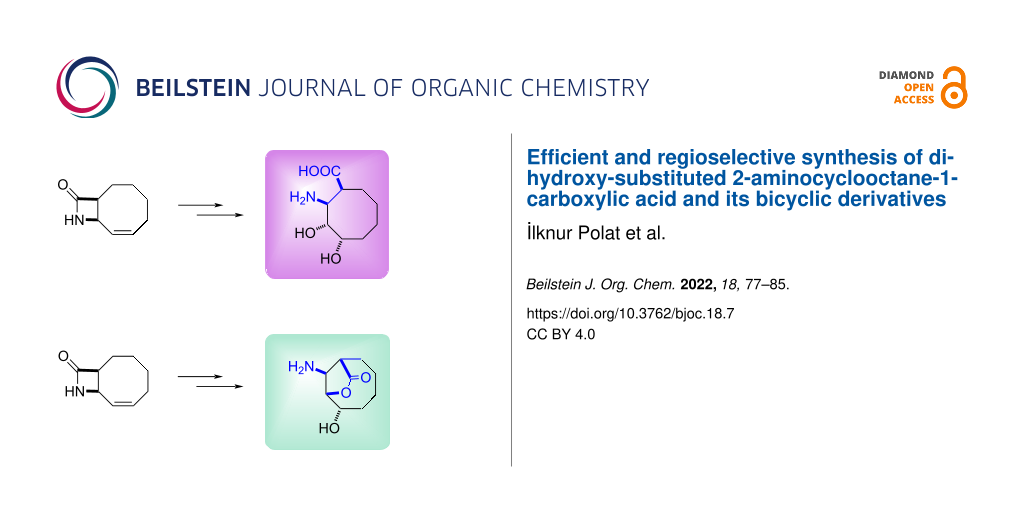
Abstract
The first synthesis of 2-amino-3,4-dihydroxycyclooctane-1-carboxylic acid, methyl 6-hydroxy-9-oxo-8-oxabicyclo[5.2.1]decan-10-yl)carbamate, and 10-amino-6-hydroxy-8-oxabicyclo[5.2.1]decan-9-one starting from cis-9-azabicyclo[6.2.0]dec-6-en-10-one is described. cis-9-Azabicyclo[6.2.0]dec-6-en-10-one was transformed into the corresponding amino ester and its protected amine. Oxidation of the double bond in the N-Boc-protected methyl 2-aminocyclooct-3-ene-1-carboxylate then delivered the targeted amino acid and its derivatives. Density-functional theory (DFT) computations were used to explain the reaction mechanism for the ring opening of the epoxide and the formation of five-membered lactones. The stereochemistry of the synthesized compounds was determined by 1D and 2D NMR spectroscopy. The configuration of methyl 6-hydroxy-9-oxo-8-oxabicyclo[5.2.1]decan-10-yl)carbamate was confirmed by X-ray diffraction.
Graphical Abstract
Introduction
Cyclic β-amino acids have for the past few decades aroused widespread synthetic interest owing to their diverse biological activities, especially applications in the field of medicinal chemistry. β-Amino acids (i.e., amino acids containing an extra methylene group in the backbone) occur naturally in peptidic structures [1-5] and have been used in peptide design to obtain mixed peptides that retain their biological activities [6,7]. Moreover, they can be used as starting substances for different heterocycles, as precursors for the synthesis of polymers, as potential pharmacons, for the synthesis of natural products or analogues, and also as building blocks in drug research [8-11]. Furthermore, some β-amino acid derivatives have antibiotic (oryzoxymycin) and antifungal activities (Figure 1) [12,13].
Figure 1: Examples of natural products containing β-amino acids.
Figure 1: Examples of natural products containing β-amino acids.
Among the cyclic β-amino acids, the most widely investigated derivatives are the five- and six-membered derivatives [8-10,14,15], but only a few synthetic methods are available for the synthesis of β-amino acids containing seven- [14-16], and eight- [14,17,18] membered rings. Only one of these studies previously reported by Fülöp and co-workers was on the synthesis of hydroxylated cyclooctane amino acids starting from 1,5-cyclooctadiene [17], the others were on the synthesis of non-hydroxylated cyclooctane amino acids [14,18]. Also in other ring systems, only non-hydroxylated cyclic amino acids and derivatives were synthesized [8-10,15,16]. Therefore, we were inspired to develop new methods for the synthesis of hydroxylated β-amino acid derivatives containing eight-membered rings. We have recently reported the synthesis of various eight-membered aminocyclitols and their derivatives [19-25]. In the present paper, we describe the synthesis of some hydroxylated β-amino acid derivatives containing eight-membered rings starting from cis,cis-1,3-cyclooctadiene.
Results and Discussion
Initially, we focused on the synthesis of β-lactam 2, which was prepared by the cycloaddition of chlorosulfonyl isocyanate (CSI) to cis,cis-1,3-cyclooctadiene, as described in the literature [26]. β-Lactam 2 was transformed into cis-amino ester 3 by cleavage of the lactam ring with HCl(g) in MeOH (Scheme 1).
Scheme 1: Synthesis of cyclic β-amino acid 6.
Scheme 1: Synthesis of cyclic β-amino acid 6.
N-Boc protection of cis-amino ester 3 with (Boc)2O in pyridine and 4-(dimethylamino)pyridine (DMAP) gave N-Boc-amino ester 4 (yield 95%). The 1H and 13C NMR spectroscopic data of 4 were in agreement with the proposed structure. Treatment of 4 with OsO4/NMO gave the expected diol 5 as a single isomer in 91% yield. We assume that the trans selectivity of hydroxylation in ester 4 is due to the steric effect of the presence of the bulky Boc group. The structure of 5 was determined with the help of 1D (1H and 13C) and 2D (COSY and HMQC) NMR spectra. The diagonal peak at 4.10 ppm has cross peaks with the protons resonating at 2.96, 3.95, and 5.39 ppm, respectively, in the COSY spectrum. The cross peak between H-2 and H-3/H-4 suggests that the proton H-2 should have a trans configuration relative to the proton H-3. Removal of the Boc protection by HCl resulted in the formation of the target β-amino acid 6, which was characterized based on its NMR spectra (Scheme 1).
The N-Boc-amino ester 4 was reacted with m-CPBA to give epoxide 7 as the sole product in 94% yield (Scheme 2). The structure of 7 was assigned based on its NMR spectra. Epoxide 7 was used as the precursor material in the synthesis of the other isomer of β-amino acid 6. The ring-opening reaction of 7 with HCl(g) in MeOH resulted in a mixture of products 8 and 9 in a 9:1 ratio (1H NMR). The product 8 in the reaction mixture was purified by recrystallization from ethanol/ether, but all attempts to purify the expected product 9 failed. The product 8 was obtained as the major product in 80% yield, and the expected product 9 was formed as the minor product in 4% yield. We propose that diol isomer mixture 9 can be formed by solvolysis. The presence of the lactone ring in 8 was determined by 2D NMR spectroscopic data (COSY and HMQC). The diagonal peak at 4.62 ppm has cross peaks with the protons resonating at 4.26 and 4.39 ppm, respectively, in the COSY spectra of 8. The cross peak between H-7 and H-6 showed strong correlation, which clearly supports the trans relation of the proton H-6. The cross peak between H-7 and H-10 also showed weak correlation, which clearly supports the cis relation of the proton H-10. In this reaction, lactonization and hydrolysis of the Boc group to the corresponding amine were observed. The formation of lactone 8 as the major product can be explained by nucleophilic attack of the neighbouring carboxyl group, which was formed by hydrolysis of the corresponding methyl ester.
Scheme 2: Epoxidation of Boc-protected amino ester 4 and hydrolysis of epoxide 7 with HCl(g)–MeOH.
Scheme 2: Epoxidation of Boc-protected amino ester 4 and hydrolysis of epoxide 7 with HCl(g)–MeOH.
For the synthesis of other isomeric β-amino acid derivatives, epoxide 7 was treated with two equivalent amounts of NaHSO4 [27] in methylene chloride/MeOH at room temperature (Scheme 3). The formation of a mixture of products 10 and 11 in a 7:3 ratio was determined by NMR spectroscopy. The reaction mixture was purified using preparative silica gel TLC on a chromatotron with ethyl acetate/hexane (50:50) as the eluent to give carbamate 10 and diol isomer mixture 11 in 65% and 25% yields, respectively. However, all attempts to isolate isomer mixture 11 failed. Again, we suggest that diol isomer mixture 11 can be formed by solvolysis. The presence of the lactone ring in 10 was determined by 2D NMR spectroscopic data (COSY and HMQC). The diagonal peak at 4.19 ppm has cross peaks with the protons resonating at 4.49, 1.53, and 2.02 ppm, respectively, in the COSY spectra of 10. The cross peak (δ 4.49 ppm) between H-6 and H-7 showed a strong correlation, which clearly supports the trans relation of the proton H-7. Furthermore, its structure was unambiguously confirmed by single crystal X-ray analysis (Figure 2) [28]. The formation of lactone 10 can again be explained by participation of the neighbouring group, as discussed above. However, during the purification on silica gel of the lactone-Boc product, its transesterification also resulted in corresponding methyl carbamate 10.
Scheme 3: Reaction of epoxide 7 with NaHSO4 in methylene chloride/MeOH.
Scheme 3: Reaction of epoxide 7 with NaHSO4 in methylene chloride/MeOH.
![[1860-5397-18-7-2]](/bjoc/content/figures/1860-5397-18-7-2.jpg?scale=2.0&max-width=1024&background=FFFFFF)
Figure 2: The X-ray crystal structure of 10.
Figure 2: The X-ray crystal structure of 10.
We then investigated the epoxide-ring-opening reaction of 7 with sodium azide, to introduce an extra amino group in position 4 on the cyclooctane skeleton. For this, epoxide 7 was treated with NaN3 in the presence of NH4Cl/DMF and this formed lactone 13 as the sole product in 80% yield (Scheme 4). The epoxide 7 was treated with NaN3 and NH4Cl/DMF to obtain compound 12. From this reaction, the formation of unexpected lactone 13 was observed. The reaction was repeated again with only NH4Cl and DMF, and the reaction resulted in formation of compound 13. This experiment shows that NaN3/DMF does not to play any role in this transformation. The structure of 13 was elucidated with the help of the 2D NMR (COSY and HMQC) experiments. Finally, removal of the Boc group from 13 with HCl(g)–MeOH resulted in the formation of cyclic β-amino acid derivative 8 in high yield.
Scheme 4: Synthesis of cyclic β-amino acid derivative 8.
Scheme 4: Synthesis of cyclic β-amino acid derivative 8.
Our suggested mechanism for the reaction of epoxide 7 with NaHSO4 in a mixture of methylene chloride/MeOH proceeded as described in Scheme 5. First, the C=O group of the ester prefers to attack the protonated epoxide to give intermediate 15. Then, water, which is available in methanol as an impurity, attacks the oxonium ion to give dealkylation product 10, which is a typical transesterification reaction.
Scheme 5: Suggested mechanism for the reaction of epoxide 7 with NaHSO4.
Scheme 5: Suggested mechanism for the reaction of epoxide 7 with NaHSO4.
To explain the formation of lactone 10, we performed a series of DFT computations using the Gaussian 16 software [29]. For this purpose, we performed geometry optimizations using the B3LYP functional [30-33]. Vibrational frequencies were computed to characterize each stationary structure. In all the computations, Pople’s polarized triple-ζ split valence basis set with diffuse functions, 6-311++G(d,p) [34-36], was utilized. The solvation model based on density (SMD) [37] was used to investigate the effects of methanol (ε = 32.613) and dichloromethane (ε = 8.93) on the computed Gibbs energies. For the TS between species A and B, the TSA-B notation is used throughout the article.
According to the given mechanism in Scheme 5, there are two possible paths for the attack of the carboxyl group on the epoxide ring. For the formation of the five-membered lactone 15 (path a), 14 → 15, the reaction proceeds via a barrierless path and the solvent corrected reaction free energies are −17.5 and −18.1 kcal mol−1 with methanol and dichloromethane, respectively. For the formation of the six-membered lactone 16 (path b), 14 → 16, the solvent corrected reaction free energy and barrier are −9.5 and 14.5 kcal mol−1 with methanol, respectively and −11.1 and 13.5 kcal mol−1 with dichloromethane, respectively (Figure 3). For the formation of the five-membered lactone 18 (path a), 17 → 18, the reaction proceeds via a barrierless path and the solvent corrected reaction free energies are −17.2 and −18.0 kcal mol−1 with methanol and dichloromethane, respectively. For the formation of the six-membered lactone 19 (path b), 17 → 19, the solvent corrected reaction free energy and barrier are −5.4 and 14.5 kcal mol−1 in methanol, respectively and −7.1 and 13.4 kcal mol−1 in dichloromethane, respectively (Figure 4). These results indicate that the formation of the methyl carbamate may occur before or after the attack by the carboxyl group. Overall, our computations demonstrate that the 14 → 15 and 17 → 18 conversions are kinetically more favourable, by about 14.0 kcal mol−1, compared to the 14 → 16 and 17 → 19 conversions. These results rule out the formations of 16 and 19 at room temperature. Therefore, we conclude that the final product is lactone 10, and the formation of lactone 20 is not feasible under the experimental conditions. Furthermore, lactone 10 is energetically more stable, by 5.0 kcal mol−1 in methanol and by 3.7 kcal mol−1 in dichloromethane, than lactone 20. The computations were in good agreement with the experimental results (Figure 3 and Figure 4).
Figure 3: Solvent-corrected relative free energy profile at 298.15 K for the reaction mechanism of 14 shown in Scheme 5.
Figure 3: Solvent-corrected relative free energy profile at 298.15 K for the reaction mechanism of 14 shown i...
Figure 4: Solvent-corrected relative free energy profile at 298.15 K for the reaction mechanism of 17 shown in Scheme 5.
Figure 4: Solvent-corrected relative free energy profile at 298.15 K for the reaction mechanism of 17 shown i...
To explain why lactone 10 is formed, a conformational analysis for the epoxide 7 was performed. It is seen that the conformation is quite appropriate for the formation of lactone 10. The hydrogen bond between the C=O group of methoxy ester and NH group of the carbamate in 7 provides an appropriate conformation for the formation of lactone 10. Additionally, according to the conformational analysis of epoxide 7, the relative free energy of 7b is higher by 10.3 kcal mol−1 than that of 7a (Figure 5).
![[1860-5397-18-7-5]](/bjoc/content/figures/1860-5397-18-7-5.jpg?scale=2.0&max-width=1024&background=FFFFFF)
Figure 5: The optimized geometries of the conformers 7a and 7b with selected interatomic distances at the B3LYP/6-311++G(d,p) level.
Figure 5: The optimized geometries of the conformers 7a and 7b with selected interatomic distances at the B3L...
Conclusion
In summary, we successfully synthesized hydroxylated cyclooctane β-amino acid 6 and its derivatives 8, 10, and 13 starting from β-lactam 2. The regioselective synthesis of lactone 8, which is a cyclic β-amino acid derivative, was achieved by oxirane ring opening in epoxide 7 with HCl(g)–MeOH, NaHSO4, or NH4Cl–DMF. The regioselectivity of oxirane ring-opening in 7 was attributed to the conformational effects. The mechanism for the formation of compound 10 was elucidated with DFT computations. Our computations demonstrate that the formation of the five-membered lactone is kinetically more favourable, and the formation of six-membered lactone is not feasible under the experimental conditions. The conformation of epoxide 7 is quite appropriate for the formation of the five-membered lactone 10, in contrast to the formation of the six-membered lactone 20. In addition, these novel compounds synthesized may be used as intermediates to design pharmacological tools.
Supporting Information
Experimental section, 1H and 13C NMR spectra for all new compounds, as well as selected 2D NMR spectra and crystallographic data for compound 10 are provided. Optimized geometries of the transition states with selected interatomic distances and cartesian coordinates for computed structures are reported.
| Supporting Information File 1: Additional experimental and computed data. | ||
| Format: PDF | Size: 1.7 MB | Download |
References
-
von Nussbaum, F.; Spiteller, P. β-Amino Acids in Nature. In Highlights in Bioorganic Chemistry: Methods and Applications; Schmuck, C.; Wennemers, H., Eds.; Wiley-VCH: Weinheim, Germany, 2004; pp 63–89. doi:10.1002/3527603727.ch1e
Return to citation in text: [1] -
Juaristi, E.; Soloshonok, V. Enantioselective Synthesis of β-Amino Acids, 2nd ed.; John Wiley & Sons: Hoboken, NJ, USA, 2005. doi:10.1002/0471698482
Return to citation in text: [1] -
Risseeuw, M.; Overhand, M.; Fleet, G. W. J.; Simone, M. I. Amino Acids 2013, 45, 613–689. doi:10.1007/s00726-013-1521-1
Return to citation in text: [1] -
Kiss, L.; Fülöp, F. Chem. Rev. 2014, 114, 1116–1169. doi:10.1021/cr300454h
Return to citation in text: [1] -
Fülöp, F.; Martinek, T. A.; Tóth, G. K. Chem. Soc. Rev. 2006, 35, 323–334. doi:10.1039/b501173f
Return to citation in text: [1] -
Norgren, A. S.; Büttner, F.; Prabpai, S.; Kongsaeree, P.; Arvidsson, P. I. J. Org. Chem. 2006, 71, 6814–6821. doi:10.1021/jo060854n
Return to citation in text: [1] -
Cardillo, G.; Gentilucci, L.; Melchiorre, P.; Spampinato, S. Bioorg. Med. Chem. Lett. 2000, 10, 2755–2758. doi:10.1016/s0960-894x(00)00562-x
Return to citation in text: [1] -
Kiss, L.; Mándity, I. M.; Fülöp, F. Amino Acids 2017, 49, 1441–1455. doi:10.1007/s00726-017-2439-9
Return to citation in text: [1] [2] [3] -
Fülöp, F. Chem. Rev. 2001, 101, 2181–2204. doi:10.1021/cr000456z
Return to citation in text: [1] [2] [3] -
Lee, M.-r.; Stahl, S. S.; Gellman, S. H. Org. Lett. 2008, 10, 5317–5319. doi:10.1021/ol802274x
Return to citation in text: [1] [2] [3] -
Rasoanaivo, P.; Langlois, N.; Potier, P. Tetrahedron Lett. 1974, 15, 3669–3672. doi:10.1016/s0040-4039(01)93205-8
Return to citation in text: [1] -
Hashimoto, T.; Kondo, S.; Naganawa, H.; Takita, T.; Maeda, K.; Umezawa, H. J. Antibiot. 1974, 27, 86–87. doi:10.7164/antibiotics.27.86
Return to citation in text: [1] -
Bunnage, M. E.; Ganesh, T.; Masesane, I. B.; Orton, D.; Steel, P. G. Org. Lett. 2003, 5, 239–242. doi:10.1021/ol0269704
Return to citation in text: [1] -
Forró, E.; Fülöp, F. Org. Lett. 2003, 5, 1209–1212. doi:10.1021/ol034096o
Return to citation in text: [1] [2] [3] [4] -
Gardiner, J.; Anderson, K. H.; Downard, A.; Abell, A. D. J. Org. Chem. 2004, 69, 3375–3382. doi:10.1021/jo049794g
Return to citation in text: [1] [2] [3] -
Fustero, S.; Bartolomé, A.; Sanz-Cervera, J. F.; Sánchez-Roselló, M.; Soler, J. G.; Ramírez de Arellano, C.; Fuentes, A. S. Org. Lett. 2003, 5, 2523–2526. doi:10.1021/ol034827k
Return to citation in text: [1] [2] -
Palkó, M.; Benedek, G.; Forró, E.; Wéber, E.; Hänninen, M.; Sillanpää, R.; Fülöp, F. Tetrahedron: Asymmetry 2010, 21, 957–961. doi:10.1016/j.tetasy.2010.05.003
Return to citation in text: [1] [2] -
Forró, E.; Árva, J.; Fülöp, F. Tetrahedron: Asymmetry 2001, 12, 643–649. doi:10.1016/s0957-4166(01)00100-8
Return to citation in text: [1] [2] -
Salamci, E. Tetrahedron Lett. 2020, 61, 151728. doi:10.1016/j.tetlet.2020.151728
Return to citation in text: [1] -
Karavaizoglu, U. N.; Salamci, E. New J. Chem. 2020, 44, 17976–17983. doi:10.1039/d0nj02697b
Return to citation in text: [1] -
Zozik, Y.; Salamci, E.; Kilic, A. Tetrahedron Lett. 2017, 58, 4822–4826. doi:10.1016/j.tetlet.2017.11.014
Return to citation in text: [1] -
Kaya, A. A.; Salamci, E.; Menzek, A.; Erdem, S. S.; Şahin, E.; Ecer, K. Tetrahedron 2017, 73, 5381–5388. doi:10.1016/j.tet.2017.07.040
Return to citation in text: [1] -
Ecer, K.; Salamci, E. Tetrahedron 2014, 70, 8389–8396. doi:10.1016/j.tet.2014.08.060
Return to citation in text: [1] -
Salamci, E. Tetrahedron 2010, 66, 4010–4015. doi:10.1016/j.tet.2010.04.052
Return to citation in text: [1] -
Salamci, E.; Zozik, Y. Beilstein J. Org. Chem. 2021, 17, 705–710. doi:10.3762/bjoc.17.59
Return to citation in text: [1] -
Kardos, M.; Kiss, L.; Fülöp, F. Asian J. Org. Chem. 2015, 4, 1155–1159. doi:10.1002/ajoc.201500286
Return to citation in text: [1] -
Das, B.; Thirupathi, P. J. Mol. Catal. A: Chem. 2007, 269, 12–16. doi:10.1016/j.molcata.2006.12.029
Return to citation in text: [1] -
Crystallographic data for the structural analysis of compound 10 reported in this paper have been deposited with the Cambridge Crystallographic Data Centre as CCDC-2064456. These data are provided free of charge via the joint CCDC/FIZ Karlsruhe deposition service http://www.ccdc.cam.ac.uk/structures.
Return to citation in text: [1] -
Gaussian 16, Revision C.01; Gaussian, Inc.: Wallingford, CT, 2019.
Return to citation in text: [1] -
Becke, A. D. J. Chem. Phys. 1993, 98, 5648–5652. doi:10.1063/1.464913
Return to citation in text: [1] -
Lee, C.; Yang, W.; Parr, R. G. Phys. Rev. B 1988, 37, 785–789. doi:10.1103/physrevb.37.785
Return to citation in text: [1] -
Vosko, S. H.; Wilk, L.; Nusair, M. Can. J. Phys. 1980, 58, 1200–1211. doi:10.1139/p80-159
Return to citation in text: [1] -
Stephens, P. J.; Devlin, F. J.; Chabalowski, C. F.; Frisch, M. J. J. Phys. Chem. 1994, 98, 11623–11627. doi:10.1021/j100096a001
Return to citation in text: [1] -
Hariharan, P. C.; Pople, J. A. Theor. Chim. Acta 1973, 28, 213–222. doi:10.1007/bf00533485
Return to citation in text: [1] -
McLean, A. D.; Chandler, G. S. J. Chem. Phys. 1980, 72, 5639–5648. doi:10.1063/1.438980
Return to citation in text: [1] -
Krishnan, R.; Binkley, J. S.; Seeger, R.; Pople, J. A. J. Chem. Phys. 1980, 72, 650–654. doi:10.1063/1.438955
Return to citation in text: [1] -
Marenich, A. V.; Cramer, C. J.; Truhlar, D. G. J. Phys. Chem. B 2009, 113, 6378–6396. doi:10.1021/jp810292n
Return to citation in text: [1]
| 34. | Hariharan, P. C.; Pople, J. A. Theor. Chim. Acta 1973, 28, 213–222. doi:10.1007/bf00533485 |
| 35. | McLean, A. D.; Chandler, G. S. J. Chem. Phys. 1980, 72, 5639–5648. doi:10.1063/1.438980 |
| 36. | Krishnan, R.; Binkley, J. S.; Seeger, R.; Pople, J. A. J. Chem. Phys. 1980, 72, 650–654. doi:10.1063/1.438955 |
| 37. | Marenich, A. V.; Cramer, C. J.; Truhlar, D. G. J. Phys. Chem. B 2009, 113, 6378–6396. doi:10.1021/jp810292n |
| 1. | von Nussbaum, F.; Spiteller, P. β-Amino Acids in Nature. In Highlights in Bioorganic Chemistry: Methods and Applications; Schmuck, C.; Wennemers, H., Eds.; Wiley-VCH: Weinheim, Germany, 2004; pp 63–89. doi:10.1002/3527603727.ch1e |
| 2. | Juaristi, E.; Soloshonok, V. Enantioselective Synthesis of β-Amino Acids, 2nd ed.; John Wiley & Sons: Hoboken, NJ, USA, 2005. doi:10.1002/0471698482 |
| 3. | Risseeuw, M.; Overhand, M.; Fleet, G. W. J.; Simone, M. I. Amino Acids 2013, 45, 613–689. doi:10.1007/s00726-013-1521-1 |
| 4. | Kiss, L.; Fülöp, F. Chem. Rev. 2014, 114, 1116–1169. doi:10.1021/cr300454h |
| 5. | Fülöp, F.; Martinek, T. A.; Tóth, G. K. Chem. Soc. Rev. 2006, 35, 323–334. doi:10.1039/b501173f |
| 8. | Kiss, L.; Mándity, I. M.; Fülöp, F. Amino Acids 2017, 49, 1441–1455. doi:10.1007/s00726-017-2439-9 |
| 9. | Fülöp, F. Chem. Rev. 2001, 101, 2181–2204. doi:10.1021/cr000456z |
| 10. | Lee, M.-r.; Stahl, S. S.; Gellman, S. H. Org. Lett. 2008, 10, 5317–5319. doi:10.1021/ol802274x |
| 14. | Forró, E.; Fülöp, F. Org. Lett. 2003, 5, 1209–1212. doi:10.1021/ol034096o |
| 15. | Gardiner, J.; Anderson, K. H.; Downard, A.; Abell, A. D. J. Org. Chem. 2004, 69, 3375–3382. doi:10.1021/jo049794g |
| 12. | Hashimoto, T.; Kondo, S.; Naganawa, H.; Takita, T.; Maeda, K.; Umezawa, H. J. Antibiot. 1974, 27, 86–87. doi:10.7164/antibiotics.27.86 |
| 13. | Bunnage, M. E.; Ganesh, T.; Masesane, I. B.; Orton, D.; Steel, P. G. Org. Lett. 2003, 5, 239–242. doi:10.1021/ol0269704 |
| 30. | Becke, A. D. J. Chem. Phys. 1993, 98, 5648–5652. doi:10.1063/1.464913 |
| 31. | Lee, C.; Yang, W.; Parr, R. G. Phys. Rev. B 1988, 37, 785–789. doi:10.1103/physrevb.37.785 |
| 32. | Vosko, S. H.; Wilk, L.; Nusair, M. Can. J. Phys. 1980, 58, 1200–1211. doi:10.1139/p80-159 |
| 33. | Stephens, P. J.; Devlin, F. J.; Chabalowski, C. F.; Frisch, M. J. J. Phys. Chem. 1994, 98, 11623–11627. doi:10.1021/j100096a001 |
| 8. | Kiss, L.; Mándity, I. M.; Fülöp, F. Amino Acids 2017, 49, 1441–1455. doi:10.1007/s00726-017-2439-9 |
| 9. | Fülöp, F. Chem. Rev. 2001, 101, 2181–2204. doi:10.1021/cr000456z |
| 10. | Lee, M.-r.; Stahl, S. S.; Gellman, S. H. Org. Lett. 2008, 10, 5317–5319. doi:10.1021/ol802274x |
| 11. | Rasoanaivo, P.; Langlois, N.; Potier, P. Tetrahedron Lett. 1974, 15, 3669–3672. doi:10.1016/s0040-4039(01)93205-8 |
| 27. | Das, B.; Thirupathi, P. J. Mol. Catal. A: Chem. 2007, 269, 12–16. doi:10.1016/j.molcata.2006.12.029 |
| 6. | Norgren, A. S.; Büttner, F.; Prabpai, S.; Kongsaeree, P.; Arvidsson, P. I. J. Org. Chem. 2006, 71, 6814–6821. doi:10.1021/jo060854n |
| 7. | Cardillo, G.; Gentilucci, L.; Melchiorre, P.; Spampinato, S. Bioorg. Med. Chem. Lett. 2000, 10, 2755–2758. doi:10.1016/s0960-894x(00)00562-x |
| 28. | Crystallographic data for the structural analysis of compound 10 reported in this paper have been deposited with the Cambridge Crystallographic Data Centre as CCDC-2064456. These data are provided free of charge via the joint CCDC/FIZ Karlsruhe deposition service http://www.ccdc.cam.ac.uk/structures. |
| 14. | Forró, E.; Fülöp, F. Org. Lett. 2003, 5, 1209–1212. doi:10.1021/ol034096o |
| 18. | Forró, E.; Árva, J.; Fülöp, F. Tetrahedron: Asymmetry 2001, 12, 643–649. doi:10.1016/s0957-4166(01)00100-8 |
| 19. | Salamci, E. Tetrahedron Lett. 2020, 61, 151728. doi:10.1016/j.tetlet.2020.151728 |
| 20. | Karavaizoglu, U. N.; Salamci, E. New J. Chem. 2020, 44, 17976–17983. doi:10.1039/d0nj02697b |
| 21. | Zozik, Y.; Salamci, E.; Kilic, A. Tetrahedron Lett. 2017, 58, 4822–4826. doi:10.1016/j.tetlet.2017.11.014 |
| 22. | Kaya, A. A.; Salamci, E.; Menzek, A.; Erdem, S. S.; Şahin, E.; Ecer, K. Tetrahedron 2017, 73, 5381–5388. doi:10.1016/j.tet.2017.07.040 |
| 23. | Ecer, K.; Salamci, E. Tetrahedron 2014, 70, 8389–8396. doi:10.1016/j.tet.2014.08.060 |
| 24. | Salamci, E. Tetrahedron 2010, 66, 4010–4015. doi:10.1016/j.tet.2010.04.052 |
| 25. | Salamci, E.; Zozik, Y. Beilstein J. Org. Chem. 2021, 17, 705–710. doi:10.3762/bjoc.17.59 |
| 17. | Palkó, M.; Benedek, G.; Forró, E.; Wéber, E.; Hänninen, M.; Sillanpää, R.; Fülöp, F. Tetrahedron: Asymmetry 2010, 21, 957–961. doi:10.1016/j.tetasy.2010.05.003 |
| 26. | Kardos, M.; Kiss, L.; Fülöp, F. Asian J. Org. Chem. 2015, 4, 1155–1159. doi:10.1002/ajoc.201500286 |
| 14. | Forró, E.; Fülöp, F. Org. Lett. 2003, 5, 1209–1212. doi:10.1021/ol034096o |
| 17. | Palkó, M.; Benedek, G.; Forró, E.; Wéber, E.; Hänninen, M.; Sillanpää, R.; Fülöp, F. Tetrahedron: Asymmetry 2010, 21, 957–961. doi:10.1016/j.tetasy.2010.05.003 |
| 18. | Forró, E.; Árva, J.; Fülöp, F. Tetrahedron: Asymmetry 2001, 12, 643–649. doi:10.1016/s0957-4166(01)00100-8 |
| 14. | Forró, E.; Fülöp, F. Org. Lett. 2003, 5, 1209–1212. doi:10.1021/ol034096o |
| 15. | Gardiner, J.; Anderson, K. H.; Downard, A.; Abell, A. D. J. Org. Chem. 2004, 69, 3375–3382. doi:10.1021/jo049794g |
| 16. | Fustero, S.; Bartolomé, A.; Sanz-Cervera, J. F.; Sánchez-Roselló, M.; Soler, J. G.; Ramírez de Arellano, C.; Fuentes, A. S. Org. Lett. 2003, 5, 2523–2526. doi:10.1021/ol034827k |
| 8. | Kiss, L.; Mándity, I. M.; Fülöp, F. Amino Acids 2017, 49, 1441–1455. doi:10.1007/s00726-017-2439-9 |
| 9. | Fülöp, F. Chem. Rev. 2001, 101, 2181–2204. doi:10.1021/cr000456z |
| 10. | Lee, M.-r.; Stahl, S. S.; Gellman, S. H. Org. Lett. 2008, 10, 5317–5319. doi:10.1021/ol802274x |
| 15. | Gardiner, J.; Anderson, K. H.; Downard, A.; Abell, A. D. J. Org. Chem. 2004, 69, 3375–3382. doi:10.1021/jo049794g |
| 16. | Fustero, S.; Bartolomé, A.; Sanz-Cervera, J. F.; Sánchez-Roselló, M.; Soler, J. G.; Ramírez de Arellano, C.; Fuentes, A. S. Org. Lett. 2003, 5, 2523–2526. doi:10.1021/ol034827k |
© 2022 Polat et al.; licensee Beilstein-Institut.
This is an open access article licensed under the terms of the Beilstein-Institut Open Access License Agreement (https://www.beilstein-journals.org/bjoc/terms), which is identical to the Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0). The reuse of material under this license requires that the author(s), source and license are credited. Third-party material in this article could be subject to other licenses (typically indicated in the credit line), and in this case, users are required to obtain permission from the license holder to reuse the material.