fused derivatives of 1-aza-9-oxafluorene.
The nitro compounds 14 and 15 were reduced to the corresponding aniline derivatives using hydrogen and Pd/C as a catalyst. The resulting aminophenols 17 and 18 were further converted to novel oxazole-fused derivatives 19 and 20, respectively, by condensation with benzaldehyde and subsequent 2,3-dichloro-5.6-dicyano-p-benzoquinone (DDQ)-mediated oxidation (Scheme 3).
Scheme 3: Synthesis of isomeric oxazole-fused derivatives.
Scheme 3: Synthesis of isomeric oxazole-fused derivatives.
Aldehyde building block 16 was a versatile starting material for further cyclization reactions. Synthesis of nitrochromenobenzofuropyridine 21 was achieved after treatment of 16 with nitrostyrene in the presence of DABCO applying our previously reported ball milling procedure [40]. The synthesis of 22 was performed using the Perkin reaction [41]. The reaction of 16 with propionic anhydride and the corresponding sodium salt in the presence of a catalytic amount of piperidine afforded pyridopsoralen 22 in 46% yield. Analogously, pyridopsoralen 23 was prepared from 16 by Knoevenagel condensation with diethyl malonate and subsequent lactonization with 62% yield (Scheme 4) [13]. To the best of our understanding, the scaffolds 21–23 are novel and may have a potential medicinal interest.
Conclusion
In conclusion, we have successfully synthesized hydroxy-substituted pyridobenzofuran 13. Furthermore, nitration of 13 yielded two regioisomers, 14 and 15, which were further converted to oxazoles 19 and 20. Formylation of 13 was regioselective in forming 16, which is a valuable building block for various condensation reactions to yield a diverse set of products, such as polycyclic fused nitrochromenes 21 as well as pyridopsoralens 22 and 23. All these novel scaffolds are interesting structures with potential medicinal applications, and we plan to expand this chemistry and carry out a bioactivity study in due course.
Supporting Information
| Supporting Information File 1: Experimental part as well as 1H and 13C NMR data. | ||
| Format: PDF | Size: 3.5 MB | Download |
References
-
Shou, Q.; Banbury, L. K.; Renshaw, D. E.; Lambley, E. H.; Mon, H.; Macfarlane, G. A.; Griesser, H. J.; Heinrich, M. M.; Wohlmuth, H. J. Nat. Prod. 2012, 75, 1612–1617. doi:10.1021/np300433r
Return to citation in text: [1] [2] -
Setzer, W. N.; Rozmus, G. F.; Setzer, M. C.; Schmidt, J. M.; Vogler, B.; Reeb, S.; Jackes, B. R.; Irvine, A. K. J. Mol. Model. 2006, 12, 703–711. doi:10.1007/s00894-005-0047-1
Return to citation in text: [1] [2] -
De Lombaert, S.; Blanchard, L.; Stamford, L. B.; Tan, J.; Wallace, E. M.; Satoh, Y.; Fitt, J.; Hoyer, D.; Simonsbergen, D.; Moliterni, J.; Marcopoulos, N.; Savage, P.; Chou, M.; Trapani, A. J.; Jeng, A. Y. J. Med. Chem. 2000, 43, 488–504. doi:10.1021/jm990507o
Return to citation in text: [1] -
Kaul, R.; Deechongkit, S.; Kelly, J. W. J. Am. Chem. Soc. 2002, 124, 11900–11907. doi:10.1021/ja020675x
Return to citation in text: [1] -
Carney, J. R.; Krenisky, J. M.; Williamson, R. T.; Luo, J. J. Nat. Prod. 2002, 65, 203–205. doi:10.1021/np010374l
Return to citation in text: [1] -
Petrassi, H. M.; Johnson, S. M.; Purkey, H. E.; Chiang, K. P.; Walkup, T.; Jiang, X.; Powers, E. T.; Kelly, J. W. J. Am. Chem. Soc. 2005, 127, 6662–6671. doi:10.1021/ja044351f
Return to citation in text: [1] -
Momotake, A.; Lindegger, N.; Niggli, E.; Barsotti, R. J.; Ellis-Davies, G. C. R. Nat. Methods 2006, 3, 35–40. doi:10.1038/nmeth821
Return to citation in text: [1] -
Fang, L.; Fang, X.; Gou, S.; Lupp, A.; Lenhardt, I.; Sun, Y.; Huang, Z.; Chen, Y.; Zhang, Y.; Fleck, C. Eur. J. Med. Chem. 2014, 76, 376–386. doi:10.1016/j.ejmech.2014.02.035
Return to citation in text: [1] -
Lin, R.-D.; Chen, M.-C.; Liu, Y.-L.; Lin, Y.-T.; Lu, M.-K.; Hsu, F.-L.; Lee, M.-H. Int. J. Mol. Sci. 2015, 16, 28598–28613. doi:10.3390/ijms161226115
Return to citation in text: [1] -
Love, B. E. Eur. J. Med. Chem. 2015, 97, 377–387. doi:10.1016/j.ejmech.2015.01.005
Return to citation in text: [1] -
Ito, T.; Sasabe, H.; Nagai, Y.; Watanabe, Y.; Onuma, N.; Kido, J. Chem. – Eur. J. 2019, 25, 7308–7314. doi:10.1002/chem.201805907
Return to citation in text: [1] -
Francisco, C. S.; Rodrigues, L. R.; Cerqueira, N. M. F. S. A.; Oliveira-Campos, A. M. F.; Rodrigues, L. M. Eur. J. Med. Chem. 2012, 47, 370–376. doi:10.1016/j.ejmech.2011.11.005
Return to citation in text: [1] -
Oliveira, A. M. A. G.; Raposo, M. M. M.; Oliveira-Campos, A. M. F.; Griffiths, J.; Machado, A. E. H. Helv. Chim. Acta 2003, 86, 2900–2907. doi:10.1002/hlca.200390237
Return to citation in text: [1] [2] -
Duan, W.; Rangan, A.; Vankayalapati, H.; Kim, M. Y.; Zeng, Q.; Sun, D.; Han, H.; Fedoroff, O. Y.; Nishioka, D.; Rha, S. Y.; Izbicka, E.; Von Hoff, D. D.; Hurley, L. H. Mol. Cancer Ther. 2001, 1, 103–120.
Return to citation in text: [1] -
Wichapong, K.; Lindner, M.; Pianwanit, S.; Kokpol, S.; Sippl, W. Eur. J. Med. Chem. 2009, 44, 1383–1395. doi:10.1016/j.ejmech.2008.09.027
Return to citation in text: [1] -
Eduardo Paiva Sena Maia, J.; de Lucas, N. C.; Cesarin-Sobrinho, D.; da Hora Machado, A. E.; Netto-Ferreira, J. C. J. Photochem. Photobiol., A 2016, 326, 21–29. doi:10.1016/j.jphotochem.2016.04.013
Return to citation in text: [1] -
Yempala, T.; Sridevi, J. P.; Yogeeswari, P.; Sriram, D.; Kantevari, S. Bioorg. Med. Chem. Lett. 2013, 23, 5393–5396. doi:10.1016/j.bmcl.2013.07.048
Return to citation in text: [1] -
Prado, S.; Ledeit, H.; Michel, S.; Koch, M.; Darbord, J. C.; Cole, S. T.; Tillequin, F.; Brodin, P. Bioorg. Med. Chem. 2006, 14, 5423–5428. doi:10.1016/j.bmc.2006.03.033
Return to citation in text: [1] -
Balasubramanian, G.; Narayanan, S.; Andiappan, L.; Sappanimuthu, T.; Thirunavukkarasu, S.; Sundaram, S.; Natarajan, S.; Sivaraman, N.; Rajagopal, S.; Nazumudeen, F. A. A.; Saxena, S.; Vishwakarma, S. L.; Narayanan, S.; Sharma, G. V. R.; Srinivasan, C. V.; Kilambi, N. Bioorg. Med. Chem. 2016, 24, 5702–5716. doi:10.1016/j.bmc.2016.09.011
Return to citation in text: [1] -
Brachwitz, K.; Hilgeroth, A. Bioorg. Med. Chem. Lett. 2002, 12, 411–413. doi:10.1016/s0960-894x(01)00769-7
Return to citation in text: [1] [2] -
Krug, M.; Voigt, B.; Baumert, C.; Lüpken, R.; Molnár, J.; Hilgeroth, A. Eur. J. Med. Chem. 2010, 45, 2683–2688. doi:10.1016/j.ejmech.2010.02.024
Return to citation in text: [1] [2] -
Krug, M.; Erlenkamp, G.; Sippl, W.; Schächtele, C.; Totzke, F.; Hilgeroth, A. Bioorg. Med. Chem. Lett. 2010, 20, 6915–6919. doi:10.1016/j.bmcl.2010.10.004
Return to citation in text: [1] [2] -
Voigt, B.; Krug, M.; Schächtele, C.; Totzke, F.; Hilgeroth, A. ChemMedChem 2008, 3, 120–126. doi:10.1002/cmdc.200700175
Return to citation in text: [1] [2] -
Tell, V.; Mahmoud, K. A.; Wichapong, K.; Schächtele, C.; Totzke, F.; Sippl, W.; Hilgeroth, A. Med. Chem. Commun. 2012, 3, 1413. doi:10.1039/c2md20201h
Return to citation in text: [1] [2] -
Brachwitz, K.; Voigt, B.; Meijer, L.; Lozach, O.; Schächtele, C.; Molnár, J.; Hilgeroth, A. J. Med. Chem. 2003, 46, 876–879. doi:10.1021/jm021090g
Return to citation in text: [1] [2] -
Voigt, B.; Meijer, L.; Lozach, O.; Schächtele, C.; Totzke, F.; Hilgeroth, A. Bioorg. Med. Chem. Lett. 2005, 15, 823–825. doi:10.1016/j.bmcl.2004.10.091
Return to citation in text: [1] [2] -
Tell, V.; Holzer, M.; Herrmann, L.; Mahmoud, K. A.; Schächtele, C.; Totzke, F.; Hilgeroth, A. Bioorg. Med. Chem. Lett. 2012, 22, 6914–6918. doi:10.1016/j.bmcl.2012.09.006
Return to citation in text: [1] -
Prado, S.; Toum, V.; Saint-Joanis, B.; Michel, S.; Koch, M.; Cole, S.; Tillequin, F.; Janin, Y. Synthesis 2007, 1566–1570. doi:10.1055/s-2007-966019
Return to citation in text: [1] -
Meerwein, H.; Büchner, E.; van Emster, K. J. Prakt. Chem. 1939, 152, 237–266. doi:10.1002/prac.19391520705
Return to citation in text: [1] -
Fujiwara, Y.; Domingo, V.; Seiple, I. B.; Gianatassio, R.; Del Bel, M.; Baran, P. S. J. Am. Chem. Soc. 2011, 133, 3292–3295. doi:10.1021/ja111152z
Return to citation in text: [1] -
Itahara, T. J. Org. Chem. 1985, 50, 5546–5550. doi:10.1021/jo00350a023
Return to citation in text: [1] -
Waldvogel, S. R.; Lips, S.; Selt, M.; Riehl, B.; Kampf, C. J. Chem. Rev. 2018, 118, 6706–6765. doi:10.1021/acs.chemrev.8b00233
Return to citation in text: [1] -
T.P, A. K.; Pandaram, S.; Ilangovan, A. Org. Chem. Front. 2019, 6, 3244–3251. doi:10.1039/c9qo00623k
Return to citation in text: [1] -
Wang, L.; Shen, J.; Yang, S.; Liu, W.; Chen, Q.; He, M. Green Chem. 2018, 20, 1290–1296. doi:10.1039/c8gc00012c
Return to citation in text: [1] -
Fürst, M. C. D.; Gans, E.; Böck, M. J.; Heinrich, M. R. Chem. – Eur. J. 2017, 23, 15312–15315. doi:10.1002/chem.201703954
Return to citation in text: [1] -
Schroll, P.; Hari, D. P.; König, B. ChemistryOpen 2012, 1, 130–133. doi:10.1002/open.201200011
Return to citation in text: [1] -
Wang, Y.; Zhu, S.; Zou, L.-H. Eur. J. Org. Chem. 2019, 2179–2201. doi:10.1002/ejoc.201900028
Return to citation in text: [1] -
Miliutina, M.; Janke, J.; Hassan, S.; Zaib, S.; Iqbal, J.; Lecka, J.; Sévigny, J.; Villinger, A.; Friedrich, A.; Lochbrunner, S.; Langer, P. Org. Biomol. Chem. 2018, 16, 717–732. doi:10.1039/c7ob02729j
Return to citation in text: [1] -
Carlson, B. W.; Miller, L. L. J. Am. Chem. Soc. 1985, 107, 479–485. doi:10.1021/ja00288a035
Return to citation in text: [1] -
Vroemans, R.; Verhaegen, Y.; Dieu, M. T. T.; Dehaen, W. Beilstein J. Org. Chem. 2018, 14, 2689–2697. doi:10.3762/bjoc.14.246
Return to citation in text: [1] -
Gia, O.; Uriarte, E.; Zagotto, G.; Baccichetti, F.; Antonello, C.; Marciani-Magno, S. J. Photochem. Photobiol., B 1992, 14, 95–104. doi:10.1016/1011-1344(92)85085-9
Return to citation in text: [1]
| 13. | Oliveira, A. M. A. G.; Raposo, M. M. M.; Oliveira-Campos, A. M. F.; Griffiths, J.; Machado, A. E. H. Helv. Chim. Acta 2003, 86, 2900–2907. doi:10.1002/hlca.200390237 |
| 1. | Shou, Q.; Banbury, L. K.; Renshaw, D. E.; Lambley, E. H.; Mon, H.; Macfarlane, G. A.; Griesser, H. J.; Heinrich, M. M.; Wohlmuth, H. J. Nat. Prod. 2012, 75, 1612–1617. doi:10.1021/np300433r |
| 2. | Setzer, W. N.; Rozmus, G. F.; Setzer, M. C.; Schmidt, J. M.; Vogler, B.; Reeb, S.; Jackes, B. R.; Irvine, A. K. J. Mol. Model. 2006, 12, 703–711. doi:10.1007/s00894-005-0047-1 |
| 14. | Duan, W.; Rangan, A.; Vankayalapati, H.; Kim, M. Y.; Zeng, Q.; Sun, D.; Han, H.; Fedoroff, O. Y.; Nishioka, D.; Rha, S. Y.; Izbicka, E.; Von Hoff, D. D.; Hurley, L. H. Mol. Cancer Ther. 2001, 1, 103–120. |
| 28. | Prado, S.; Toum, V.; Saint-Joanis, B.; Michel, S.; Koch, M.; Cole, S.; Tillequin, F.; Janin, Y. Synthesis 2007, 1566–1570. doi:10.1055/s-2007-966019 |
| 12. | Francisco, C. S.; Rodrigues, L. R.; Cerqueira, N. M. F. S. A.; Oliveira-Campos, A. M. F.; Rodrigues, L. M. Eur. J. Med. Chem. 2012, 47, 370–376. doi:10.1016/j.ejmech.2011.11.005 |
| 13. | Oliveira, A. M. A. G.; Raposo, M. M. M.; Oliveira-Campos, A. M. F.; Griffiths, J.; Machado, A. E. H. Helv. Chim. Acta 2003, 86, 2900–2907. doi:10.1002/hlca.200390237 |
| 29. | Meerwein, H.; Büchner, E.; van Emster, K. J. Prakt. Chem. 1939, 152, 237–266. doi:10.1002/prac.19391520705 |
| 11. | Ito, T.; Sasabe, H.; Nagai, Y.; Watanabe, Y.; Onuma, N.; Kido, J. Chem. – Eur. J. 2019, 25, 7308–7314. doi:10.1002/chem.201805907 |
| 23. | Voigt, B.; Krug, M.; Schächtele, C.; Totzke, F.; Hilgeroth, A. ChemMedChem 2008, 3, 120–126. doi:10.1002/cmdc.200700175 |
| 24. | Tell, V.; Mahmoud, K. A.; Wichapong, K.; Schächtele, C.; Totzke, F.; Sippl, W.; Hilgeroth, A. Med. Chem. Commun. 2012, 3, 1413. doi:10.1039/c2md20201h |
| 1. | Shou, Q.; Banbury, L. K.; Renshaw, D. E.; Lambley, E. H.; Mon, H.; Macfarlane, G. A.; Griesser, H. J.; Heinrich, M. M.; Wohlmuth, H. J. Nat. Prod. 2012, 75, 1612–1617. doi:10.1021/np300433r |
| 2. | Setzer, W. N.; Rozmus, G. F.; Setzer, M. C.; Schmidt, J. M.; Vogler, B.; Reeb, S.; Jackes, B. R.; Irvine, A. K. J. Mol. Model. 2006, 12, 703–711. doi:10.1007/s00894-005-0047-1 |
| 3. | De Lombaert, S.; Blanchard, L.; Stamford, L. B.; Tan, J.; Wallace, E. M.; Satoh, Y.; Fitt, J.; Hoyer, D.; Simonsbergen, D.; Moliterni, J.; Marcopoulos, N.; Savage, P.; Chou, M.; Trapani, A. J.; Jeng, A. Y. J. Med. Chem. 2000, 43, 488–504. doi:10.1021/jm990507o |
| 4. | Kaul, R.; Deechongkit, S.; Kelly, J. W. J. Am. Chem. Soc. 2002, 124, 11900–11907. doi:10.1021/ja020675x |
| 5. | Carney, J. R.; Krenisky, J. M.; Williamson, R. T.; Luo, J. J. Nat. Prod. 2002, 65, 203–205. doi:10.1021/np010374l |
| 6. | Petrassi, H. M.; Johnson, S. M.; Purkey, H. E.; Chiang, K. P.; Walkup, T.; Jiang, X.; Powers, E. T.; Kelly, J. W. J. Am. Chem. Soc. 2005, 127, 6662–6671. doi:10.1021/ja044351f |
| 7. | Momotake, A.; Lindegger, N.; Niggli, E.; Barsotti, R. J.; Ellis-Davies, G. C. R. Nat. Methods 2006, 3, 35–40. doi:10.1038/nmeth821 |
| 8. | Fang, L.; Fang, X.; Gou, S.; Lupp, A.; Lenhardt, I.; Sun, Y.; Huang, Z.; Chen, Y.; Zhang, Y.; Fleck, C. Eur. J. Med. Chem. 2014, 76, 376–386. doi:10.1016/j.ejmech.2014.02.035 |
| 9. | Lin, R.-D.; Chen, M.-C.; Liu, Y.-L.; Lin, Y.-T.; Lu, M.-K.; Hsu, F.-L.; Lee, M.-H. Int. J. Mol. Sci. 2015, 16, 28598–28613. doi:10.3390/ijms161226115 |
| 10. | Love, B. E. Eur. J. Med. Chem. 2015, 97, 377–387. doi:10.1016/j.ejmech.2015.01.005 |
| 25. | Brachwitz, K.; Voigt, B.; Meijer, L.; Lozach, O.; Schächtele, C.; Molnár, J.; Hilgeroth, A. J. Med. Chem. 2003, 46, 876–879. doi:10.1021/jm021090g |
| 26. | Voigt, B.; Meijer, L.; Lozach, O.; Schächtele, C.; Totzke, F.; Hilgeroth, A. Bioorg. Med. Chem. Lett. 2005, 15, 823–825. doi:10.1016/j.bmcl.2004.10.091 |
| 27. | Tell, V.; Holzer, M.; Herrmann, L.; Mahmoud, K. A.; Schächtele, C.; Totzke, F.; Hilgeroth, A. Bioorg. Med. Chem. Lett. 2012, 22, 6914–6918. doi:10.1016/j.bmcl.2012.09.006 |
| 18. | Prado, S.; Ledeit, H.; Michel, S.; Koch, M.; Darbord, J. C.; Cole, S. T.; Tillequin, F.; Brodin, P. Bioorg. Med. Chem. 2006, 14, 5423–5428. doi:10.1016/j.bmc.2006.03.033 |
| 20. | Brachwitz, K.; Hilgeroth, A. Bioorg. Med. Chem. Lett. 2002, 12, 411–413. doi:10.1016/s0960-894x(01)00769-7 |
| 21. | Krug, M.; Voigt, B.; Baumert, C.; Lüpken, R.; Molnár, J.; Hilgeroth, A. Eur. J. Med. Chem. 2010, 45, 2683–2688. doi:10.1016/j.ejmech.2010.02.024 |
| 17. | Yempala, T.; Sridevi, J. P.; Yogeeswari, P.; Sriram, D.; Kantevari, S. Bioorg. Med. Chem. Lett. 2013, 23, 5393–5396. doi:10.1016/j.bmcl.2013.07.048 |
| 22. | Krug, M.; Erlenkamp, G.; Sippl, W.; Schächtele, C.; Totzke, F.; Hilgeroth, A. Bioorg. Med. Chem. Lett. 2010, 20, 6915–6919. doi:10.1016/j.bmcl.2010.10.004 |
| 16. | Eduardo Paiva Sena Maia, J.; de Lucas, N. C.; Cesarin-Sobrinho, D.; da Hora Machado, A. E.; Netto-Ferreira, J. C. J. Photochem. Photobiol., A 2016, 326, 21–29. doi:10.1016/j.jphotochem.2016.04.013 |
| 15. | Wichapong, K.; Lindner, M.; Pianwanit, S.; Kokpol, S.; Sippl, W. Eur. J. Med. Chem. 2009, 44, 1383–1395. doi:10.1016/j.ejmech.2008.09.027 |
| 19. | Balasubramanian, G.; Narayanan, S.; Andiappan, L.; Sappanimuthu, T.; Thirunavukkarasu, S.; Sundaram, S.; Natarajan, S.; Sivaraman, N.; Rajagopal, S.; Nazumudeen, F. A. A.; Saxena, S.; Vishwakarma, S. L.; Narayanan, S.; Sharma, G. V. R.; Srinivasan, C. V.; Kilambi, N. Bioorg. Med. Chem. 2016, 24, 5702–5716. doi:10.1016/j.bmc.2016.09.011 |
| 32. | Waldvogel, S. R.; Lips, S.; Selt, M.; Riehl, B.; Kampf, C. J. Chem. Rev. 2018, 118, 6706–6765. doi:10.1021/acs.chemrev.8b00233 |
| 30. | Fujiwara, Y.; Domingo, V.; Seiple, I. B.; Gianatassio, R.; Del Bel, M.; Baran, P. S. J. Am. Chem. Soc. 2011, 133, 3292–3295. doi:10.1021/ja111152z |
| 40. | Vroemans, R.; Verhaegen, Y.; Dieu, M. T. T.; Dehaen, W. Beilstein J. Org. Chem. 2018, 14, 2689–2697. doi:10.3762/bjoc.14.246 |
| 41. | Gia, O.; Uriarte, E.; Zagotto, G.; Baccichetti, F.; Antonello, C.; Marciani-Magno, S. J. Photochem. Photobiol., B 1992, 14, 95–104. doi:10.1016/1011-1344(92)85085-9 |
| 39. | Carlson, B. W.; Miller, L. L. J. Am. Chem. Soc. 1985, 107, 479–485. doi:10.1021/ja00288a035 |
| 20. | Brachwitz, K.; Hilgeroth, A. Bioorg. Med. Chem. Lett. 2002, 12, 411–413. doi:10.1016/s0960-894x(01)00769-7 |
| 21. | Krug, M.; Voigt, B.; Baumert, C.; Lüpken, R.; Molnár, J.; Hilgeroth, A. Eur. J. Med. Chem. 2010, 45, 2683–2688. doi:10.1016/j.ejmech.2010.02.024 |
| 22. | Krug, M.; Erlenkamp, G.; Sippl, W.; Schächtele, C.; Totzke, F.; Hilgeroth, A. Bioorg. Med. Chem. Lett. 2010, 20, 6915–6919. doi:10.1016/j.bmcl.2010.10.004 |
| 23. | Voigt, B.; Krug, M.; Schächtele, C.; Totzke, F.; Hilgeroth, A. ChemMedChem 2008, 3, 120–126. doi:10.1002/cmdc.200700175 |
| 24. | Tell, V.; Mahmoud, K. A.; Wichapong, K.; Schächtele, C.; Totzke, F.; Sippl, W.; Hilgeroth, A. Med. Chem. Commun. 2012, 3, 1413. doi:10.1039/c2md20201h |
| 25. | Brachwitz, K.; Voigt, B.; Meijer, L.; Lozach, O.; Schächtele, C.; Molnár, J.; Hilgeroth, A. J. Med. Chem. 2003, 46, 876–879. doi:10.1021/jm021090g |
| 26. | Voigt, B.; Meijer, L.; Lozach, O.; Schächtele, C.; Totzke, F.; Hilgeroth, A. Bioorg. Med. Chem. Lett. 2005, 15, 823–825. doi:10.1016/j.bmcl.2004.10.091 |
| 37. | Wang, Y.; Zhu, S.; Zou, L.-H. Eur. J. Org. Chem. 2019, 2179–2201. doi:10.1002/ejoc.201900028 |
| 38. | Miliutina, M.; Janke, J.; Hassan, S.; Zaib, S.; Iqbal, J.; Lecka, J.; Sévigny, J.; Villinger, A.; Friedrich, A.; Lochbrunner, S.; Langer, P. Org. Biomol. Chem. 2018, 16, 717–732. doi:10.1039/c7ob02729j |
| 33. | T.P, A. K.; Pandaram, S.; Ilangovan, A. Org. Chem. Front. 2019, 6, 3244–3251. doi:10.1039/c9qo00623k |
| 34. | Wang, L.; Shen, J.; Yang, S.; Liu, W.; Chen, Q.; He, M. Green Chem. 2018, 20, 1290–1296. doi:10.1039/c8gc00012c |
| 35. | Fürst, M. C. D.; Gans, E.; Böck, M. J.; Heinrich, M. R. Chem. – Eur. J. 2017, 23, 15312–15315. doi:10.1002/chem.201703954 |
| 36. | Schroll, P.; Hari, D. P.; König, B. ChemistryOpen 2012, 1, 130–133. doi:10.1002/open.201200011 |
© 2021 Singh et al.; licensee Beilstein-Institut.
This is an Open Access article under the terms of the Creative Commons Attribution License (https://creativecommons.org/licenses/by/4.0). Please note that the reuse, redistribution and reproduction in particular requires that the author(s) and source are credited and that individual graphics may be subject to special legal provisions.
The license is subject to the Beilstein Journal of Organic Chemistry terms and conditions: (https://www.beilstein-journals.org/bjoc/terms)