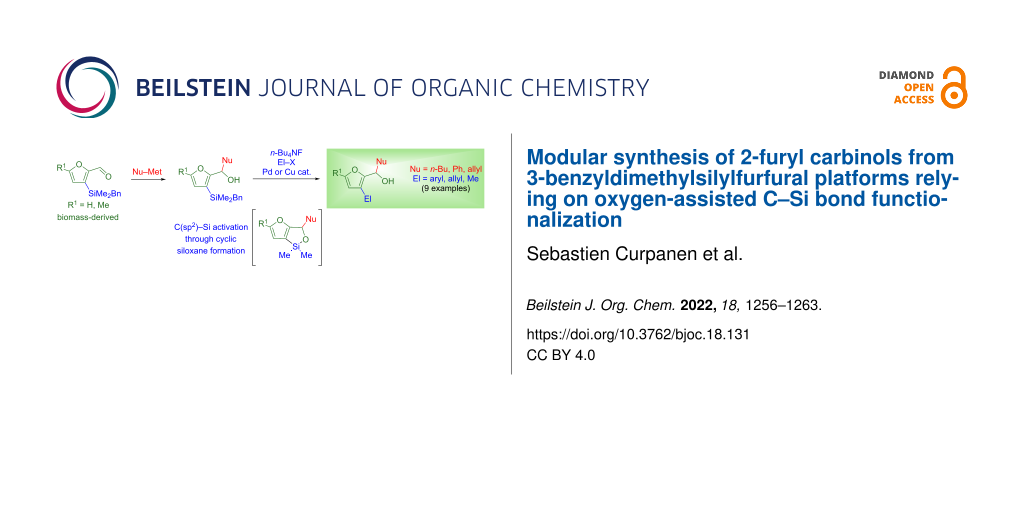
Abstract
3-Silylated furfurals, readily prepared in three steps from biomass-derived furfural and 5-methylfurfural, are converted into 3-silylated 2-furyl carbinols upon condensation with organomagnesium or organolithium reagents. The hydroxy unit of the carbinol adducts can be exploited to promote C3(sp2)–Si bond functionalization through intramolecular activation. Two approaches were contemplated for this purpose. Activation by alkoxides of the C3–SiEt3 or C3–SiMe2t-Bu bonds was ineffective. Conversely, treatment of the C3-benzyldimethylsilyl-appended derivatives with tetrabutylammonium fluoride led to cyclic siloxanes, which revealed to be competent donors for copper-catalyzed cross-coupling reactions, such as arylation reactions catalyzed by Pd2(dba)3/CuI, as well as allylation and methylation reactions catalyzed by CuI⋅PPh3. C3-Benzyldimethylsilyl-appended furfurals are thus introduced as versatile platforms, providing a modular access to 3-substituted 2-furyl carbinols from renewable feedstock.
Graphical Abstract
Introduction
Progress towards the use of nonedible renewable feedstock to replace fossil resources as starting material for high-value chemicals is an important endeavor of modern synthesis [1-3]. As part of this effort, exploitation of furfural and corresponding derivatives attracts continuous attention [4-7]. In this area, we have actively investigated catalytic C–H activation reactions [8-14], and as part of this work, we have recently developed an iridium-based protocol for the catalytic C3–H silylation of furfurylimines [15]. This method allows to install a C–Si bond poised for further functionalization on the furfural unit, and thereby leads to synthetic platforms useful to access elaborated furans. This prospect was demonstrated with platforms relying on the SiMe(OSiMe3)2 unit, which were readily converted through Pd- or Cu-catalyzed electrophilic substitution reactions into an array of furfurals decorated at C3 with carbon- or heteroatom-containing substituents (Scheme 1). Conversely, all of our subsequent efforts to achieve cross-coupling reactions from C3-silylated furfurals with triorganosilane units have failed so far.
Scheme 1: C3–Si bond functionalization of biomass-derived 3-silylated furfural platforms.
Scheme 1: C3–Si bond functionalization of biomass-derived 3-silylated furfural platforms.
2-Furyl carbinols represent a useful class of furanic synthetic intermediates that have given rise to a number of synthetically relevant transformations, such as the Piancatelli and aza-Piancatelli reactions [16,17] or the Achmatowicz rearrangement [18]. Addition reactions of nucleophiles to the C–O double bond of furfurals represent an obvious synthetic approach to 2-furyl carbinols. We reasoned that for carbinols derived from C3-triorganosilyl-substituted furfurals, the OH unit could be exploited to assist C–Si bond activation [19]. Thereby, C3-triorganosilyl-substituted furfurals could be suitable platforms to develop a two-step modular approach to 3-substituted 2-furyl carbinols, entailing nucleophilic addition to the aldehyde function and oxygen-assisted electrophilic substitution of the C–Si bond (Scheme 1).
Results and Discussion
Synthesis of 3-silylated 2-furyl carbinols
C3-silylated furfurals 1a–c and 2c are accessible from furfural or 5-methylfurfural [20], respectively, according to our previously reported protocol for selective catalytic C3 silylation [15]. The addition of organolithium [21] or Grignard reagents [22,23] to these substrates was uneventful and allowed for the preparation of 2-furylalkyl (see 3a–c, 4c), -aryl (see 5c, 6c), and -allyl carbinols (see 7c) having furan rings with various triorganosilyl substituents at C3 in a synthetically useful yield and on an appropriate scale (Scheme 2).
Scheme 2: Preparation of 3-silylated 2-furyl carbinols.
Scheme 2: Preparation of 3-silylated 2-furyl carbinols.
With the 3-silylated 2-furyl carbinol substrates at hand, we then considered C–Si bond activation strategies relying on the assistance of the oxygen atom to promote electrophilic substitution reactions with carbon electrophiles.
C3–Si bond functionalization through intramolecular activation by alkoxides
We first contemplated the possibility to promote C3–Si bond functionalization through intramolecular activation by alkoxides [15]. It was reported that lithium alkoxides A undergo 1,4-silyl migration (Brook rearrangement) to generate C2-lithiated furans C, which in turn can react in the presence of electrophiles to deliver product D, resulting from the overall C2–Si functionalization (Scheme 3, top) [24,25]. The key to the viability of this process is the formation of pentavalent silicon intermediate B. This suggested that a related pentavalent intermediate F could be similarly accessible from E, and thus affording the C3-lithiated furan derivative G upon 1,4-silyl migration as well as the electrophilic substitution product H in the presence of an appropriate electrophile (Scheme 3, bottom).
Scheme 3: C–Si bond functionalization of 2,3-disubstituted furyl carbinols by 1,4-silyl migration.
Scheme 3: C–Si bond functionalization of 2,3-disubstituted furyl carbinols by 1,4-silyl migration.
However, treatment of aldehyde 1b with n-BuLi, followed by addition of benzaldehyde in THF/DMPU [25], afforded only the addition product 3b without any detectable formation of product 9, expected from 1,4-silyl migration/electrophilic substitution of 8 (Scheme 4, top).
Scheme 4: Attempts of C3–Si bond functionalization promoted by intramolecular activation via alkoxide.
Scheme 4: Attempts of C3–Si bond functionalization promoted by intramolecular activation via alkoxide.
Conversely, treatment of alcohol 3a with t-BuOCu in the presence of allyl chloride, according to conditions developed by Takeda et al. for the reaction of γ-trimethylsilyl-substituted allylic alcohols [26,27] or ortho-silylated aryl carbinols [28], did provide C3-functionalized compound 10, albeit in very low yield (6%) and along with the O-allylated product 11 (11%), as well as products 12 (4%) and 13 (4%) [29] (Scheme 4, bottom). In spite of extensive experimentation to find better conditions, this result could not be improved.
Nevertheless, important information came from an experiment involving furyl carbinol 4c, having a benzyldimethylsilyl unit (Scheme 5). Here, treatment with t-BuOCu⋅LiI in THF/HMPA in the presence of benzaldehyde led to the formation of adduct 14 (in 75% yield), which arose from the addition of a benzyl carbanion 17 to benzaldehyde. The generation of such a nucleophile strongly suggests the formation of pentavalent silicon intermediate 15 [27], which then produced a (stabilized) benzylic carbanion (by exocyclic cleavage) rather than a 3-furanyl anion (by endocyclic cleavage). As a consequence, the failure to promote C3–Si activation of intermediates E (SiR3 = SiEt3 or SiMe2t-Bu) according to our initially envisaged scenario depicted in Scheme 3, did not seem to be related to the difficulty to form pentavalent silicon intermediates F. Instead, the reason was probably a too low intrinsic stability of the C3-lithiated furans G, which thwarted 1,4-silyl migration. Conversely, this experiment provided evidence that cyclic siloxanes (e.g., 16), which are potential nucleophilic partners for cross-coupling chemistry [30,31], could be accessed from 2-[(3-benzyldimethylsilyl)furyl] carbinols.
Scheme 5: Alkoxide-promoted cyclic siloxane formation from 2-[(3-benzyldimethylsilyl)furyl] carbinol 4c.
Scheme 5: Alkoxide-promoted cyclic siloxane formation from 2-[(3-benzyldimethylsilyl)furyl] carbinol 4c.
C3–Si bond functionalization through siloxane formation
In an attempt to isolate cyclic siloxane 16, we then considered a protocol reported by Anderson and co-workers to prepare cyclic siloxanes by debenzylation/cyclization of benzyldimethysilyl-substituted allylic alcohols [32]. To that end, carbinol 4c was treated with TBAF⋅3H2O (1.0 equiv), which resulted in the formation of 16 in 86% yield (along with toluene, Scheme 6). Alike 5-membered cyclic dimethyl(alkenyl)siloxanes, 16 was found to be highly sensitive towards silica gel column chromatography, and all of our attempts to isolate it failed. For this reason, we went on to consider C3–Si functionalization strategies of alcohols 4c–7c relying on the formation of cyclic siloxanes and subsequent in situ cross-coupling.
Scheme 6: TBAF-promoted cyclic siloxane formation from 2-[(3-benzyldimethylsilyl)furyl] carbinol 4c.
Scheme 6: TBAF-promoted cyclic siloxane formation from 2-[(3-benzyldimethylsilyl)furyl] carbinol 4c.
We first briefly investigated Pd-catalyzed arylation reactions (Scheme 7). Fluoride-promoted arylation reactions of benzyldimethyl(alkenyl)silanes have been reported, and it is established that they proceed through the cleavage of the benzyl moiety from the benzyldimethylsilyl groups, leading to either dimethylsilanols or cyclic siloxanes (as for substrates with pending hydroxy units) [32-35]. To the best of our knowledge, no analogous cross-coupling reactions from aryl- or heteroaryl-substituted benzyldimethylsilanes have been disclosed. We were thus delighted to find that using Pd2(dba)3 as precatalyst (2.5 mol %) in combination with CuI (20 mol %), cross-coupling between 4c and iodobenzene was achieved, giving 18 in reasonably good yield (70%). 4-Iodoanisole could also be coupled (giving 19 in 57% yield), but not electron-deficient 1-iodo-4-nitrobenzene. At this point, it should be mentioned that treatment of aldehyde 2c under the same reaction conditions led predominantly to decomposition products and only 5-methylfurfural (resulting from protodesilylation) was recovered in low yield (≈20%). Hence, intramolecular hydroxy activation seems decisive to obtain productive cross-coupling reactions with this system. Also, it is important to underscore that the copper cocatalyst was crucial for the success of this transformation as without, only protodesilylation products were obtained. This behavior is in contrast to that of dimethyl(alkenyl)siloxanes, which do not require copper to undergo cross-coupling [30,32].
Scheme 7: Pd-catalyzed arylation of 2-[(3-benzyldimethylsilyl)furyl] carbinol 4c.
Scheme 7: Pd-catalyzed arylation of 2-[(3-benzyldimethylsilyl)furyl] carbinol 4c.
This requirement for copper prompted us to test copper-catalyzed C(sp2)–C(sp3) cross-coupling reactions, as reported by Takeda et al., to achieve allylation reactions of benzyldimethyl(alkenyl)silanes [36]. Treatment of 4c with methallyl chloride in the presence of TBAF⋅(t-BuOH)4 (2.4 equiv), CuI (1.5 equiv), and P(OEt)3 (1.5 equiv) in DMF at room temperature delivered, after hydrolysis, the cross-coupling compound 21 in 68% yield, along with 9% of protodesilylation product 22 (Table 1, entry 1). As indicated by Takeda et al., the amount and source of TBAF proved crucial: use of either 1.2 equiv of TBAF⋅(t-BuOH)4 instead of 2.4 equiv (Table 1, entry 2), or use of a (commercially available) 1-molar TBAF solution in THF (Table 1, entry 3), gave an increased amount of protodesilylation product 22 at the expense of 21. By contrast, byproduct 22 was not observed reducing the reaction time from 24 h to 2 h, but the yield of product 21 remained the same (Table 1, entry 4). In spite of the satisfactory yield of 21, these conditions were synthetically impractical as we were unable to conveniently separate the final product from the phosphite ligand. The use of a ligand for copper revealed to be necessary, given that the cross-coupling product 21 was obtained in only 40% yield (along with 23% of 22) in the absence of P(OEt)3 (Table 1, entry 5). The solution was the replacement of triethylphosphite by triphenylphosphine, which allowed to suppress the formation of the protodesilylated product and to obtain product 21 in 80% yield (Table 1, entry 6). Similar results were obtained using the preformed complex CuI⋅PPh3 (1.5 equiv), so that compound 21 could be isolated in 65% yield (Table 1, entry 7). Importantly, the use of copper in a substoichiometric amount (20 mol %) was also suitable, allowing to isolate 21 in an even better 78% yield (Table 1, entry 9). It should also be mentioned that the use of THF instead of DMF as solvent led to worse results (Table 1, entries 7 and 8).
Table 1: Optimization of the reaction conditions for Cu-catalyzed methallylation of 2-[(3-benzyldimethylsilyl)furyl] carbinol 4c.
|
|
|||||
| entry | Cu source | ligand | conditions | 21a (%) | 22a (%) |
| 1 | CuI (1.5 equiv) | P(OEt)3 (1.5 equiv) | DMF, 24 h | 68 | 9 |
| 2b | CuI (1.5 equiv) | P(OEt)3 (1.5 equiv) | DMF, 24 h | 38 | 32 |
| 3c | CuI (1.5 equiv) | P(OEt)3 (1.5 equiv) | DMF, 24 h | 41 | 20 |
| 4 | CuI (1.5 equiv) | P(OEt)3 (1.5 equiv) | DMF, 2 h | 68 | 0 |
| 5 | CuI (1.5 equiv) | — | DMF, 2 h | 40 | 23 |
| 6 | CuI (1.5 equiv) | PPh3 (1.5 equiv) | DMF, 2 h | 80 | 0 |
| 7 | CuI⋅PPh3 (1.5 equiv) | DMF, 2 h | 79 (65)d | 0 | |
| 8 | CuI⋅PPh3 (1.5 equiv) | THF, 2 h | 71 | 20 | |
| 9 | CuI⋅PPh3 (20 mol %) | DMF, 2 h | 84 (78)d | 0 | |
aYield measured prior to purification by 1H NMR analysis using Me2SO2 as internal standard. bTBAF⋅(t-BuOH)4 (1.2 equiv) was used. cTBAF (1 molar solution in THF, 2.4 equiv) was used. dIsolated yield.
Investigating the scope of this transformation (Scheme 8), we established that the reaction performed well with other 5-methylfuryl carbinols. As such, products 23 and 24, arising from phenyl- and allyl-substituted substrates 6c and 7c, respectively, were isolated in 56% and 65% yield, respectively. Somewhat lower yields were noted with butyl- and phenyl-substituted carbinols 3c and 5c, respectively, bearing C5-unsubstituted furan rings, which gave products 25 and 26 in 42% and 40% yield, respectively. Electrophiles other than methallyl chloride could also be used. C3-Allylation of substrate 4c, leading to 27, was achieved in 52% yield by reaction with allyl chloride and in a better 61% yield using allyl bromide. It should be mentioned that competing protodesilylation could not always be fully suppressed in the above described reactions. Furthermore, purification was troublesome for products 25–27, which could only be isolated in the presence of the protodesilylated side products (see Supporting Information File 1 for details). We also contemplated the use of alkyl iodides as electrophiles. Methylation with methyl iodide was efficient, as shown through the preparation of 28 in 61% yield from 4c. In contrast, higher alkyl iodides, such as ethyl iodide, failed to provide the alkylation product (i.e., 29) and only protodesilylation was observed (even at 50 °C).
Scheme 8: Cu-catalyzed allylation and methylation of 2-[(3-benzyldimethylsilyl)furyl] carbinols. aCuI⋅PPh3 (120 mol %). bCuI/P(OEt)3 (1.5 equiv), rt or 50 °C.
Scheme 8: Cu-catalyzed allylation and methylation of 2-[(3-benzyldimethylsilyl)furyl] carbinols. aCuI⋅PPh3 (1...
Conclusion
In conclusion, we have shown that 3-silylated 2-furyl carbinols are readily accessible in three steps from furfural and 5-methylfurfural, and the hydroxy unit of these adducts can be used to promote C(sp2)–Si bond functionalization. Although intramolecular activation by alkoxides did not prove useful, C3–Si bond functionalization is achieved from benzyldimethylsilyl units upon siloxane formation in the presence of TBAF. Protocols for fluoride-promoted Pd/Cu-catalyzed arylation, as well as Cu-catalyzed allylation and methylation, have been developed. Overall, we have demonstrated that C3-benzyldimethylsilyl-appended furfurals are useful platforms that offer modular access to 3-substituted 2-furyl carbinols. This strategy represents a new, simple, and selective way to decorate the biomass-derived furan nucleus, allowing to reach synthetically relevant building blocks.
Experimental
Procedure for the addition of n-BuLi to C3-silylated furfurals (preparation of compounds 3a–c and 4c)
In a flame-dried round-bottom flask under argon was placed the appropriate C3-silylated furfural [15] and dissolved in freshly distilled THF (0.3 M). The solution was cooled to −78 °C, and then n-BuLi (1.2 equiv in hexane) was added dropwise. The reaction mixture was allowed to stir at −78 °C for 30 min and then quenched with aq saturated NH4Cl/NH3 2:1 solution. Et2O was added, and the aqueous layer was extracted three times. The combined organic layer was washed with brine, dried over MgSO4, filtered, concentrated under reduced pressure, and purified by silica gel column chromatography.
Procedure for the addition of Grignard reagents to C3-silylated furfurals (preparation of compounds 5c, 6c, and 7c)
In a flame-dried round-bottom flask under argon was placed the appropriate C3-silylated furfural [15] and dissolved in freshly distilled THF (0.2 M solution). The solution was cooled to 0 °C, and then the Grignard reagent (1.3 equiv in Et2O) was added dropwise (the rate of addition was equal to, or lower than 0.125 mL/min). The mixture was allowed to stir at 0 °C for 1 h and then allowed to reach room temperature. Upon consumption of the starting material, the reaction mixture was quenched with saturated NH4Cl. CH2Cl2 was added, and the aqueous layer was extracted three times using CH2Cl2. The combined organic layer was dried over Na2SO4, filtered, concentrated under reduced pressure. and purified by silica gel column chromatography.
Procedure for the Pd/Cu-catalyzed arylation of C3-benzyldimethylsilyl-substituted 2-furyl carbinol 4c (preparation of compounds 18 and 19)
A flame-dried Schlenk tube was charged with CuI (20 mol %) and Pd2(dba)3 (2.5 mol %) and heated gently under vacuum using a heat gun. In another Schlenk tube, the appropriate iodoarene (1.5 equiv) and 4c (1 equiv) were dissolved in freshly distilled THF (0.63 mL). The solution was degassed in three freeze–pump–thaw cycles, and degassed anhydrous TBAF (1 M in THF, 2.2 equiv) was added. The mixture was allowed to stir for 10 min at 0 °C and transferred via cannula to the Schlenk tube containing the catalytic mixture. The resulting mixture was stirred for 2 h at rt, then filtered through a short pad of silica gel, and concentrated. The residue was purified by silica gel column chromatography.
Procedure for the Cu-catalyzed allylation and methylation of C3-benzyldimethylsilyl-substituted 2-furyl carbinols (preparation of compounds 21 and 23–28)
CuI⋅PPh3 [37] (20 or 120 mol %) was introduced to a flame-dried microwave vial, which was then placed under an argon atmosphere and sealed. In a Schlenk tube, the appropriate C3-benzyldimethylsilyl-substituted 2-furyl carbinol (0.3 mmol, 1 equiv) was dissolved in CH2Cl2 (1 mL), concentrated under reduced pressure, and placed under argon. DMF (3.0 mL) was then added, and the solution was degassed in three freeze–pump–thaw cycles. TBAF⋅(t-BuOH)4 [38] (402 mg, 0.72 mmol) was then added along with the corresponding electrophile (3 equiv). The mixture was stirred for 2–3 min at rt and then cannulated into the vial containing the copper complex. The mixture was stirred for 2 h at 30 °C and then quenched with aq KOH (6 M, 1 mL). The mixture was extracted with cyclohexane/CH2Cl2 9:1 (4 × 10 mL), and the combined organic layer was washed with water (10 mL), dried over MgSO4, and concentrated under reduced pressure. The residue was purified by silica gel column chromatography.
Supporting Information
| Supporting Information File 1: General information, characterization data, and copies of NMR spectra. | ||
| Format: PDF | Size: 1.1 MB | Download |
References
-
Bender, T. A.; Dabrowski, J. A.; Gagné, M. R. Nat. Rev. Chem. 2018, 2, 35–46. doi:10.1038/s41570-018-0005-y
Return to citation in text: [1] -
Bozell, J. J.; Petersen, G. R. Green Chem. 2010, 12, 539–554. doi:10.1039/b922014c
Return to citation in text: [1] -
Mika, L. T.; Cséfalvay, E.; Németh, Á. Chem. Rev. 2018, 118, 505–613. doi:10.1021/acs.chemrev.7b00395
Return to citation in text: [1] -
Chen, S.; Wojcieszak, R.; Dumeignil, F.; Marceau, E.; Royer, S. Chem. Rev. 2018, 118, 11023–11117. doi:10.1021/acs.chemrev.8b00134
Return to citation in text: [1] -
Zhang, X.; Xu, S.; Li, Q.; Zhou, G.; Xia, H. RSC Adv. 2021, 11, 27042–27058. doi:10.1039/d1ra04633k
Return to citation in text: [1] -
Xu, C.; Paone, E.; Rodríguez-Padrón, D.; Luque, R.; Mauriello, F. Chem. Soc. Rev. 2020, 49, 4273–4306. doi:10.1039/d0cs00041h
Return to citation in text: [1] -
Bielski, R.; Grynkiewicz, G. Green Chem. 2021, 23, 7458–7487. doi:10.1039/d1gc02402g
Return to citation in text: [1] -
Pezzetta, C.; Veiros, L. F.; Oble, J.; Poli, G. Chem. – Eur. J. 2017, 23, 8385–8389. doi:10.1002/chem.201701850
Return to citation in text: [1] -
Siopa, F.; Ramis Cladera, V.-A.; Afonso, C. A. M.; Oble, J.; Poli, G. Eur. J. Org. Chem. 2018, 6101–6106. doi:10.1002/ejoc.201800767
Return to citation in text: [1] -
Ravasco, J. M. J. M.; Monteiro, C. M.; Siopa, F.; Trindade, A. F.; Oble, J.; Poli, G.; Simeonov, S. P.; Afonso, C. A. M. ChemSusChem 2019, 12, 4629–4635. doi:10.1002/cssc.201902051
Return to citation in text: [1] -
Sala, R.; Roudesly, F.; Veiros, L. F.; Broggini, G.; Oble, J.; Poli, G. Adv. Synth. Catal. 2020, 362, 2486–2493. doi:10.1002/adsc.202000249
Return to citation in text: [1] -
Gupta, K.; Rai, R. K.; Singh, S. K. ChemCatChem 2018, 10, 2326–2349. doi:10.1002/cctc.201701754
Return to citation in text: [1] -
Mariscal, R.; Maireles-Torres, P.; Ojeda, M.; Sádaba, I.; López Granados, M. Energy Environ. Sci. 2016, 9, 1144–1189. doi:10.1039/c5ee02666k
Return to citation in text: [1] -
Karlinskii, B. Y.; Ananikov, V. P. ChemSusChem 2021, 14, 558–568. doi:10.1002/cssc.202002397
Return to citation in text: [1] -
Curpanen, S.; Poli, G.; Perez-Luna, A.; Oble, J. Asian J. Org. Chem. 2022, e202200199. doi:10.1002/ajoc.202200199
Return to citation in text: [1] [2] [3] [4] [5] -
Piutti, C.; Quartieri, F. Molecules 2013, 18, 12290–12312. doi:10.3390/molecules181012290
Return to citation in text: [1] -
Verrier, C.; Moebs-Sanchez, S.; Queneau, Y.; Popowycz, F. Org. Biomol. Chem. 2018, 16, 676–687. doi:10.1039/c7ob02962d
Return to citation in text: [1] -
Ghosh, A. K.; Brindisi, M. RSC Adv. 2016, 6, 111564–111598. doi:10.1039/c6ra22611f
Return to citation in text: [1] -
Curpanen, S.; Poli, G.; Oble, J.; Perez-Luna, A. Eur. J. Org. Chem. 2021, 1055–1071. doi:10.1002/ejoc.202001458
Return to citation in text: [1] -
Yang, W.; Sen, A. ChemSusChem 2011, 4, 349–352. doi:10.1002/cssc.201000369
See for the synthesis of 5-methylfurfural from biomass-derived carbohydrates.
Return to citation in text: [1] -
Kazancioglu, E. A.; Kazancioglu, M. Z.; Fistikci, M.; Secen, H.; Altundas, R. Org. Lett. 2013, 15, 4790–4793. doi:10.1021/ol402163u
Return to citation in text: [1] -
Hashmi, A. S. K.; Häffner, T.; Yang, W.; Pankajakshan, S.; Schäfer, S.; Schultes, L.; Rominger, F.; Frey, W. Chem. – Eur. J. 2012, 18, 10480–10486. doi:10.1002/chem.201200306
Return to citation in text: [1] -
Lebœuf, D.; Schulz, E.; Gandon, V. Org. Lett. 2014, 16, 6464–6467. doi:10.1021/ol5032987
Return to citation in text: [1] -
Bures, E.; Spinazzé, P. G.; Beese, G.; Hunt, I. R.; Rogers, C.; Keay, B. A. J. Org. Chem. 1997, 62, 8741–8749. doi:10.1021/jo971097j
Return to citation in text: [1] -
Devarie-Baez, N. O.; Kim, W.-S.; Smith, A. B., III; Xian, M. Org. Lett. 2009, 11, 1861–1864. doi:10.1021/ol900434k
Return to citation in text: [1] [2] -
Taguchi, H.; Ghoroku, K.; Tadaki, M.; Tsubouchi, A.; Takeda, T. Org. Lett. 2001, 3, 3811–3814. doi:10.1021/ol016837w
Return to citation in text: [1] -
Taguchi, H.; Ghoroku, K.; Tadaki, M.; Tsubouchi, A.; Takeda, T. J. Org. Chem. 2002, 67, 8450–8456. doi:10.1021/jo025973r
Return to citation in text: [1] [2] -
Taguchi, H.; Takami, K.; Tsubouchi, A.; Takeda, T. Tetrahedron Lett. 2004, 45, 429–432. doi:10.1016/j.tetlet.2003.10.094
Return to citation in text: [1] -
We rationalized the formation of products 12 and 13 from product 11. The sequence is depicted in Supporting Information File 1 and involves metalation to form an α-furyl anion, which then undergoes a [2,3]-Wittig rearrangement, followed by dehydoxylation through β-hydride transfer.
Return to citation in text: [1] -
Denmark, S. E.; Yang, S.-M. Org. Lett. 2001, 3, 1749–1752. doi:10.1021/ol015950j
Return to citation in text: [1] [2] -
Denmark, S. E.; Yang, S.-M. Tetrahedron 2004, 60, 9695–9708. doi:10.1016/j.tet.2004.06.149
Return to citation in text: [1] -
Gudmundsson, H. G.; Kuper, C. J.; Cornut, D.; Urbitsch, F.; Elbert, B. L.; Anderson, E. A. J. Org. Chem. 2019, 84, 14868–14882. doi:10.1021/acs.joc.9b01664
Return to citation in text: [1] [2] [3] -
Trost, B. M.; Machacek, M. R.; Ball, Z. T. Org. Lett. 2003, 5, 1895–1898. doi:10.1021/ol034463w
Return to citation in text: [1] -
Denmark, S. E.; Tymonko, S. A. J. Am. Chem. Soc. 2005, 127, 8004–8005. doi:10.1021/ja0518373
Return to citation in text: [1] -
Denmark, S. E.; Liu, J. H.-C. J. Am. Chem. Soc. 2007, 129, 3737–3744. doi:10.1021/ja067854p
Return to citation in text: [1] -
Takeda, T.; Matsumura, R.; Wasa, H.; Tsubouchi, A. Asian J. Org. Chem. 2014, 3, 838–841. doi:10.1002/ajoc.201402060
Return to citation in text: [1] -
Wang, Y.; Xiao, Y.; Yang Tan, T. T.; Ng, S.-C. Tetrahedron Lett. 2008, 49, 5190–5191. doi:10.1016/j.tetlet.2008.06.048
Return to citation in text: [1] -
Kim, D. W.; Jeong, H.-J.; Lim, S. T.; Sohn, M.-H. Angew. Chem., Int. Ed. 2008, 47, 8404–8406. doi:10.1002/anie.200803150
Return to citation in text: [1]
| 1. | Bender, T. A.; Dabrowski, J. A.; Gagné, M. R. Nat. Rev. Chem. 2018, 2, 35–46. doi:10.1038/s41570-018-0005-y |
| 2. | Bozell, J. J.; Petersen, G. R. Green Chem. 2010, 12, 539–554. doi:10.1039/b922014c |
| 3. | Mika, L. T.; Cséfalvay, E.; Németh, Á. Chem. Rev. 2018, 118, 505–613. doi:10.1021/acs.chemrev.7b00395 |
| 16. | Piutti, C.; Quartieri, F. Molecules 2013, 18, 12290–12312. doi:10.3390/molecules181012290 |
| 17. | Verrier, C.; Moebs-Sanchez, S.; Queneau, Y.; Popowycz, F. Org. Biomol. Chem. 2018, 16, 676–687. doi:10.1039/c7ob02962d |
| 26. | Taguchi, H.; Ghoroku, K.; Tadaki, M.; Tsubouchi, A.; Takeda, T. Org. Lett. 2001, 3, 3811–3814. doi:10.1021/ol016837w |
| 27. | Taguchi, H.; Ghoroku, K.; Tadaki, M.; Tsubouchi, A.; Takeda, T. J. Org. Chem. 2002, 67, 8450–8456. doi:10.1021/jo025973r |
| 15. | Curpanen, S.; Poli, G.; Perez-Luna, A.; Oble, J. Asian J. Org. Chem. 2022, e202200199. doi:10.1002/ajoc.202200199 |
| 28. | Taguchi, H.; Takami, K.; Tsubouchi, A.; Takeda, T. Tetrahedron Lett. 2004, 45, 429–432. doi:10.1016/j.tetlet.2003.10.094 |
| 8. | Pezzetta, C.; Veiros, L. F.; Oble, J.; Poli, G. Chem. – Eur. J. 2017, 23, 8385–8389. doi:10.1002/chem.201701850 |
| 9. | Siopa, F.; Ramis Cladera, V.-A.; Afonso, C. A. M.; Oble, J.; Poli, G. Eur. J. Org. Chem. 2018, 6101–6106. doi:10.1002/ejoc.201800767 |
| 10. | Ravasco, J. M. J. M.; Monteiro, C. M.; Siopa, F.; Trindade, A. F.; Oble, J.; Poli, G.; Simeonov, S. P.; Afonso, C. A. M. ChemSusChem 2019, 12, 4629–4635. doi:10.1002/cssc.201902051 |
| 11. | Sala, R.; Roudesly, F.; Veiros, L. F.; Broggini, G.; Oble, J.; Poli, G. Adv. Synth. Catal. 2020, 362, 2486–2493. doi:10.1002/adsc.202000249 |
| 12. | Gupta, K.; Rai, R. K.; Singh, S. K. ChemCatChem 2018, 10, 2326–2349. doi:10.1002/cctc.201701754 |
| 13. | Mariscal, R.; Maireles-Torres, P.; Ojeda, M.; Sádaba, I.; López Granados, M. Energy Environ. Sci. 2016, 9, 1144–1189. doi:10.1039/c5ee02666k |
| 14. | Karlinskii, B. Y.; Ananikov, V. P. ChemSusChem 2021, 14, 558–568. doi:10.1002/cssc.202002397 |
| 24. | Bures, E.; Spinazzé, P. G.; Beese, G.; Hunt, I. R.; Rogers, C.; Keay, B. A. J. Org. Chem. 1997, 62, 8741–8749. doi:10.1021/jo971097j |
| 25. | Devarie-Baez, N. O.; Kim, W.-S.; Smith, A. B., III; Xian, M. Org. Lett. 2009, 11, 1861–1864. doi:10.1021/ol900434k |
| 4. | Chen, S.; Wojcieszak, R.; Dumeignil, F.; Marceau, E.; Royer, S. Chem. Rev. 2018, 118, 11023–11117. doi:10.1021/acs.chemrev.8b00134 |
| 5. | Zhang, X.; Xu, S.; Li, Q.; Zhou, G.; Xia, H. RSC Adv. 2021, 11, 27042–27058. doi:10.1039/d1ra04633k |
| 6. | Xu, C.; Paone, E.; Rodríguez-Padrón, D.; Luque, R.; Mauriello, F. Chem. Soc. Rev. 2020, 49, 4273–4306. doi:10.1039/d0cs00041h |
| 7. | Bielski, R.; Grynkiewicz, G. Green Chem. 2021, 23, 7458–7487. doi:10.1039/d1gc02402g |
| 25. | Devarie-Baez, N. O.; Kim, W.-S.; Smith, A. B., III; Xian, M. Org. Lett. 2009, 11, 1861–1864. doi:10.1021/ol900434k |
| 15. | Curpanen, S.; Poli, G.; Perez-Luna, A.; Oble, J. Asian J. Org. Chem. 2022, e202200199. doi:10.1002/ajoc.202200199 |
| 22. | Hashmi, A. S. K.; Häffner, T.; Yang, W.; Pankajakshan, S.; Schäfer, S.; Schultes, L.; Rominger, F.; Frey, W. Chem. – Eur. J. 2012, 18, 10480–10486. doi:10.1002/chem.201200306 |
| 23. | Lebœuf, D.; Schulz, E.; Gandon, V. Org. Lett. 2014, 16, 6464–6467. doi:10.1021/ol5032987 |
| 20. |
Yang, W.; Sen, A. ChemSusChem 2011, 4, 349–352. doi:10.1002/cssc.201000369
See for the synthesis of 5-methylfurfural from biomass-derived carbohydrates. |
| 15. | Curpanen, S.; Poli, G.; Perez-Luna, A.; Oble, J. Asian J. Org. Chem. 2022, e202200199. doi:10.1002/ajoc.202200199 |
| 19. | Curpanen, S.; Poli, G.; Oble, J.; Perez-Luna, A. Eur. J. Org. Chem. 2021, 1055–1071. doi:10.1002/ejoc.202001458 |
| 18. | Ghosh, A. K.; Brindisi, M. RSC Adv. 2016, 6, 111564–111598. doi:10.1039/c6ra22611f |
| 21. | Kazancioglu, E. A.; Kazancioglu, M. Z.; Fistikci, M.; Secen, H.; Altundas, R. Org. Lett. 2013, 15, 4790–4793. doi:10.1021/ol402163u |
| 30. | Denmark, S. E.; Yang, S.-M. Org. Lett. 2001, 3, 1749–1752. doi:10.1021/ol015950j |
| 31. | Denmark, S. E.; Yang, S.-M. Tetrahedron 2004, 60, 9695–9708. doi:10.1016/j.tet.2004.06.149 |
| 29. | We rationalized the formation of products 12 and 13 from product 11. The sequence is depicted in Supporting Information File 1 and involves metalation to form an α-furyl anion, which then undergoes a [2,3]-Wittig rearrangement, followed by dehydoxylation through β-hydride transfer. |
| 27. | Taguchi, H.; Ghoroku, K.; Tadaki, M.; Tsubouchi, A.; Takeda, T. J. Org. Chem. 2002, 67, 8450–8456. doi:10.1021/jo025973r |
| 37. | Wang, Y.; Xiao, Y.; Yang Tan, T. T.; Ng, S.-C. Tetrahedron Lett. 2008, 49, 5190–5191. doi:10.1016/j.tetlet.2008.06.048 |
| 38. | Kim, D. W.; Jeong, H.-J.; Lim, S. T.; Sohn, M.-H. Angew. Chem., Int. Ed. 2008, 47, 8404–8406. doi:10.1002/anie.200803150 |
| 15. | Curpanen, S.; Poli, G.; Perez-Luna, A.; Oble, J. Asian J. Org. Chem. 2022, e202200199. doi:10.1002/ajoc.202200199 |
| 15. | Curpanen, S.; Poli, G.; Perez-Luna, A.; Oble, J. Asian J. Org. Chem. 2022, e202200199. doi:10.1002/ajoc.202200199 |
| 30. | Denmark, S. E.; Yang, S.-M. Org. Lett. 2001, 3, 1749–1752. doi:10.1021/ol015950j |
| 32. | Gudmundsson, H. G.; Kuper, C. J.; Cornut, D.; Urbitsch, F.; Elbert, B. L.; Anderson, E. A. J. Org. Chem. 2019, 84, 14868–14882. doi:10.1021/acs.joc.9b01664 |
| 36. | Takeda, T.; Matsumura, R.; Wasa, H.; Tsubouchi, A. Asian J. Org. Chem. 2014, 3, 838–841. doi:10.1002/ajoc.201402060 |
| 32. | Gudmundsson, H. G.; Kuper, C. J.; Cornut, D.; Urbitsch, F.; Elbert, B. L.; Anderson, E. A. J. Org. Chem. 2019, 84, 14868–14882. doi:10.1021/acs.joc.9b01664 |
| 32. | Gudmundsson, H. G.; Kuper, C. J.; Cornut, D.; Urbitsch, F.; Elbert, B. L.; Anderson, E. A. J. Org. Chem. 2019, 84, 14868–14882. doi:10.1021/acs.joc.9b01664 |
| 33. | Trost, B. M.; Machacek, M. R.; Ball, Z. T. Org. Lett. 2003, 5, 1895–1898. doi:10.1021/ol034463w |
| 34. | Denmark, S. E.; Tymonko, S. A. J. Am. Chem. Soc. 2005, 127, 8004–8005. doi:10.1021/ja0518373 |
| 35. | Denmark, S. E.; Liu, J. H.-C. J. Am. Chem. Soc. 2007, 129, 3737–3744. doi:10.1021/ja067854p |
© 2022 Curpanen et al.; licensee Beilstein-Institut.
This is an open access article licensed under the terms of the Beilstein-Institut Open Access License Agreement (https://www.beilstein-journals.org/bjoc/terms), which is identical to the Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0). The reuse of material under this license requires that the author(s), source and license are credited. Third-party material in this article could be subject to other licenses (typically indicated in the credit line), and in this case, users are required to obtain permission from the license holder to reuse the material.