Abstract
A straightforward electro-conversion of cumene into acetophenone has been reported using boron-doped diamond (BDD) electrodes. This particular conversion is driven by the addition reaction of a cathodically generated hydroperoxide anion to an anodically generated cumyl cation, where the BDD’s wide potential window enables the direct anodic oxidation of cumene into the cumyl cation. Since electricity is directly employed as the oxidizing and reducing reagents, the present protocol is easy to use, suitable for scale-up, and inherently safe.
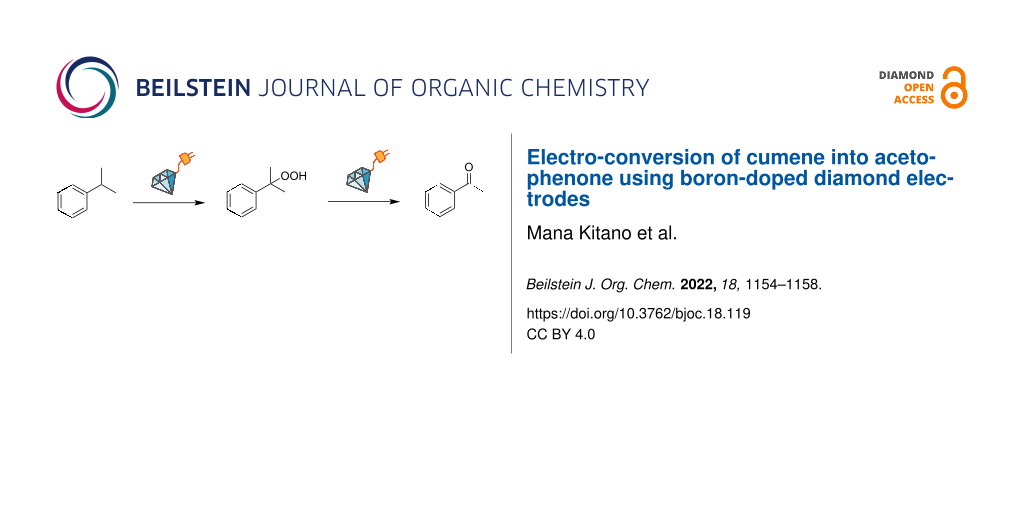
Graphical Abstract
Introduction
Selective oxidation of aromatic alkyl side chains is an important molecular transformation process to obtain various rubbers, resins, fine chemicals, and other industrial products [1,2]: terephthalic acid from p-xylene, cumene hydroperoxide/dicumyl peroxide/phenol from cumene, acetophenone from ethylbenzene, and others. Generally, molecular oxygen has been utilized in the straightforward oxidation of aromatic alkyls. However, since molecular oxygen is highly stable, activation of the molecular oxygen itself is necessary, which requires a specific catalyst and/or harsh conditions such as high temperature and pressure. Recent environmental and sustainable concerns lead to a growing demand for the development of greener oxidation processes. For example, even for the cumene process that involves the oxidation reaction of cumene, first reported in 1944 [3], a wide variety of catalytic systems are still being reported [4-12].
Electro-organic synthesis refers to an organic synthetic method combined with electrochemistry [13,14]. A striking feature in electro-organic synthesis is the use of electricity as a reagent, which allows to reduce the reagent waste to a minimum. Obviously, as this characteristic matches well with the increasing demands to realize a sustainable society. In electro-organic synthesis, electrode materials are one of the most significant parameters because reactions occur at the anode and/or cathode. Boron-doped diamond (BDD) is a relatively new electrode material [15,16] and shows a wide potential window, which can be applied to the transformation of compounds with high redox potentials. Therefore, BDD electrodes would enable a straightforward oxidation reaction of aromatic alkyls, which is difficult to achieve with other conventional electrode materials.
Herein, we report the straightforward electro-conversion of cumene, one of the most important and extensively investigated aromatic alkyls, by BDD electrodes. Acetophenone was obtained as the main product when BDD was used as the anode. The role of electrode materials was investigated with electrochemical measurements. Only the BDD anode with a wide potential window can oxidize cumene directly to afford a cumyl cation as the reaction intermediate. Furthermore, acetophenone is produced via cumene hydroperoxide, and this molecular conversion is found to proceed electrochemically.
Results and Discussion
First, we carried out the electrolysis of cumene (1) in 0.1 M Bu4NBF4/MeCN under constant current conditions in an undivided beaker-type cell (Table 1, entry 1). The main product was acetophenone (3) and α-cumyl alcohol (4) was also obtained. When the anion of the supporting electrolyte was changed to the perchlorate ion (Table 1, entries 2–4), isolated yields of 3 were increased, in which using Et4NClO4 gave the best result. Next, the current density (j) and the amount of charge (Q; referring to mole of 1) were investigated (Table 1, entries 5–7). As the isolated yield of 3 was not particularly improved by changing j and Q, we set the optimum conditions as j of 2.1 mA/cm2 and Q of 5 F. On the other hand, when the combination of anode and cathode was graphite/graphite or Ni/Ni (Table 1, entries 8 and 9), almost no acetophenone was obtained. Therefore, it is suggested that the BDD anode is essential in the electro-conversion of 1 into 3, and the cathode material has no significant effect. Here, the low total yields would be due to the formation of highly polar compounds. We were not able to obtain them as isolated compounds. In addition, 1H NMR and FTIR spectra for the crude compound did not show characteristic peaks derived from carboxylic acid and amide.
Table 1: Electro-conversion of 1.
|
|
|||||||||
| Entrya | Anode | Cathode |
Supporting
electrolyte |
jb | Qc | Isolated yields (%) | |||
| 1 | 2 | 3 | 4 | ||||||
| 1 | BDD | BDD | Bu4NBF4 | 2.1 | 5 | n.d. | n.d. | 18 | 14 |
| 2 | BDD | BDD | Bu4NClO4 | 2.1 | 5 | n.d. | 1 | 27 | 9 |
| 3 | BDD | BDD | LiClO4 | 2.1 | 5 | n.d. | n.d. | 19 | 13 |
| 4 | BDD | BDD | Et4NClO4 | 2.1 | 5 | trace | 1 | 34 | 11 |
| 5 | BDD | BDD | Et4NClO4 | 2.1 | 3 | 3 | 4 | 17 | 28 |
| 6 | BDD | BDD | Et4NClO4 | 2.1 | 7.5 | n.d. | n.d. | 32 | n.d. |
| 7 | BDD | BDD | Et4NClO4 | 1.05 | 5 | 5 | 2 | 23 | 26 |
| 8 | graphite | graphite | Et4NClO4 | 2.1 | 5 | 4 | n.d. | 1 | 4 |
| 9 | Ni | Ni | Et4NClO4 | 2.1 | 5 | 4 | n.d. | n.d. | n.d. |
| 10 | BDD | graphite | Et4NClO4 | 2.1 | 5 | trace | trace | 33 | 5 |
| 11 | graphite | BDD | Et4NClO4 | 2.1 | 5 | 5 | n.d. | trace | 20 |
aReaction conditions: 1 mmol cumene (1), 5 mL MeCN, 0.1 M supporting electrolyte, undivided beaker-type cell, rt; bcurrent density (mA/cm2); camount of charge (F) referring to mole of 1. n.d. = not detected.
In order to clarify the role of the anode material, we carried out electrochemical measurements (Figure 1). Cyclic voltammetry was performed using BDD as a working electrode. A clear oxidation peak of 1 was observed at around 2.40 V (vs Ag/Ag+) (Figure 1a), which is comparable to a previous report using a Pt disk electrode [17]. On the other hand, no clear oxidation peak was observed when using graphite or Ni as a working electrode. This is because potential windows of graphite and Ni are too narrow to oxidize 1 directly, as can be seen in Figure 1b. Overall, the electrochemical measurements indicate the BDD’s wide potential window enables direct oxidation of 1 to produce a key reaction intermediate to afford 3.
![[1860-5397-18-119-1]](/bjoc/content/figures/1860-5397-18-119-1.png?scale=2.0&max-width=1024&background=FFFFFF)
Figure 1: (a) Cyclic voltammograms of a BDD electrode in MeCN solution containing cumene (1; 5 mM) and Et4NClO4 (0.1 M). The gray dashed line shows the voltammogram in the solution without cumene. (b) Linear sweep voltammograms of BDD (red), graphite (blue), and Ni (green) electrodes in MeCN solution containing Et4NClO4 (0.1 M). Scan rate: 100 mV/s.
Figure 1: (a) Cyclic voltammograms of a BDD electrode in MeCN solution containing cumene (1; 5 mM) and Et4NClO...
A series of electrolysis experiments was performed to propose a reaction mechanism (Table 2). First, we carried out the electrolysis of 1 in MeCN–MeOH to confirm whether the reaction intermediate is a radical or cationic species (Table 2, entry 1). As a result, methyl cumyl ether, a methoxy adduct to the benzyl position of 1, was obtained as the main product in 21% yield. Therefore, it is indicated that the reaction intermediate is the cumyl cation. Second, we carried out the electrolysis of 1 in MeCN–H2O to confirm whether the oxygen source is dissolved oxygen or residual water. When dehydrated MeCN was used, 3 was obtained as the main product (Table 2, entry 2). On the other hand, the isolated yield of 3 was decreased by the addition of H2O (Table 2, entries 3 and 4). This is probably because the addition of H2O promoted the generation of hydroxyl radicals, and a decomposition reaction became dominant. These results indicated that the oxygen source is not residual water in MeCN, but dissolved oxygen. The role of dissolved oxygen was further investigated. As the reaction did not proceed without electricity, it is suggested that the superoxide generated on the cathode is involved in the reaction, rather than dissolved molecular oxygen itself. Therefore, we treated 1 with KO2 and 18-crown-6 to examine whether the reaction proceeds only with the superoxide. As a result, only the starting material, 1, was recovered, which indicates that 3 is produced by a concerted reaction of the direct oxidation of 1 on the anode and the reduction of dissolved oxygen on the cathode.
Table 2: Control electrolysis experiments of 1a.
|
|
||||||
| Entry | Solvent | Isolated yields (%) | ||||
| 1 | 2 | 3 | 4 | 5 | ||
| 1 | MeCN–MeOH 9:1 | trace | 4 | trace | 12 | 21 |
| 2 | MeCN (dehydrated) | trace | 1 | 29 | 15 | n.a. |
| 3 | MeCN–H2O 9:1 | n.d. | 6 | 9 | 20 | n.a. |
| 4 | MeCN–H2O 1:1 | n.d. | trace | trace | 15 | n.a. |
aReaction conditions: BDD anode and cathode, 1 mmol cumene (1), 5 mL solvent, 0.1 M Et4NClO4, 2.1 mA/cm2 and Q of 5 F (referring to mole of 1), undivided beaker-type cell, rt. n.d. = not detected, n.a. = not applicable.
Figure 2 shows a proposed mechanism. Anodic oxidation of cumene on the BDD electrode with a wide potential window preferentially affords the cumyl cation as the reaction intermediate. On the other hand, cathodic reduction of dissolved oxygen produces the superoxide and even the hydroperoxide anion. Addition of the hydroperoxide anion to the cumyl cation yields cumene hydroperoxide, which is further converted into acetophenone. This reaction pathway is supported by the following two facts. One is that cumene hydroperoxide was obtained as a byproduct, and the other is that electrolysis of cumene hydroperoxide as a starting material afforded acetophenone [18]. It should be noted that the tertiary carbon at the benzyl position is a key for the present molecular transformation, since acetophenone was yielded in 19% as the main product by the electrolysis of sec-butylbenzene as a starting material, while propylbenzene was not. Moreover, the electrolysis under a flow of oxygen did not improve the yields, which indicates that the BDD cathode can utilize the electrogenerated oxygen species efficiently, as we have reported previously [19].
Figure 2: Proposed reaction mechanism of electro-conversion of cumene (1) into acetophenone (3).
Figure 2: Proposed reaction mechanism of electro-conversion of cumene (1) into acetophenone (3).
Conclusion
We have demonstrated a straightforward electro-conversion of cumene into acetophenone using boron-doped diamond (BDD) electrodes. The BDD’s wide potential window enabled the direct anodic oxidation of cumene to afford a key reaction intermediate, which cannot be realized by other electrodes such as graphite and Ni. Electrosynthesis is a sustainable, scalable, and cost-efficient protocol; a specific catalyst is not required, and reagent waste can be avoided. In addition, the present work offers new perspectives for an electrosynthetic strategy toward oxidation reactions of aromatic alkyls.
Experimental
General protocol for electro-conversion of cumene
Electrolysis was carried out by using an IKA screening system (IKA, Germany). A solution of cumene (1, 0.12 g, 1.00 mmol) and supporting electrolyte (0.1 M) in 5 mL solvent was transferred into the electrolysis cell equipped with electrodes (purchased from IKA, Germany; 0.3 × 1.0 × 7.0 cm; immersed 1.8 cm into solution). A constant current electrolysis was performed at room temperature. After application of the desired amount of charge, the electrolysis was stopped, and the solvent was removed in vacuo. The residue was purified by silica gel column chromatography (CH2Cl2).
Supporting Information
| Supporting Information File 1: Characterization data and 1H NMR spectra of isolated compounds 2, 3, 4, and 5. | ||
| Format: PDF | Size: 384.7 KB | Download |
References
-
Suresh, A. K.; Sharma, M. M.; Sridhar, T. Ind. Eng. Chem. Res. 2000, 39, 3958–3997. doi:10.1021/ie0002733
Return to citation in text: [1] -
Carrà, S.; Santacesaria, E. Catal. Rev.: Sci. Eng. 1980, 22, 75–140. doi:10.1080/03602458008066530
Return to citation in text: [1] -
Hock, H.; Lang, S. Ber. Dtsch. Chem. Ges. B 1944, 77, 257–264. doi:10.1002/cber.19440770321
Return to citation in text: [1] -
Bryant, J. R.; Matsuo, T.; Mayer, J. M. Inorg. Chem. 2004, 43, 1587–1592. doi:10.1021/ic035298j
Return to citation in text: [1] -
Kaizer, J.; Klinker, E. J.; Oh, N. Y.; Rohde, J.-U.; Song, W. J.; Stubna, A.; Kim, J.; Münck, E.; Nam, W.; Que, L., Jr. J. Am. Chem. Soc. 2004, 126, 472–473. doi:10.1021/ja037288n
Return to citation in text: [1] -
Brutchey, R. L.; Drake, I. J.; Bell, A. T.; Tilley, T. D. Chem. Commun. 2005, 3736–3738. doi:10.1039/b506426k
Return to citation in text: [1] -
Minisci, F.; Recupero, F.; Cecchetto, A.; Gambarotti, C.; Punta, C.; Paganelli, R.; Pedulli, G. F.; Fontana, F. Org. Process Res. Dev. 2004, 8, 163–168. doi:10.1021/op034137w
Return to citation in text: [1] -
Bonchio, M.; Carraro, M.; Gardan, M.; Scorrano, G.; Drioli, E.; Fontananova, E. Top. Catal. 2006, 40, 133–140. doi:10.1007/s11244-006-0115-5
Return to citation in text: [1] -
Liao, S.; Peng, F.; Yu, H.; Wang, H. Appl. Catal., A 2014, 478, 1–8. doi:10.1016/j.apcata.2014.03.024
Return to citation in text: [1] -
Safa, M. A.; Al-Shamary, T.; Al-Majren, R.; Bouresli, R.; Ma, X. Energy Fuels 2017, 31, 7464–7470. doi:10.1021/acs.energyfuels.7b01272
Return to citation in text: [1] -
Mu, C.; Cao, Y.; Wang, H.; Yu, H.; Peng, F. Chem. Eng. Sci. 2018, 177, 391–398. doi:10.1016/j.ces.2017.11.016
Return to citation in text: [1] -
Yang, M.; Qiu, G.; Huang, C.; Han, X.; Li, Y.; Chen, B. Ind. Eng. Chem. Res. 2019, 58, 19785–19793. doi:10.1021/acs.iecr.9b03476
Return to citation in text: [1] -
Fuchigami, T.; Inagi, S.; Atobe, M. Fundamentals and Applications of Organic Electrochemistry: Synthesis, Materials, Devices; John Wiley & Sons: Chichester, UK, 2015. doi:10.1002/9781118670750
Return to citation in text: [1] -
Hammerich, O.; Speiser, B. Organic Electrochemistry, 5th ed.; CRC Press: Boca Raton, FL, USA, 2015. doi:10.1201/b19122
Return to citation in text: [1] -
Macpherson, J. V. Phys. Chem. Chem. Phys. 2015, 17, 2935–2949. doi:10.1039/c4cp04022h
Return to citation in text: [1] -
Yang, N.; Yu, S.; Macpherson, J. V.; Einaga, Y.; Zhao, H.; Zhao, G.; Swain, G. M.; Jiang, X. Chem. Soc. Rev. 2019, 48, 157–204. doi:10.1039/c7cs00757d
Return to citation in text: [1] -
Tajima, T.; Kurihara, H.; Nakajima, A.; Fuchigami, T. J. Electroanal. Chem. 2005, 580, 155–160. doi:10.1016/j.jelechem.2005.03.024
Return to citation in text: [1] -
(Electrolysis) 45% yield; (without electricity) recovery of cumene hydroperoxide.
Return to citation in text: [1] -
Zhang, Y.; Sugai, T.; Yamamoto, T.; Yamamoto, N.; Kutsumura, N.; Einaga, Y.; Nishiyama, S.; Saitoh, T.; Nagase, H. ChemElectroChem 2019, 6, 4194–4198. doi:10.1002/celc.201801308
Return to citation in text: [1]
| 1. | Suresh, A. K.; Sharma, M. M.; Sridhar, T. Ind. Eng. Chem. Res. 2000, 39, 3958–3997. doi:10.1021/ie0002733 |
| 2. | Carrà, S.; Santacesaria, E. Catal. Rev.: Sci. Eng. 1980, 22, 75–140. doi:10.1080/03602458008066530 |
| 15. | Macpherson, J. V. Phys. Chem. Chem. Phys. 2015, 17, 2935–2949. doi:10.1039/c4cp04022h |
| 16. | Yang, N.; Yu, S.; Macpherson, J. V.; Einaga, Y.; Zhao, H.; Zhao, G.; Swain, G. M.; Jiang, X. Chem. Soc. Rev. 2019, 48, 157–204. doi:10.1039/c7cs00757d |
| 13. | Fuchigami, T.; Inagi, S.; Atobe, M. Fundamentals and Applications of Organic Electrochemistry: Synthesis, Materials, Devices; John Wiley & Sons: Chichester, UK, 2015. doi:10.1002/9781118670750 |
| 14. | Hammerich, O.; Speiser, B. Organic Electrochemistry, 5th ed.; CRC Press: Boca Raton, FL, USA, 2015. doi:10.1201/b19122 |
| 4. | Bryant, J. R.; Matsuo, T.; Mayer, J. M. Inorg. Chem. 2004, 43, 1587–1592. doi:10.1021/ic035298j |
| 5. | Kaizer, J.; Klinker, E. J.; Oh, N. Y.; Rohde, J.-U.; Song, W. J.; Stubna, A.; Kim, J.; Münck, E.; Nam, W.; Que, L., Jr. J. Am. Chem. Soc. 2004, 126, 472–473. doi:10.1021/ja037288n |
| 6. | Brutchey, R. L.; Drake, I. J.; Bell, A. T.; Tilley, T. D. Chem. Commun. 2005, 3736–3738. doi:10.1039/b506426k |
| 7. | Minisci, F.; Recupero, F.; Cecchetto, A.; Gambarotti, C.; Punta, C.; Paganelli, R.; Pedulli, G. F.; Fontana, F. Org. Process Res. Dev. 2004, 8, 163–168. doi:10.1021/op034137w |
| 8. | Bonchio, M.; Carraro, M.; Gardan, M.; Scorrano, G.; Drioli, E.; Fontananova, E. Top. Catal. 2006, 40, 133–140. doi:10.1007/s11244-006-0115-5 |
| 9. | Liao, S.; Peng, F.; Yu, H.; Wang, H. Appl. Catal., A 2014, 478, 1–8. doi:10.1016/j.apcata.2014.03.024 |
| 10. | Safa, M. A.; Al-Shamary, T.; Al-Majren, R.; Bouresli, R.; Ma, X. Energy Fuels 2017, 31, 7464–7470. doi:10.1021/acs.energyfuels.7b01272 |
| 11. | Mu, C.; Cao, Y.; Wang, H.; Yu, H.; Peng, F. Chem. Eng. Sci. 2018, 177, 391–398. doi:10.1016/j.ces.2017.11.016 |
| 12. | Yang, M.; Qiu, G.; Huang, C.; Han, X.; Li, Y.; Chen, B. Ind. Eng. Chem. Res. 2019, 58, 19785–19793. doi:10.1021/acs.iecr.9b03476 |
| 3. | Hock, H.; Lang, S. Ber. Dtsch. Chem. Ges. B 1944, 77, 257–264. doi:10.1002/cber.19440770321 |
| 19. | Zhang, Y.; Sugai, T.; Yamamoto, T.; Yamamoto, N.; Kutsumura, N.; Einaga, Y.; Nishiyama, S.; Saitoh, T.; Nagase, H. ChemElectroChem 2019, 6, 4194–4198. doi:10.1002/celc.201801308 |
| 18. | (Electrolysis) 45% yield; (without electricity) recovery of cumene hydroperoxide. |
| 17. | Tajima, T.; Kurihara, H.; Nakajima, A.; Fuchigami, T. J. Electroanal. Chem. 2005, 580, 155–160. doi:10.1016/j.jelechem.2005.03.024 |
© 2022 Kitano et al.; licensee Beilstein-Institut.
This is an open access article licensed under the terms of the Beilstein-Institut Open Access License Agreement (https://www.beilstein-journals.org/bjoc/terms), which is identical to the Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0). The reuse of material under this license requires that the author(s), source and license are credited. Third-party material in this article could be subject to other licenses (typically indicated in the credit line), and in this case, users are required to obtain permission from the license holder to reuse the material.