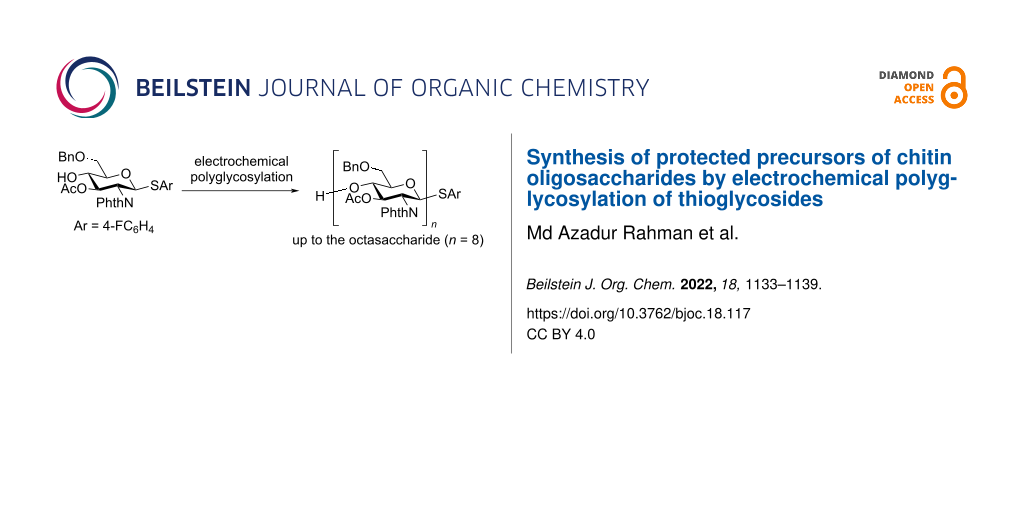
Abstract
The synthesis of protected precursors of chitin oligosaccharides by electrochemical polyglycosylation of thioglycosides as monomer is described. Oligosaccharides up to the hexasaccharide were synthesized under optimized reaction conditions. Further, a modified method enabled the synthesis of oligosaccharides up to the octasaccharide by repeating electrolysis with additional monomers. The mechanism of the electrochemical polyglycosylation is also discussed, based on the oxidation potential of the monomer and oligosaccharides.
Graphical Abstract
Introduction
Chitin oligosaccharides are partial structures of chitin, which is an abundant β-1,4-linked polysaccharide composed of N-acetylglucosamine as repeating unit (Figure 1) [1]. Biological activities of longer oligosaccharides, such as octasaccharide, have been paid much attention for many years. However, it is difficult to obtain pure oligosaccharides by isolation from natural sources or by synthesis via chemical glycosylation [2]. Total syntheses of chitin and chitosan oligosaccharides based on conventional chemical glycosylation of protected monosaccharides as building blocks have already been reported. Convergent synthesis using oligosaccharide building blocks can reduce the number of steps in the total synthesis. However, it requires manipulation of the anomeric leaving groups and deprotection of the protected hydroxy group at the 4-position prior to glycosylation. Although automated electrochemical assembly, which is a one-pot iterative synthesis of oligosaccharides based on electrochemical preactivation of building blocks, is an alternative method for the synthesis of chitin oligosaccharides [3,4], it is also time-consuming and too sophisticated to prepare oligosaccharides composed of a single repeating structure. Thus, we assume that the electrochemical polyglycosylation via the electrochemical activation of thioglycosides is a practical approach for the preparation of chitin oligosaccharides. Hashimoto and co-workers have already reported the synthesis of protected precursors of chitin oligosaccharides by polyglycosylation of thioglycosides [5]. However, this is one of a few examples of chemical synthesis of chitin oligosaccharides through polyglycosylation of a glucosamine monosaccharide [6]. Recently, we have reported electrochemical polyglycosylation using a glucosamine derivative as a monomer [7]. This is another example of polyglycosylation of a glucosamine monosaccharide. However, N-acetylglucosamines are linked by α-1,4-glycosidic bonds. Here, we report electrochemical polyglycosylation of thioglycosides to produce protected precursors of chitin oligosaccharides.
Figure 1: Structures of chitin and chitosan oligosaccharides.
Figure 1: Structures of chitin and chitosan oligosaccharides.
Results and Discussion
Optimization of electrochemical polyglycosylation
We initiated our study with the optimization of the arylthio group of thioglycoside 1, carrying an unprotected 4-OH group, an acetyl-protected 3-OH unit, a benzyl-protected 6-OH group, and a phthaloyl-protected 2-NH2 unit (Figure 2) [3]. Electrochemical polyglycosylation was performed by a sequential two-step process, which involved anodic oxidation at −80 °C and glycosylation at −50 °C. The crude product of the reaction was purified by gel permeation chromatography (GPC), and the monosaccharides 1a–d and oligosaccharides 2a–d (n = 2)–7a–d (n = 7) were isolated. Only thioglycoside 1a (Ar = 4-FC6H4, Eox = 1.70 V vs SCE) gave oligosaccharides up to hexasaccharide 6a, although the yield of pentasaccharide 5a (3%) and hexasaccharide 6a (1%) was very low. For thioglycoside 1b (Ar = 4-ClC6H4, Eox = 1.68 V vs SCE), the highest conversion (79%) and the highest yield of tetrasaccharide 4b (14%) were observed. Contrary, thioglycoside 1c (Ar = 4-MeC6H4, Eox = 1.47 V vs SCE), which had the lowest oxidation potential, showed the lowest conversion (51%) and the lowest yield of tetrasaccharide 4c (2%) [8]. This being the case, lower conversion of the building block 1c and lower yield of oligosaccharides 2c–4c indicated that thus-generated oligosaccharides 2c–4c, with a lower oxidation potential, also consumed electricity and converted to the corresponding hydroxy-substituted sugars, as observed by MS analysis. Thioglycoside 1d (Ar = 2,4-F2C6H3, Eox = 1.73 V vs SCE), which had the highest oxidation potential, also showed low conversion (63%). However, it gave pentasaccharide 5d in the highest yield (6%) among these four thioglycosides. Based on these results, we optimized the reaction using thioglycoside 1a, which afforded oligosaccharides 2a–6a and recovered monosaccharide 1a in the highest total yield (88%).
Figure 2: Effect of the anomeric leaving group on the yield of oligosaccharides.
Figure 2: Effect of the anomeric leaving group on the yield of oligosaccharides.
Reaction parameters of the electrochemical polyglycosylation, such as amount of electricity and electrolyte, were also optimized using thioglycoside 1a (see Supporting Information File 1 for details). The complete conversion of monosaccharide 1a was observed with 0.6 F/mol. However, 0.525 F/mol was chosen as the optimal amount of electricity to prevent formation of byproducts, such as hydroxy-substituted sugars that carry an anomeric hydroxy group instead of the ArS group. Although we tested other ammonium triflates, such as tetraethylammonium triflate and 1-butyl-1-methylpyrrolidinium triflate as electrolytes, both electrolytes gave oligosaccharides in lower yield.
Next, we investigated the influence of the glycosylation temperature (T2), and it was revealed that glycosylation proceeded even at −80 °C (Figure 3, in pink). Although higher conversion of thioglycoside 1a was observed at higher temperature, we did not test a glycosylation temperature above −30 °C because of the low stability of the glycosylation intermediate at an elevated temperature [9]. It is important to note that heptasaccharide 7a, which was never obtained at −50 °C, was produced at −40 °C (in blue) and −30 °C (in black), although the yield of 7a was very low (1%). These results indicated that the glycosylation temperature was an important parameter for obtaining longer oligosaccharides, and glycosylation might proceed during the anodic oxidation at −80 °C.
Figure 3: Influence of the glycosylation temperature (T2) on the yield of oligosaccharides.
Figure 3: Influence of the glycosylation temperature (T2) on the yield of oligosaccharides.
The temperature of anodic oxidation (T1) was also investigated together with the glycosylation temperature (T2) because glycosylation must occur during the anodic oxidation at elevated temperature (Figure 4). Indeed, formation of oligosaccharides longer than tetrasaccharide 4a was increased at elevated temperature. The highest total yield of oligosaccharides 2a–7a was obtained at T1 = −60 °C and T2 = −30 °C, although heptasaccharide 7a was not produced. MALDI–TOF MS spectra indicated the formation of byproducts derived from longer oligosaccharides at T1 = −30 °C and T2 = −30 °C (Figure 5). The relative intensity of the molecular ion peaks of hydroxy-substituted sugars of oligosaccharides and/or trehalose pseudo-oligosaccharides, which were major byproducts at the elevated temperature, became stronger in the corresponding peaks of longer oligosaccharides, such as hexasaccharide 6a and heptasaccharide 7a. Proposed structures of byproducts of trisaccharide 3a, including hydroxy-substituted sugar 9 and trehalose product 10, are shown in Figure 6. These byproducts were obtained as inseparable mixture because of the same molecular weight and similar polarity. Moreover, the trehalose product of longer oligosaccharides has more than two possible structures. For example, there are two pseudo-tetrasaccharide structures 11a and 11b, which would be hard to separate by preparative-scale purification techniques.
Figure 4: Influence of temperatures of anodic oxidation (T1) and glycosylation (T2).
Figure 4: Influence of temperatures of anodic oxidation (T1) and glycosylation (T2).
![[1860-5397-18-117-5]](/bjoc/content/figures/1860-5397-18-117-5.png?scale=2.0&max-width=1024&background=FFFFFF)
Figure 5: MALDI–TOF MS spectra of oligosaccharides.
Figure 5: MALDI–TOF MS spectra of oligosaccharides.
Figure 6: Proposed structures of byproducts of electrochemical polyglycosylation.
Figure 6: Proposed structures of byproducts of electrochemical polyglycosylation.
Reaction mechanism
There are two possible pathways for chain elongation in electrochemical polyglycosylation (Figure 7). In path a, monosaccharide 1a is converted to the corresponding glycosyl triflate 12, and 4-OH of oligosaccharides 2a–6a reacts with 12 [9]. In path b, oligosaccharides 2a–6a are converted to the corresponding glycosyl triflates 13–17, and 4-OH of monosaccharide 1a reacts with 13–17. It is difficult to exclude the possibility of reactions between oligosaccharides. However, polyglycosylation has been carried out with a slightly excessive amount of electricity (0.525 F/mol), and monosaccharide 1a has always been recovered in more than 15% [10]. Moreover, longer oligosaccharides might be less reactive on grounds of mass transfer because electrochemical activation occurs at the surface of the anode and the substrates must move to the surface of the electrode.
Figure 7: Proposed mechanisms of electrochemical polyglycosylation.
Figure 7: Proposed mechanisms of electrochemical polyglycosylation.
To confirm the reactivity of oligosaccharides, we measured oxidation potentials of monosaccharide 1a, disaccharide 2a, and trisaccharide 3a using a rotating disk electrode (RDE) made of glassy carbon (Figure 8). The oxidation potential of oligosaccharides 2a and 3a (Eox = 1.76 and 1.74 V vs SCE) was higher than that of monosaccharide 1a (Eox = 1.70 V vs SCE). We also examined electrochemical activation of tetrasaccharide 4a to obtain octasaccharide 8a through the dimerization of 4a. However, a trace amount of 8a was formed together with byproducts, and the recovered amount of tetrasaccharide 4a was 64% (Scheme 1). These results strongly suggested that path a in Figure 7 is the most probable mechanism of the reaction.
Figure 8: Oxidative potential of monosaccharide 1a, disaccharide 2a, and trisaccharide 3a.
Figure 8: Oxidative potential of monosaccharide 1a, disaccharide 2a, and trisaccharide 3a.
Scheme 1: Electrochemical dimerization of tetrasaccharide 4a.
Scheme 1: Electrochemical dimerization of tetrasaccharide 4a.
Modification of electrochemical polyglycosylation protocol
The optimized conditions of the electrochemical polyglycosylation can afford oligosaccharides up to the hexasaccharide 6a. However, we were also interested in longer oligosaccharides, such as the heptasaccharide 7a and the octasaccharide 8a because of the biological activities [11]. Accordingly, the higher reactivity of monosaccharide 1a compared to the oligosaccharides encouraged us to modify the electrochemical polyglycosylation protocol (Figure 9). We developed a modified electrochemical polyglycosylation method by repeating the addition of monosaccharide 1a and anodic oxidation as a single cycle. To prove our concept, we ran the electrochemical polyglycosylation under the optimized conditions, and one equivalent of monosaccharide 1a was added before the second anodic oxidation. After the second cycle, we could isolate heptasaccharide 7a (3%, 1.5 μmol, 4.7 mg) together with an increased amount of hexasaccharide 6a (5%, 3.1 μmol, 8.2 mg). We ran the process up to the third cycle and isolated octasaccharide 8a (3%, 2.3 μmol, 7.6 mg), which was never isolated after the first cycle and the second cycle. These results also supported the proposed reaction mechanism path a in Figure 7.
Figure 9: Influence of cycle number on the yield of longer oligosaccharides 5a (n = 5)–8a (n = 8). Conditions of anodic oxidation: constant current (8.0 mA, 0.52 F/mol), temperature −60 °C for anodic oxidation and −30 °C for glycosylation, building block 1a (0.20 mmol, 109 mg) per cycle as 0.2 M solution in dry CH2Cl2.
Figure 9: Influence of cycle number on the yield of longer oligosaccharides 5a (n = 5)–8a (n = 8). Conditions...
Conclusion
In conclusion, we have developed a practical method to synthesize longer-chain oligosaccharides within a short period of time through electrochemical polyglycosylation. A rational reaction mechanism was proposed based on oxidation potentials of the oligosaccharides, and further modification of the protocol was examined. By repeating cycles in one pot, the modified method enabled us to prepare longer oligosaccharides up to the octasaccharide. Further optimizations of reaction parameters, such as concentration, size and shape of electrodes for large-scale production of oligosaccharides, and deprotection of oligosaccharides thus obtained are in progress in our laboratory.
Experimental
Electrochemical polyglycosylation (see Figure 4, T1 = −60 °C and T2 = −30 °C) was performed using our second-generation automated electrochemical synthesizer equipped with the H-type electrolysis cell. Thioglycoside 1a (0.20 mmol, 109 mg), Bu4NOTf (1.0 mmol, 393 mg), and dry CH2Cl2 (10 mL) were added to the anodic chamber. Triflic acid (0.2 mmol, 17.6 μL) and CH2Cl2 (10 mL) were added to the cathodic chamber. Electrolysis was performed at −60 °C under constant current conditions until 0.52 F/mol of electricity had been consumed. Then, the reaction temperature was elevated to −30 °C, and the temperature was kept for 1 h. The reaction was quenched with Et3N (0.30 mL), and the reaction mixture was diluted with Et2O and EtOAc and washed with water to remove the electrolyte. The combined organic layer was dried with Na2SO4, and the solvent was removed under reduced pressure. The thus-obtained crude product (110 mg) was purified by preparative GPC using CHCl3 as eluent.
Supporting Information
| Supporting Information File 1: Additional experimental details and compound characterization data. | ||
| Format: PDF | Size: 8.0 MB | Download |
References
-
Fittolani, G.; Tyrikos-Ergas, T.; Vargová, D.; Chaube, M. A.; Delbianco, M. Beilstein J. Org. Chem. 2021, 17, 1981–2025. doi:10.3762/bjoc.17.129
Return to citation in text: [1] -
Yang, Y.; Yu, B. Tetrahedron 2014, 70, 1023–1046. doi:10.1016/j.tet.2013.11.064
Return to citation in text: [1] -
Nokami, T.; Hayashi, R.; Saigusa, Y.; Shimizu, A.; Liu, C.-Y.; Mong, K.-K. T.; Yoshida, J. Org. Lett. 2013, 15, 4520–4523. doi:10.1021/ol402034g
Return to citation in text: [1] [2] -
Isoda, Y.; Sasaki, N.; Kitamura, K.; Takahashi, S.; Manmode, S.; Takeda-Okuda, N.; Tamura, J.; Nokami, T.; Itoh, T. Beilstein J. Org. Chem. 2017, 13, 919–924. doi:10.3762/bjoc.13.93
Return to citation in text: [1] -
Hashimoto, H.; Abe, Y.; Horito, S.; Yoshimura, J. J. Carbohydr. Chem. 1989, 8, 307–311. doi:10.1080/07328308908048012
Return to citation in text: [1] -
Kadokawa, J.; Watanabe, Y.; Karasu, M.; Tagaya, H.; Chiba, K. Macromol. Rapid Commun. 1996, 17, 367–372. doi:10.1002/marc.1996.030170601
Return to citation in text: [1] -
Endo, H.; Ochi, M.; Rahman, M. A.; Hamada, T.; Kawano, T.; Nokami, T. Chem. Commun. 2022, 58, 7948–7951. doi:10.1039/d2cc02287g
Return to citation in text: [1] -
Nokami, T.; Isoda, Y.; Sasaki, N.; Takaiso, A.; Hayase, S.; Itoh, T.; Hayashi, R.; Shimizu, A.; Yoshida, J. Org. Lett. 2015, 17, 1525–1528. doi:10.1021/acs.orglett.5b00406
Return to citation in text: [1] -
Nokami, T.; Shibuya, A.; Tsuyama, H.; Suga, S.; Bowers, A. A.; Crich, D.; Yoshida, J. J. Am. Chem. Soc. 2007, 129, 10922–10928. doi:10.1021/ja072440x
Return to citation in text: [1] [2] -
The theoretical amount of electricity necessary for 100% conversion of the monosaccharide building block into disaccharide is 0.5 F/mol. This theoretical value was based on the hypothesis of selective activation of the monosaccharide.
Return to citation in text: [1] -
Li, K.; Xing, R.; Liu, S.; Li, P. J. Agric. Food Chem. 2020, 68, 12203–12211. doi:10.1021/acs.jafc.0c05316
Return to citation in text: [1]
| 1. | Fittolani, G.; Tyrikos-Ergas, T.; Vargová, D.; Chaube, M. A.; Delbianco, M. Beilstein J. Org. Chem. 2021, 17, 1981–2025. doi:10.3762/bjoc.17.129 |
| 6. | Kadokawa, J.; Watanabe, Y.; Karasu, M.; Tagaya, H.; Chiba, K. Macromol. Rapid Commun. 1996, 17, 367–372. doi:10.1002/marc.1996.030170601 |
| 5. | Hashimoto, H.; Abe, Y.; Horito, S.; Yoshimura, J. J. Carbohydr. Chem. 1989, 8, 307–311. doi:10.1080/07328308908048012 |
| 3. | Nokami, T.; Hayashi, R.; Saigusa, Y.; Shimizu, A.; Liu, C.-Y.; Mong, K.-K. T.; Yoshida, J. Org. Lett. 2013, 15, 4520–4523. doi:10.1021/ol402034g |
| 4. | Isoda, Y.; Sasaki, N.; Kitamura, K.; Takahashi, S.; Manmode, S.; Takeda-Okuda, N.; Tamura, J.; Nokami, T.; Itoh, T. Beilstein J. Org. Chem. 2017, 13, 919–924. doi:10.3762/bjoc.13.93 |
| 2. | Yang, Y.; Yu, B. Tetrahedron 2014, 70, 1023–1046. doi:10.1016/j.tet.2013.11.064 |
| 9. | Nokami, T.; Shibuya, A.; Tsuyama, H.; Suga, S.; Bowers, A. A.; Crich, D.; Yoshida, J. J. Am. Chem. Soc. 2007, 129, 10922–10928. doi:10.1021/ja072440x |
| 10. | The theoretical amount of electricity necessary for 100% conversion of the monosaccharide building block into disaccharide is 0.5 F/mol. This theoretical value was based on the hypothesis of selective activation of the monosaccharide. |
| 8. | Nokami, T.; Isoda, Y.; Sasaki, N.; Takaiso, A.; Hayase, S.; Itoh, T.; Hayashi, R.; Shimizu, A.; Yoshida, J. Org. Lett. 2015, 17, 1525–1528. doi:10.1021/acs.orglett.5b00406 |
| 11. | Li, K.; Xing, R.; Liu, S.; Li, P. J. Agric. Food Chem. 2020, 68, 12203–12211. doi:10.1021/acs.jafc.0c05316 |
| 3. | Nokami, T.; Hayashi, R.; Saigusa, Y.; Shimizu, A.; Liu, C.-Y.; Mong, K.-K. T.; Yoshida, J. Org. Lett. 2013, 15, 4520–4523. doi:10.1021/ol402034g |
| 7. | Endo, H.; Ochi, M.; Rahman, M. A.; Hamada, T.; Kawano, T.; Nokami, T. Chem. Commun. 2022, 58, 7948–7951. doi:10.1039/d2cc02287g |
| 9. | Nokami, T.; Shibuya, A.; Tsuyama, H.; Suga, S.; Bowers, A. A.; Crich, D.; Yoshida, J. J. Am. Chem. Soc. 2007, 129, 10922–10928. doi:10.1021/ja072440x |
© 2022 Rahman et al.; licensee Beilstein-Institut.
This is an open access article licensed under the terms of the Beilstein-Institut Open Access License Agreement (https://www.beilstein-journals.org/bjoc/terms), which is identical to the Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0). The reuse of material under this license requires that the author(s), source and license are credited. Third-party material in this article could be subject to other licenses (typically indicated in the credit line), and in this case, users are required to obtain permission from the license holder to reuse the material.