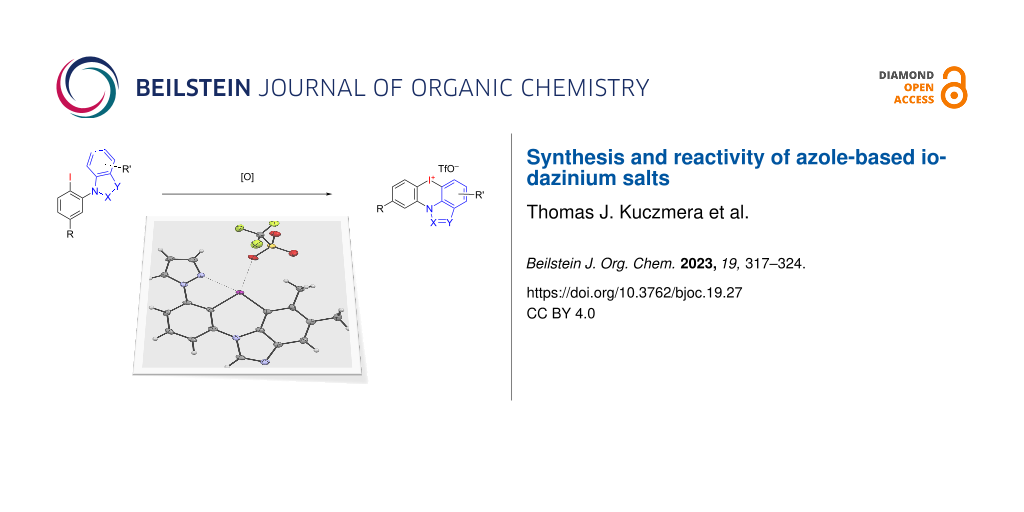
Abstract
A systematic investigation of imidazo- and pyrazoloiodazinium salts is presented. Besides a robust synthetic protocol that allowed us to synthesize these novel cyclic iodonium salts in their mono- and dicationic forms, we gained in-depth structural information through single-crystal analysis and demonstrated the ring opening of the heterocycle-bridged iodonium species. For an exclusive set of dicationic imidazoiodaziniums, we show highly delicate post-oxidation functionalizations retaining the hypervalent iodine center.
Graphical Abstract
Introduction
The chemistry of hypervalent iodine compounds, in particular aryl-λ3-iodanes, is highly versatile, and a wide range of applications is meanwhile established in organic synthesis [1-5]. They can be applied as mild oxidants [6-8], in phenol dearomatizations [9] or in α-oxygenation reactions [10]. In a complemental reactivity, diaryliodonium salts are potent electrophilic aryl donors [11-16]. Their cyclic derivatives have a proven utility as precursors for the synthesis of hetero- and carbocycles [17-21], and their pronounced σ-holes [22] render them efficient halogen-bond donors (XB donors in XB catalysis) [23]. Despite their great potential in organic synthesis and catalysis, their structural variation is still limited. In particular, heteroarene-bridged cyclic iodonium salts are rare. Examples include the benzisoxazole-containing iodonium salt 1 described by Lisichkina and Tolstaya (Figure 1) [24,25]. Our group is interested in the chemistry of hypervalent iodine species in all their variety, particularly those containing N-heterocycles either as tethered stabilizing ligands or as an inclusive part of a cyclic iodonium salt [26-31]. We prepared five-membered, N-heterocycle-containing iodoliums 2 and investigated their reactivity and utility in XB catalysis. We also established one-pot methods for generating six-membered carbon-, oxygen-, and nitrogen-bridged iodonium salts, such as the iodazinium triflate 3 [32,33]. Based on these promising findings, we further wanted to elaborate this chemistry and herein we present the first synthesis and application of more sophisticated imidazo- and pyrazoloiodazinium salts.
Figure 1: Nitrogen-containing iodolium and iodonium salts.
Figure 1: Nitrogen-containing iodolium and iodonium salts.
Results and Discussion
Initially, we focused on developing a mild oxidation procedure starting from iodoarene precursors. Previous studies on five-membered heteroaromatic iodonium salts revealed m-chloroperoxybenzoic acid (mCPBA) as the oxidant of choice in the presence of triflic acid (TfOH) [27,29]. Based on these promising results, the conditions were optimized using o-benzimidazole-substituted iodoarenes 4aa and 4ah (Table 1).
Table 1: Optimization of reaction conditions for the synthesis of azoiodazinium salts 5aa and 5ah.a
|
|
|||||||
| Entry | R |
mCPBA
(equiv) |
TfOH
(equiv) |
T
(°C) |
t
(d) |
Solvent |
Yield
(%) |
|---|---|---|---|---|---|---|---|
| 1 | H | 1.1 | 3.0 | 50 | 3 | MeCN | 0 |
| 2 | H | 1.1 | 3.0 | 50 | 3 | DCE | 23 |
| 3 | H | 1.1 | 2.5 | 50 | 3 | DCE | 69 |
| 4 | H | 1.1 | 2.0 | 50 | 3 | DCE | 19 |
| 5 | H | 1.1 | 2.5 | 40 | 3 | DCM | 69 |
| 6 | H | 1.5 | 2.5 | 40 | 3 | DCM | 53 |
| 7 | Cl | 1.1 | 2.5 | 40 | 3 | DCM | 9b |
| 8 | Cl | 1.3 | 5.0 | 65 | 14 | DCM | 52 |
aIodoarene 4aa or 4ah (200 µmol) and mCPBA were dissolved in the given solvent (1 mL) in a screw cap vial, TfOH was added, and the reaction mixture was stirred under the corresponding conditions. For full table, see Supporting Information File 1. bIncomplete conversion, product not clean.
While running the reaction in MeCN as solvent resulted in no product formation, the reaction of 4aa in DCE at 50 °C gave the product 5aa in 23% yield (Table 1, entries 1 and 2). A larger amount of TfOH turned out to increase the solubility of the product and therefore impeded the purification process. However, an excess of acid is required for the electrophilic aromatic substitution to take place. With 2.5 equivalents of TfOH as the optimum amount of acid the product 5aa was obtained in a yield of 69% (Table 1, entry 3). Similar results were observed with DCM at 40 °C (Table 1, entry 5). A higher amount of mCPBA did not lead to a better yield due to more washing required to remove the m-chlorobenzoic acid (Table 1, entry 6). When we employed the chlorinated, electron-deficient iodoarene 4ah, the yield of the product 5ah dropped significantly (Table 1, entry 7). Combining an electron-deficient heterocycle and an iodoarene with electron-withdrawing substituents results in a significantly decreased reactivity. Thus, for those substrates, harsher reaction conditions were required. A slight adaption of the original conditions to elevated temperatures (65 °C) and prolonged reaction times of 14 d finally resulted in the formation of product 5ah in 52% yield using DCM as the solvent (Table 1, entry 8).
Next, various substituted iodoarenes 4 were oxidized and cyclized using the optimized conditions to generate a diverse set of azoiodazinium salts 5 (Figure 2). The ortho-methylated salt 5ab was obtained in a low yield of 19%, and the fluorinated derivative 5ac could be obtained in 55%. Unfortunately, the MeO-substituted derivative 5ad did not form. Except for the acetamide 5ae, which could not be obtained due to decomposition, other meta- and para-substituted derivatives 5af–ak, among them derivatives with strong electron-withdrawing functionalities, could be synthesized in 39–69% yield. The electron-rich salt 5al was obtained in 75% yield using modified reaction conditions B. The harsher conditions were probably required due to a sterically hindered rotation of the benzimidazole moiety in the plane of the iodophenyl, which could also be observed in two rotamers of the starting iodoarene 4al (see Supporting Information File 1).
Figure 2: Synthesis of a set of azoiodazinium salts 5. Method A: Iodoarene 4 (200 µmol) and mCPBA (1.1 equiv) were dissolved/suspended in DCM (1 mL), TfOH (2.5 equiv) was added, and the reaction mixture was stirred for 72 h at 40 °C. Method B: Iodoarene 4 (200 µmol) and mCPBA (1.3 equiv) were dissolved/suspended in DCM (1 mL), TfOH (5.0 equiv) was added, and the reaction mixture was stirred for 14 d at 65 °C. a6.00 mmol scale, T = 50 °C, bT = 40 °C, cT = 80 °C, t = 6 d, dt = 7 d, e0.3 equiv of DCM were included in the product.
Figure 2: Synthesis of a set of azoiodazinium salts 5. Method A: Iodoarene 4 (200 µmol) and mCPBA (1.1 equiv)...
Next, we investigated substrates 4 having various substituents in the benzimidazole motif. Starting from 2-bromo-derivative 4am, the moisture-sensitive, brominated salt 5am was obtained in an excellent yield of 85%. Also, the C2-alkyl- and phenyl-substituted benzimidazoles gave the expected products 5an–ap in 50–60% yield. The pyrazole-substituted salt 5aq was obtained with 65% yield. Using an electron-rich 5,6-dimethylbenzimidazole substrate yielded dimethylated product 5ar in 85% yield. Unfortunately, even under harsher reaction conditions, the corresponding electron-deficient dibrominated salt 5as could not be obtained. This further demonstrated the crucial influence of the electronic properties on the reactivity of those substrates. Especially, electron deficiency is particularly counterproductive for the final cyclization step. To prove the influence of the electron-rich dimethylated benzimidazole, this moiety combined with the chlorinated and brominated iodoarene gave the corresponding salts 5at and 5au in good yields under milder reaction conditions, in particular in direct comparison to the unsubstituted analogs 5ah–aj. N-Substituted iodoarenes were then used to create dicationic iodonium salts. The N-Me and N-Ph-iodonium-benzimidazolium salts 5av and 5aw were obtained in 47% and 36% yield, respectively. The introduction of additional ortho-methyl groups resulted in the formation of the σ-hole-protected N-substituted salts 5ax–az in up to 97% yield. Next, the iodonium center was stabilized through an additional N-coordination via ortho-pyrazole substitution, giving the iodonium salts 5ba and 5bb in 88% and 50% yield. When replacing imidazoles by indazoles the oxidation was not as efficient giving the products 5bc and 5bd with only 24% and 44% yields. In the latter case, the initially generated hydroxy-iodonium salt is stabilized via the indazole nitrogen [26] and the steric hindrance by the methyl group is likely destabilizing this intermediate by an out-of-plane distortion [28,34] and hence accelerating the cyclization. The dicationic indazole salt 5be was isolated in 30% yield and the benzyl-bridged, seven-membered salt 5bf could not be obtained under our optimized reaction conditions.
Single crystal structures of selected salts were obtained to gain a better understanding of the bonding situation and the coordination states in these novel azoiodazinium salts (Figure 3). An N4–I1 distance of 2.540 Å with a typical T-shape structure (N4–I1–C1 angle 185.74°) implies a significant interaction between the N-heterocycle and the iodine atom for the ortho-pyrazole-substituted derivative 5bb [35]. However, the presence of an ortho-methyl group significantly disturbs the triflate coordination to the other iodine σ-hole, which results in a C15–I1–O5 angle of 145.50°. In contrast to other six-membered iodonium salts, this molecule is nearly in plane with an I1–C1–C15–N4 dihedral angle of 2.03° [30,32]. For the dicationic salt 5av, we observed a coordination of the triflates along the C–I axis with distances of 2.705 Å (I1–O1) and 2.898 Å (I1–O5). For the ortho-methyl-substituted analogue 5ax, no halogen bonding to the triflates was observed, indicating an effective steric protection of the σ-holes [36]. Instead, there were only two weak interactions with one of the triflates (I1–O3: 3.354 Å, I1–O5, 3.078 Å).
![[1860-5397-19-27-3]](/bjoc/content/figures/1860-5397-19-27-3.png?scale=2.0&max-width=1024&background=FFFFFF)
Figure 3: Single crystal structures (ORTEP drawing with 50% probability) of the pyrazole-coordinated salt 5bb (dimer, a second structure was omitted for clarity. For full structure, see Supporting Information File 1; CCDC 2216124) and the two N-methylated, dicationic salts 5av (CCDC 2216134) and 5ax (CCDC 2216127). Selected bond lengths and angles: For 5bb: N4–I1: 2.540 Å, I1–O5: 2.905 Å, N4–I1–C1: 185.74°, C15–I1–O5: 145.50°; for 5av: I1–O1: 2.705 Å, I1–O5: 2.898 Å, C8–I1–O1: 173.80°, C6–I1–O5: 167.75°, C6–I1–C8: 94.05°; for 5ax: I1–O3: 3.354 Å, I1–O5, 3.078 Å, C1–I1–O3: 154.71°, C8–I1–O5: 148.91°, C1–I1–C8: 94.53°.
Figure 3: Single crystal structures (ORTEP drawing with 50% probability) of the pyrazole-coordinated salt 5bb...
We finally investigated the further reactivity of the synthesized azoiodazinium salts to elaborate their potential as synthetic building blocks (Scheme 1). Treatment of 5aa with Ac2O led to a non-selective ring opening at both C–I bonds giving the iodinated N-arylbenzimidazoles 6a and 6b as a mixture with 54% and 25% yield [37]. A ring opening/closing cascade reaction with elemental sulfur resulted in the formation of the imidazo[4,5,1-kl]phenothiazine (7a) in 47% yield.
Scheme 1: Derivatizations of the iodonium salt 5aa. a) Ac2O, CuSO4·5H2O, NaOAc, AcOH, 120 °C, 5 h; b) S8/Se/Te, Cs2CO3, DMSO, rt–100 °C, 2.5–24 h; c) I: PhNH2, Cu(OAc)2·H2O, Na2CO3, iPrOH, 40 °C, 17 h, II: CuI, Cs2CO3, DMF, 120 °C, 40 h; d) TsNH2, (CH2OH)2, iPrOH, Na2CO3, 100 °C, 24 h, e) KX, H2O, EtOH, reflux; f) CuI, DMEDA, 1,4-dioxane, TBAI, rt, 24 h; g) MeOTf, DMF, 40 °C, 24 h.
Scheme 1: Derivatizations of the iodonium salt 5aa. a) Ac2O, CuSO4·5H2O, NaOAc, AcOH, 120 °C, 5 h; b) S8/Se/T...
The corresponding phenoselenazine 7b and the phenotellurazine 7c were isolated in lower yields of 16% and 6%, likely due to undesired oxidations of selenium and tellurium [38]. Substitutions with nitrogen nucleophiles were performed, giving the N-phenylphenazine 8 in 26% yield [39,40] and the N-tosyl derivative 9 in 18% yield [41]. Anion exchange reactions to iodide and bromide were performed giving the salts 10a and 10b in excellent yields [27]. A copper-catalyzed iodination gave the diiodinated product 11 in quantitative yield [42]. Finally, N-methylation of 5aa was performed, to yield the dicationic salt 5av in 56% yield without decomposition of the iodonium center [43]. In this reaction, however, no complete conversion could be achieved, even by adding excess MeOTf.
Inspired by the latter results, we were interested to investigate other post-oxidation functionalizations on the benzimidazole ring while keeping the highly reactive hypervalent iodine center intact. Treatment of the ortho-pyrazole-substituted salt 5bb with MeOTf resulted in a selective benzimidazole N-methylation. A reaction on the pyrazole nitrogen is impeded due to its coordination with the iodane’s σ-hole (Scheme 2a). Besides nitrogen-substitution, the benzimidazole C-2 position of the dicationic salts is a reactive site for oxidative transformations [44,45]. The reaction of the iodine-protected benzimidazolium salts 5ax–az and 12 with different oxygen sources revealed K2CO3 and NCS as the optimal system to form the benzimidazole-2-ones 13a–d in 18–48% yield, with the best result obtained when using the stabilized salt 12 (Scheme 2b) [44]. Here, no counter-ion exchange to chloride was observed. The favored counter ion is determined by the pKa value of the corresponding acids but not by halogen bonding due to the steric hindrance at the iodines’ σ-holes. The reaction of TsNH2 in combination with NaOCl as an oxidant was investigated next. Under these conditions, the N-Me salts 5ax and 12 gave the desired products 14a and 14b in 55% and 26% yield, respectively. The corresponding N-Ph- and N-Mes-derivatives 5ay and 5az failed to give products 14c and 14d and only underwent undesired ring openings.
Scheme 2: Post-functionalization of mono- and dicationic iodonium salts under preservation of the hypervalent iodine center.
Scheme 2: Post-functionalization of mono- and dicationic iodonium salts under preservation of the hypervalent...
Treating 12 with BocNH2 resulted in the formation of protected guanidine 15 in 80% yield (Scheme 2c), which would not be possible to obtain via an oxidative cyclization of the corresponding iodine(I) species due to a carbamate cleavage with acid. The other dicationic salts underwent ring openings in this reaction. This reactivity demonstrates the highly stabilizing effect of N-heterocycles on hypervalent iodine species. Furthermore, the formed 2-aminobenzimidazoles reveal new access to potential bioactive compounds [46,47]. Even the formation of the free guanidine 16 via cleavage of the Boc-group was possible in quantitative yield.
Conclusion
In this work, we prepared azoiodaziniums as a new class of six-membered heterocyclic iodonium salts with a wide range of substituents. Derivatizations of the reactive iodonium center allow for the formation of new heterocyclic compounds based on azol-based iodazinium as reactive intermediates. Most interestingly, functionalization of the heteroarene salts was achieved without an undesired attack of the delicate C–I bond at the hypervalent iodine center.
Experimental
General procedure for the synthesis of azoiodazinium salts (method A): To a stirred solution of the corresponding (2-iodophenyl)-1H-benzo[d]imidazole or -indazole (200 µmol, 1.0 equiv) and mCPBA (85%, 44.8 mg, 220 µmol, 1.1 equiv) in DCM (1 mL) was added TfOH (44.2 µL, 500 µmol, 2.5 equiv) and the resulting solution was stirred for 72 h at 40 °C. The solvent was removed under reduced pressure. The residue was suspended in Et2O (1 mL) or another solvent if necessary, stored at 4 °C for 30 min, filtered, washed with Et2O (3 × 1 mL) and dried in vacuo.
Supporting Information
| Supporting Information File 1: Experimental procedures, characterization data and copies of spectra. | ||
| Format: PDF | Size: 14.4 MB | Download |
References
-
Yoshimura, A.; Zhdankin, V. V. Chem. Rev. 2016, 116, 3328–3435. doi:10.1021/acs.chemrev.5b00547
Return to citation in text: [1] -
Wirth, T., Ed. Hypervalent Iodine Chemistry; Topics in Current Chemistry; Springer International Publishing: Cham, Switzerland, 2016. doi:10.1007/978-3-319-33733-3
Return to citation in text: [1] -
Boelke, A.; Finkbeiner, P.; Nachtsheim, B. J. Beilstein J. Org. Chem. 2018, 14, 1263–1280. doi:10.3762/bjoc.14.108
Return to citation in text: [1] -
Dohi, T.; Kita, Y. Chem. Commun. 2009, 2073–2085. doi:10.1039/b821747e
Return to citation in text: [1] -
Moriarty, R. M. J. Org. Chem. 2005, 70, 2893–2903. doi:10.1021/jo050117b
Return to citation in text: [1] -
Ochiai, M. Chem. Rec. 2007, 7, 12–23. doi:10.1002/tcr.20104
Return to citation in text: [1] -
Dohi, T.; Takenaga, N.; Goto, A.; Fujioka, H.; Kita, Y. J. Org. Chem. 2008, 73, 7365–7368. doi:10.1021/jo8012435
Return to citation in text: [1] -
Mizar, P.; Laverny, A.; El-Sherbini, M.; Farid, U.; Brown, M.; Malmedy, F.; Wirth, T. Chem. – Eur. J. 2014, 20, 9910–9913. doi:10.1002/chem.201403891
Return to citation in text: [1] -
Kürti, L.; Herczegh, P.; Visy, J.; Simonyi, M.; Antus, S.; Pelter, A. J. Chem. Soc., Perkin Trans. 1 1999, 379–380. doi:10.1039/a809206k
Return to citation in text: [1] -
Moriarty, R. M.; Prakash, O.; Duncan, M. P. J. Chem. Soc., Chem. Commun. 1985, 420. doi:10.1039/c39850000420
Return to citation in text: [1] -
Phipps, R. J.; Gaunt, M. J. Science 2009, 323, 1593–1597. doi:10.1126/science.1169975
Return to citation in text: [1] -
Ghosh, R.; Stridfeldt, E.; Olofsson, B. Chem. – Eur. J. 2014, 20, 8888–8892. doi:10.1002/chem.201403523
Return to citation in text: [1] -
Scattolin, T.; Bortolamiol, E.; Visentin, F.; Palazzolo, S.; Caligiuri, I.; Perin, T.; Canzonieri, V.; Demitri, N.; Rizzolio, F.; Togni, A. Chem. – Eur. J. 2020, 26, 11868–11876. doi:10.1002/chem.202002199
Return to citation in text: [1] -
Grushin, V. V. Chem. Soc. Rev. 2000, 29, 315–324. doi:10.1039/a909041j
Return to citation in text: [1] -
Chatterjee, N.; Goswami, A. Eur. J. Org. Chem. 2017, 3023–3032. doi:10.1002/ejoc.201601651
Return to citation in text: [1] -
Collette, J.; McGreer, D.; Crawford, R.; Chubb, F.; Sandin, R. B. J. Am. Chem. Soc. 1956, 78, 3819–3820. doi:10.1021/ja01596a070
Return to citation in text: [1] -
Zhu, D.; Wu, Z.; Liang, L.; Sun, Y.; Luo, B.; Huang, P.; Wen, S. RSC Adv. 2019, 9, 33170–33179. doi:10.1039/c9ra07288h
Return to citation in text: [1] -
Jiang, M.; Guo, J.; Liu, B.; Tan, Q.; Xu, B. Org. Lett. 2019, 21, 8328–8333. doi:10.1021/acs.orglett.9b03106
Return to citation in text: [1] -
Liu, Z.; Zhu, D.; Luo, B.; Zhang, N.; Liu, Q.; Hu, Y.; Pi, R.; Huang, P.; Wen, S. Org. Lett. 2014, 16, 5600–5603. doi:10.1021/ol502654a
Return to citation in text: [1] -
Zhu, K.; Xu, K.; Fang, Q.; Wang, Y.; Tang, B.; Zhang, F. ACS Catal. 2019, 9, 4951–4957. doi:10.1021/acscatal.9b00695
Return to citation in text: [1] -
Reddy Kandimalla, S.; Prathima Parvathaneni, S.; Sabitha, G.; Subba Reddy, B. V. Eur. J. Org. Chem. 2019, 1687–1714. doi:10.1002/ejoc.201801469
Return to citation in text: [1] -
Robidas, R.; Reinhard, D.; Huber, S. M.; Legault, C. ChemPhysChem 2023, 24, e2022006. doi:10.1002/cphc.202200634
Return to citation in text: [1] -
Heinen, F.; Reinhard, D. L.; Engelage, E.; Huber, S. M. Angew. Chem., Int. Ed. 2021, 60, 5069–5073. doi:10.1002/anie.202013172
Return to citation in text: [1] -
Batsanov, A. S.; Petrov, V. N.; Struchkov, Y. T.; Egorova, L. D.; Lisichkina, I. N.; Tolstaya, T. P. Bull. Acad. Sci. USSR, Div. Chem. Sci. (Engl. Transl.) 1985, 34, 2138–2143. doi:10.1007/bf00963250
Return to citation in text: [1] -
Tolstaya, T. P.; Egorova, L. D.; Lisichkina, I. N. Chem. Heterocycl. Compd. 1985, 21, 392–396. doi:10.1007/bf00504396
Return to citation in text: [1] -
Boelke, A.; Lork, E.; Nachtsheim, B. J. Chem. – Eur. J. 2018, 24, 18653–18657. doi:10.1002/chem.201804957
Return to citation in text: [1] [2] -
Boelke, A.; Kuczmera, T. J.; Caspers, L. D.; Lork, E.; Nachtsheim, B. J. Org. Lett. 2020, 22, 7261–7266. doi:10.1021/acs.orglett.0c02593
Return to citation in text: [1] [2] [3] -
Boelke, A.; Nachtsheim, B. J. Adv. Synth. Catal. 2020, 362, 184–191. doi:10.1002/adsc.201901356
Return to citation in text: [1] [2] -
Boelke, A.; Kuczmera, T. J.; Lork, E.; Nachtsheim, B. J. Chem. – Eur. J. 2021, 27, 13128–13134. doi:10.1002/chem.202101961
Return to citation in text: [1] [2] -
Boelke, A.; Sadat, S.; Lork, E.; Nachtsheim, B. J. Chem. Commun. 2021, 57, 7434–7437. doi:10.1039/d1cc03097c
Return to citation in text: [1] [2] -
Kuczmera, T. J.; Boelke, A.; Nachtsheim, B. J. Eur. J. Org. Chem. 2022, 28–32. doi:10.1002/ejoc.202200276
Return to citation in text: [1] -
Damrath, M.; Caspers, L. D.; Duvinage, D.; Nachtsheim, B. J. Org. Lett. 2022, 24, 2562–2566. doi:10.1021/acs.orglett.2c00691
Return to citation in text: [1] [2] -
Caspers, L. D.; Spils, J.; Damrath, M.; Lork, E.; Nachtsheim, B. J. J. Org. Chem. 2020, 85, 9161–9178. doi:10.1021/acs.joc.0c01125
Return to citation in text: [1] -
Guilbault, A.-A.; Basdevant, B.; Wanie, V.; Legault, C. Y. J. Org. Chem. 2012, 77, 11283–11295. doi:10.1021/jo302393u
Return to citation in text: [1] -
Batsanov, S. S. Inorg. Mater. 2001, 37, 871–885. doi:10.1023/a:1011625728803
Return to citation in text: [1] -
Heinen, F.; Engelage, E.; Dreger, A.; Weiss, R.; Huber, S. M. Angew. Chem., Int. Ed. 2018, 57, 3830–3833. doi:10.1002/anie.201713012
Return to citation in text: [1] -
Hu, Z.; Tang, Y.; Yu, B. J. Am. Chem. Soc. 2019, 141, 4806–4810. doi:10.1021/jacs.9b00210
Return to citation in text: [1] -
Wang, M.; Fan, Q.; Jiang, X. Org. Lett. 2016, 18, 5756–5759. doi:10.1021/acs.orglett.6b03078
Return to citation in text: [1] -
Zhu, D.; Liu, Q.; Luo, B.; Chen, M.; Pi, R.; Huang, P.; Wen, S. Adv. Synth. Catal. 2013, 355, 2172–2178. doi:10.1002/adsc.201300271
Return to citation in text: [1] -
Zhu, L.; Guo, P.; Li, G.; Lan, J.; Xie, R.; You, J. J. Org. Chem. 2007, 72, 8535–8538. doi:10.1021/jo0712289
Return to citation in text: [1] -
Zhu, D.; Chen, M.; Li, M.; Luo, B.; Zhao, Y.; Huang, P.; Xue, F.; Rapposelli, S.; Pi, R.; Wen, S. Eur. J. Med. Chem. 2013, 68, 81–88. doi:10.1016/j.ejmech.2013.07.029
Return to citation in text: [1] -
Wu, B.; Yoshikai, N. Angew. Chem., Int. Ed. 2015, 54, 8736–8739. doi:10.1002/anie.201503134
Return to citation in text: [1] -
Wonner, P.; Vogel, L.; Düser, M.; Gomes, L.; Kniep, F.; Mallick, B.; Werz, D. B.; Huber, S. M. Angew. Chem., Int. Ed. 2017, 56, 12009–12012. doi:10.1002/anie.201704816
Return to citation in text: [1] -
Lima, H. M.; Lovely, C. J. Org. Lett. 2011, 13, 5736–5739. doi:10.1021/ol2022438
Return to citation in text: [1] [2] -
Das, R.; Banerjee, M.; Rai, R. K.; Karri, R.; Roy, G. Org. Biomol. Chem. 2018, 16, 4243–4260. doi:10.1039/c8ob00535d
Return to citation in text: [1] -
Prasher, P.; Sharma, M. Drug Dev. Res. 2022, 83, 296–300. doi:10.1002/ddr.21933
Return to citation in text: [1] -
Aguayo-Ortiz, R.; Cano-González, L.; Castillo, R.; Hernández-Campos, A.; Dominguez, L. Chem. Biol. Drug Des. 2017, 90, 40–51. doi:10.1111/cbdd.12926
Return to citation in text: [1]
| 1. | Yoshimura, A.; Zhdankin, V. V. Chem. Rev. 2016, 116, 3328–3435. doi:10.1021/acs.chemrev.5b00547 |
| 2. | Wirth, T., Ed. Hypervalent Iodine Chemistry; Topics in Current Chemistry; Springer International Publishing: Cham, Switzerland, 2016. doi:10.1007/978-3-319-33733-3 |
| 3. | Boelke, A.; Finkbeiner, P.; Nachtsheim, B. J. Beilstein J. Org. Chem. 2018, 14, 1263–1280. doi:10.3762/bjoc.14.108 |
| 4. | Dohi, T.; Kita, Y. Chem. Commun. 2009, 2073–2085. doi:10.1039/b821747e |
| 5. | Moriarty, R. M. J. Org. Chem. 2005, 70, 2893–2903. doi:10.1021/jo050117b |
| 11. | Phipps, R. J.; Gaunt, M. J. Science 2009, 323, 1593–1597. doi:10.1126/science.1169975 |
| 12. | Ghosh, R.; Stridfeldt, E.; Olofsson, B. Chem. – Eur. J. 2014, 20, 8888–8892. doi:10.1002/chem.201403523 |
| 13. | Scattolin, T.; Bortolamiol, E.; Visentin, F.; Palazzolo, S.; Caligiuri, I.; Perin, T.; Canzonieri, V.; Demitri, N.; Rizzolio, F.; Togni, A. Chem. – Eur. J. 2020, 26, 11868–11876. doi:10.1002/chem.202002199 |
| 14. | Grushin, V. V. Chem. Soc. Rev. 2000, 29, 315–324. doi:10.1039/a909041j |
| 15. | Chatterjee, N.; Goswami, A. Eur. J. Org. Chem. 2017, 3023–3032. doi:10.1002/ejoc.201601651 |
| 16. | Collette, J.; McGreer, D.; Crawford, R.; Chubb, F.; Sandin, R. B. J. Am. Chem. Soc. 1956, 78, 3819–3820. doi:10.1021/ja01596a070 |
| 10. | Moriarty, R. M.; Prakash, O.; Duncan, M. P. J. Chem. Soc., Chem. Commun. 1985, 420. doi:10.1039/c39850000420 |
| 30. | Boelke, A.; Sadat, S.; Lork, E.; Nachtsheim, B. J. Chem. Commun. 2021, 57, 7434–7437. doi:10.1039/d1cc03097c |
| 32. | Damrath, M.; Caspers, L. D.; Duvinage, D.; Nachtsheim, B. J. Org. Lett. 2022, 24, 2562–2566. doi:10.1021/acs.orglett.2c00691 |
| 9. | Kürti, L.; Herczegh, P.; Visy, J.; Simonyi, M.; Antus, S.; Pelter, A. J. Chem. Soc., Perkin Trans. 1 1999, 379–380. doi:10.1039/a809206k |
| 26. | Boelke, A.; Lork, E.; Nachtsheim, B. J. Chem. – Eur. J. 2018, 24, 18653–18657. doi:10.1002/chem.201804957 |
| 6. | Ochiai, M. Chem. Rec. 2007, 7, 12–23. doi:10.1002/tcr.20104 |
| 7. | Dohi, T.; Takenaga, N.; Goto, A.; Fujioka, H.; Kita, Y. J. Org. Chem. 2008, 73, 7365–7368. doi:10.1021/jo8012435 |
| 8. | Mizar, P.; Laverny, A.; El-Sherbini, M.; Farid, U.; Brown, M.; Malmedy, F.; Wirth, T. Chem. – Eur. J. 2014, 20, 9910–9913. doi:10.1002/chem.201403891 |
| 28. | Boelke, A.; Nachtsheim, B. J. Adv. Synth. Catal. 2020, 362, 184–191. doi:10.1002/adsc.201901356 |
| 34. | Guilbault, A.-A.; Basdevant, B.; Wanie, V.; Legault, C. Y. J. Org. Chem. 2012, 77, 11283–11295. doi:10.1021/jo302393u |
| 24. | Batsanov, A. S.; Petrov, V. N.; Struchkov, Y. T.; Egorova, L. D.; Lisichkina, I. N.; Tolstaya, T. P. Bull. Acad. Sci. USSR, Div. Chem. Sci. (Engl. Transl.) 1985, 34, 2138–2143. doi:10.1007/bf00963250 |
| 25. | Tolstaya, T. P.; Egorova, L. D.; Lisichkina, I. N. Chem. Heterocycl. Compd. 1985, 21, 392–396. doi:10.1007/bf00504396 |
| 32. | Damrath, M.; Caspers, L. D.; Duvinage, D.; Nachtsheim, B. J. Org. Lett. 2022, 24, 2562–2566. doi:10.1021/acs.orglett.2c00691 |
| 33. | Caspers, L. D.; Spils, J.; Damrath, M.; Lork, E.; Nachtsheim, B. J. J. Org. Chem. 2020, 85, 9161–9178. doi:10.1021/acs.joc.0c01125 |
| 23. | Heinen, F.; Reinhard, D. L.; Engelage, E.; Huber, S. M. Angew. Chem., Int. Ed. 2021, 60, 5069–5073. doi:10.1002/anie.202013172 |
| 27. | Boelke, A.; Kuczmera, T. J.; Caspers, L. D.; Lork, E.; Nachtsheim, B. J. Org. Lett. 2020, 22, 7261–7266. doi:10.1021/acs.orglett.0c02593 |
| 29. | Boelke, A.; Kuczmera, T. J.; Lork, E.; Nachtsheim, B. J. Chem. – Eur. J. 2021, 27, 13128–13134. doi:10.1002/chem.202101961 |
| 22. | Robidas, R.; Reinhard, D.; Huber, S. M.; Legault, C. ChemPhysChem 2023, 24, e2022006. doi:10.1002/cphc.202200634 |
| 17. | Zhu, D.; Wu, Z.; Liang, L.; Sun, Y.; Luo, B.; Huang, P.; Wen, S. RSC Adv. 2019, 9, 33170–33179. doi:10.1039/c9ra07288h |
| 18. | Jiang, M.; Guo, J.; Liu, B.; Tan, Q.; Xu, B. Org. Lett. 2019, 21, 8328–8333. doi:10.1021/acs.orglett.9b03106 |
| 19. | Liu, Z.; Zhu, D.; Luo, B.; Zhang, N.; Liu, Q.; Hu, Y.; Pi, R.; Huang, P.; Wen, S. Org. Lett. 2014, 16, 5600–5603. doi:10.1021/ol502654a |
| 20. | Zhu, K.; Xu, K.; Fang, Q.; Wang, Y.; Tang, B.; Zhang, F. ACS Catal. 2019, 9, 4951–4957. doi:10.1021/acscatal.9b00695 |
| 21. | Reddy Kandimalla, S.; Prathima Parvathaneni, S.; Sabitha, G.; Subba Reddy, B. V. Eur. J. Org. Chem. 2019, 1687–1714. doi:10.1002/ejoc.201801469 |
| 26. | Boelke, A.; Lork, E.; Nachtsheim, B. J. Chem. – Eur. J. 2018, 24, 18653–18657. doi:10.1002/chem.201804957 |
| 27. | Boelke, A.; Kuczmera, T. J.; Caspers, L. D.; Lork, E.; Nachtsheim, B. J. Org. Lett. 2020, 22, 7261–7266. doi:10.1021/acs.orglett.0c02593 |
| 28. | Boelke, A.; Nachtsheim, B. J. Adv. Synth. Catal. 2020, 362, 184–191. doi:10.1002/adsc.201901356 |
| 29. | Boelke, A.; Kuczmera, T. J.; Lork, E.; Nachtsheim, B. J. Chem. – Eur. J. 2021, 27, 13128–13134. doi:10.1002/chem.202101961 |
| 30. | Boelke, A.; Sadat, S.; Lork, E.; Nachtsheim, B. J. Chem. Commun. 2021, 57, 7434–7437. doi:10.1039/d1cc03097c |
| 31. | Kuczmera, T. J.; Boelke, A.; Nachtsheim, B. J. Eur. J. Org. Chem. 2022, 28–32. doi:10.1002/ejoc.202200276 |
| 38. | Wang, M.; Fan, Q.; Jiang, X. Org. Lett. 2016, 18, 5756–5759. doi:10.1021/acs.orglett.6b03078 |
| 36. | Heinen, F.; Engelage, E.; Dreger, A.; Weiss, R.; Huber, S. M. Angew. Chem., Int. Ed. 2018, 57, 3830–3833. doi:10.1002/anie.201713012 |
| 37. | Hu, Z.; Tang, Y.; Yu, B. J. Am. Chem. Soc. 2019, 141, 4806–4810. doi:10.1021/jacs.9b00210 |
| 44. | Lima, H. M.; Lovely, C. J. Org. Lett. 2011, 13, 5736–5739. doi:10.1021/ol2022438 |
| 46. | Prasher, P.; Sharma, M. Drug Dev. Res. 2022, 83, 296–300. doi:10.1002/ddr.21933 |
| 47. | Aguayo-Ortiz, R.; Cano-González, L.; Castillo, R.; Hernández-Campos, A.; Dominguez, L. Chem. Biol. Drug Des. 2017, 90, 40–51. doi:10.1111/cbdd.12926 |
| 43. | Wonner, P.; Vogel, L.; Düser, M.; Gomes, L.; Kniep, F.; Mallick, B.; Werz, D. B.; Huber, S. M. Angew. Chem., Int. Ed. 2017, 56, 12009–12012. doi:10.1002/anie.201704816 |
| 44. | Lima, H. M.; Lovely, C. J. Org. Lett. 2011, 13, 5736–5739. doi:10.1021/ol2022438 |
| 45. | Das, R.; Banerjee, M.; Rai, R. K.; Karri, R.; Roy, G. Org. Biomol. Chem. 2018, 16, 4243–4260. doi:10.1039/c8ob00535d |
| 27. | Boelke, A.; Kuczmera, T. J.; Caspers, L. D.; Lork, E.; Nachtsheim, B. J. Org. Lett. 2020, 22, 7261–7266. doi:10.1021/acs.orglett.0c02593 |
| 42. | Wu, B.; Yoshikai, N. Angew. Chem., Int. Ed. 2015, 54, 8736–8739. doi:10.1002/anie.201503134 |
| 39. | Zhu, D.; Liu, Q.; Luo, B.; Chen, M.; Pi, R.; Huang, P.; Wen, S. Adv. Synth. Catal. 2013, 355, 2172–2178. doi:10.1002/adsc.201300271 |
| 40. | Zhu, L.; Guo, P.; Li, G.; Lan, J.; Xie, R.; You, J. J. Org. Chem. 2007, 72, 8535–8538. doi:10.1021/jo0712289 |
| 41. | Zhu, D.; Chen, M.; Li, M.; Luo, B.; Zhao, Y.; Huang, P.; Xue, F.; Rapposelli, S.; Pi, R.; Wen, S. Eur. J. Med. Chem. 2013, 68, 81–88. doi:10.1016/j.ejmech.2013.07.029 |
© 2023 Kuczmera et al.; licensee Beilstein-Institut.
This is an open access article licensed under the terms of the Beilstein-Institut Open Access License Agreement (https://www.beilstein-journals.org/bjoc/terms), which is identical to the Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0). The reuse of material under this license requires that the author(s), source and license are credited. Third-party material in this article could be subject to other licenses (typically indicated in the credit line), and in this case, users are required to obtain permission from the license holder to reuse the material.