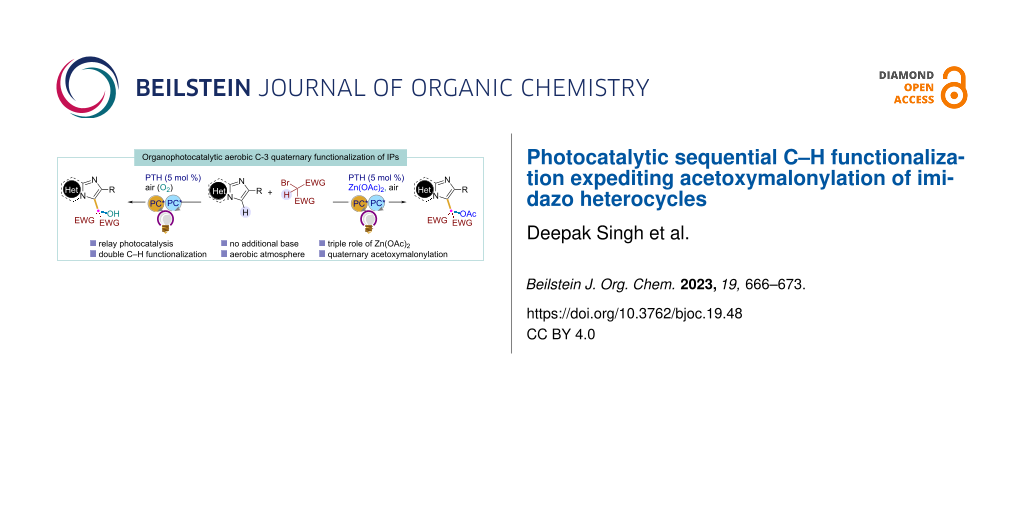
Abstract
The importance of functionalized imidazo heterocycles has often been featured in several impactful research both from academia and industry. Herein, we report a direct C-3 acetoxymalonylation of imidazo heterocycles using relay C–H functionalization enabled by organophotocatalysis starring zinc acetate in the triple role of an activator, ion scavenger as well as an acetylating reagent. The mechanistic investigation revealed a sequential sp2 and sp3 C–H activation, followed by functionalization driven by zinc acetate coupled with the photocatalyst PTH. A variety of imidazo[1,2-a]pyridines and related heterocycles were explored as substrates along with several active methylene reagents, all generating the products with excellent yields and regioselectivity, thus confirming excellent functional group tolerability.
Graphical Abstract
Introduction
Among all N-fused heterocycles, imidazo[1,2-a]pyridines (IPs) are the prevalent moieties in several bioactive pharmaceuticals and natural products [1-4]. Moreover, due to their susceptibility towards 'exited-state intramolecular proton transfer' phenomena, IPs are also effective in optoelectronics and materials sciences [5,6]. C-3-functionalized imidazo[1,2-a]pyridines are particularly familiar due to their biological and medicinal attributes [7-11]. Not surprisingly, the C-3 functionalization of IPs is a continuing interest of research in the synthetic community [12-16].
Despite many successful strategies in this field, the regioselective C–H functionalization is still challenging for chemists to combine a C(sp3) carbon of incoming functionalities and C(sp2) carbon of the IP core. The direct C-3 alkylation of imidazopyridines using active malonates and related moieties has been achieved by different routes [17-20]. However, these reactions rely either on harsh reaction conditions or require the preactivation of substrates, which limits their synthetic efficiency. A photocatalytic quaternary C-3 alkylation has also been reported recently (Scheme 1A) [21,22]. During the course of our study, the Wu group reported a solvent-controlled chemodivergent formation of C-3 ethoxycarbonylmethylated and hydroxyalkylated IPs under visible light using water or alcohol as the source of the oxygenated group under degassed conditions [22]. However, all these photochemical methods require the usage of a substantial amount of base, the preactivation with a boron complex (B2pin2), and using an expensive metal-based photocatalyst [fac-Ir(ppy)3] under inert atmosphere. We have recently demonstrated that aerial oxygen could be captured by alkyl radicals to install a keto-functionality onto alkenes in an organophotocatalytic way [23]. We aimed to extend this aerobic oxygenation approach to imidazo heterocycles II to install the hydroxymalonate unit onto I through sequential photoredox C–H functionalization.
Scheme 1: Strategies of C-3 functionalizations of IPs and present work.
Scheme 1: Strategies of C-3 functionalizations of IPs and present work.
Till date, there is no report of the direct incorporation of a quaternary hydroxyalkyl, specifically a hydroxymalonyl group at the C-3 position of IPs using air as the sole oxygen source.
Keeping in mind the progress in photochemical relay catalysis [24] and the attention paid to photocatalytic carbon-bond functionalization in the past several years [25], here we developed an organophotoredox-catalyzed C–H functionalization of imidazo[1,2-a]pyridines and related heterocycles with active bromomethylenes under mild conditions (Scheme 1B). Importantly, using simple Zn(OAc)2 as the additive, the first photocatalytic direct acetoxymalonylation of imidazo heterocycles was developed under aerobic conditions. Here, the additive Zn(OAc)2 plays a crucial triple role as activator of IPs, halide scavenger, and acetylating agent.
Results and Discussion
Optimization
In the quest for the optimal reaction conditions, we started our investigations with 2-phenylimidazo[1,2-a]pyridine (1a) and diethyl bromomalonate (2a) as model substrates. Initially, the reaction was carried out between 1a and 2a in dry CH3CN as solvent under N2 atmosphere using 4CzIPN as the photocatalyst. Irradiating the reaction mixture for 10 h under blue LEDs (450 nm) led to the isolation of products 5 (54%) and 6 (28%) (Table 1, entry 1). However, the same reaction, under aerobic conditions, delivered compounds 3a (47%) and 6 (22%) (Table 1, entry 2). Keeping in mind the ability of Zn(OAc)2 as a bromide ion scavenger [26], we used Zn(OAc)2 (2 equiv) as an additive to prevent the formation of the bromo product 6. While the additive successfully prevented the formation of compound 6, we were delighted to isolate the unexpected acetylated product 4a with a promising yield of 38% (Table 1, entry 3), reflecting the ability of Zn(OAc)2 to act as an acetylating agent. While screening other organophotocatalysts, we detected no desired product 4a (Table 1, entries 4–6) [27], except for photocatalyst 10-phenylphenothiazine (PTH) under violet LEDs which uplifted the yield up to 52% (Table 1, entry 7). Now with the optimal catalyst in hand, we screened some common solvents, out of which 1,2-DCE positively impacted the yield (Table 1, entries 8–11). However, the best result was obtained when 3.0 equiv of Zn(OAc)2 was used as an additive (Table 1, entry 12). To check the viability of other acetylating agents, Zn(OAc)2 was replaced with AcOH, generating the desired product in a comparatively lower yield (Table 1, entry 13). Finally, control experiments without a catalyst (Table 1, entry 14), light (entry 15) or acetylation agent (entry 16) failed to provide the desired product 4a, displaying the necessity of each component for developing the reaction.
Table 1: Reaction optimization.a
|
|
||||
| Entry | Catalyst | Solvent | Additive |
Yield (%)b
3a:4a:5:6 |
| 1c | 4-CzIPN | CH3CN | – | 0:0:54:28 |
| 2 | 4-CzIPN | CH3CN | – | 47:0:0:22 |
| 3 | 4-CzIPN | CH3CN | Zn(OAc)2 | 0:38:0:0 |
| 4 | Rose Bengal | CH3CN | Zn(OAc)2 | – |
| 5 | eosin-Y | CH3CN | Zn(OAc)2 | – |
| 6 | rhodamine-B | CH3CN | Zn(OAc)2 | – |
| 7d | PTH | CH3CN | Zn(OAc)2 | 0:52:0:0 |
| 8 | PTH | 1,4-dioxane | Zn(OAc)2 | 0:34:0:0 |
| 9 | PTH | DMF | Zn(OAc)2 | 0:25:0:0 |
| 10 | PTH | toluene | Zn(OAc)2 | 0:18:0:0 |
| 11 | PTH | 1,2-DCE | Zn(OAc)2 | 0:70:0:0 |
| 12e | PTH | 1,2-DCE | Zn(OAc)2 | 0:94:0:0 |
| 13 | PTH | 1,2-DCE | AcOH | 0:64:0:0 |
| 14 | – | 1,2-DCE | Zn(OAc)2 | – |
| 15f | PTH | 1,2-DCE | Zn(OAc)2 | – |
| 16 | PTH | 1,2-DCE | – | 57:0:0:24 |
aReaction conditions: 1a (0.2 mmol), 2a (0.4 mmol), catalyst (5 mol %), additive (0.4 mmol) in dry solvent (2 mL) under aerobic conditions, irradiation with 12 W blue LEDs for 10 h. bIsolated yield. cUnder N2 atmosphere. dIrradiation with violet LEDs (λmax = 390 nm). e3.0 equiv of zinc acetate used. fIn the dark, without light source.
Substrate scope
With suitable reaction conditions (Table 1, entry 12), we systematically investigated the scope of this acetoxymalonylation strategy with substrate 2a (Scheme 2). Several imidazo[1,2-a]pyridines with diverse aryl substituents in the C-2 position were acetoxymalonylated, providing the desired products 4a–k regioselectively in good to excellent yields. Reflection of electronic properties was shown by the substituents attached to the aryl ring – as electron-releasing groups (Me, OMe) showed little more reactivity than electron-withdrawing groups (CN) at the same position (4b, 4f, and 4g). Halogen-substituted IPs also followed the general reactivity trend of the respective halogens (4c–e). Excellent reactivity was found for o-F and m-Br-substituted IPs (4h and 4i). Similarly, IPs associated with biphenyl and naphthyl groups in the C-2 position were also suitable substrates giving the corresponding products 4j and 4k in 77% and 82% yield, respectively. However, the yield of the products varied when different groups with diverse electronic properties were present in the pyridine ring of the IP moieties (4l–q). With substrates having a methyl substitution at C-7 and C-8 of the pyridine ring, the yields and regioselectivity were still excellent (4l and 4m), but reduced significantly upon introducing a halogen group onto the pyridine ring. Except for the 6-bromo-substituted compound (4o), all other substrates having a halogen substituent in the pyridine ring showed reduced yields (4n, and 4p,q). The number of substituents also seemed to negatively affect the yield, as observed for products 4p and 4q, featuring two substituents each on the pyridine ring. Moreover, IPs with a non-aromatic C-2 substituent like an ester group were also included (4r). We also explored bromo analogues of other active methylenes such as ethyl cyanoacetate, ethyl acetoacetate, dimethyl, and diisopropyl malonates, as extension of diethyl malonate (4s,t and 4x,y). Lastly, we explored a few heterocycles that resemble imidazo[1,2-a]pyridine to vindicate the generality of this method. Gratifyingly, 6-phenylimidazo[2,1-b]thiazole, 2-phenylbenzo[d]imidazo[2,1-b]thiazole, and 2-phenylimidazo[1,2-a]pyrimidine participated well under the standard reaction conditions, generating the corresponding acetoxymalonylated products 4u–w in good to excellent yields.
Scheme 2: Substrate scope. Conditions: unless otherwise noted, all reactions were carried out with 1 (0.2 mmol), 2 (0.4 mmol), PTH (5 mol %), Zn(OAc)2 (0.6 mmol), dry 1,2-DCE (2 mL), irradiation with LEDs (λmax = 390 nm), under air for 10 h.
Scheme 2: Substrate scope. Conditions: unless otherwise noted, all reactions were carried out with 1 (0.2 mmo...
Several control experiments were performed to gain insights into the mechanistic pathway of this reaction. Firstly, a radical scavenging experiment using the radical scavenger TEMPO was performed (Scheme 3A). Upon analyzing the reaction mixture of 1a and 2a under standard conditions in the presence of TEMPO, we found only a trace of the desired product 4a. At the same time, a TEMPO-DEM adduct 7 and TEMPO-OAc adduct 8 were identified by the HRMS analysis of the crude reaction mixture, indicating the involvement of a malonyl radical and an acetyl radical in the course of the reaction (see Supporting Information File 1 for details). Additionally, when an aliphatic alkene, 5-hexen-1-ol was introduced into the reaction mixture under standard conditions without Zn(OAc)2, an ATRA product 9 was isolated, further confirming the involvement of a malonyl radical generated by the cleavage of the C–Br bond of 2a [28]. Next, an attempt was made to identify the key intermediate of the reaction (Scheme 3B). When compound 5 was subjected to the acetylation reaction individually with Zn(OAc)2 and AcOH under optimized reaction conditions, the acetylated product 4a was produced with excellent conversion (>90%). These results suggest the involvement of compound 5 as an intermediate, and Zn(OAc)2 or AcOH may be effective acetylating agents via generation of acetyl radicals. Control experiments under degassed conditions with or without water only delivered a trace amount (<5%) of the desired products, indicating that aerial oxygen plays a crucial role in the second catalytic cycle for the conversion of 5 to 3a or 4a (Scheme 3C). To determine the role of zinc acetate, a standard reaction of 1a and 2a in the absence of Zn(OAc)2 was conducted (Scheme 3D). The results showed the formation of hydroxymalonated product 3a (57%) and bromo derivative 6 (24%). Notably, the hydroxymalonated product 3a under the reaction conditions was not converted to the acetylated derivative 4a, confirming 3a is not the intermediate for the final product 4a. So, Zn(OAc)2 is crucial in shutting down the formation of 6 by scavenging free bromide in the reaction as ZnBr2 salt (confirmed by HRMS). In addition, an excellent yield of the final product 4a [4a: 94% vs (6, 24% + 3a, 57%)] with additive indicates that zinc acetate plays a crucial role in activating IP towards the photoredox coupling reaction. Shifting of protons in the 1H NMR spectrum of 2-phenylimidazo[1,2-a]pyridine (1a) in the presence of Zn(OAc)2 in CDCl3 indicates a weak interaction of Zn(OAc)2 with 1a (see Supporting Information File 1 for details) [20,21]. Finally, the reaction of 5 with benzoic acid and zinc acetate (in a 1:1 ratio) under standard reaction protocol resulted in the competitive formation of products 4a and 10 (Scheme 3E), indicating the susceptibility of other acids towards this method. These results, along with the Stern–Volmer fluorescence quenching study (Scheme 3F), expressed that the photoredox reaction started with the reductive generation of a malonyl radical from bromomalonate by interaction with the photocatalyst.
Analyzing all the observations from the above mechanistic studies, we propose a plausible mechanism involving sequential activation and functionalization of sp2 and sp3 C–H bonds via relay catalysis (Scheme 4). The relay can be divided into two cycles; the first cycle (cycle-1) deals with the C(sp2)–H functionalization at the C-3 position of the imidazo heterocycles, while the second cycle (cycle-2) is all about the C(sp3)–H functionalization at the newly incorporated active methylene center.
Cycle-1 is initiated with the reduction of bromomalonate 2a by the photoexcited catalyst PC* to malonyl radical I. This is followed by the Minisci-type addition of radical I to the imidazopyridine, preactivated by Lewis acidic Zn(OAc)2 [29]. PC∙+ then oxidizes the resulting radical II to carbocation III which rearomatizes by losing a proton to generate the intermediate IV and closing the first catalytic cycle. Meanwhile, the bromide ions in the medium undergo anion exchange with the Zn(OAc)2 to release free acetate ions, along with the conversion into ZnBr2 (confirmed by HRMS). These in situ-generated free acetate ions function as a base, deprotonating carbocation III to produce the intermediate IV and AcOH.
The first step of cycle-2 involves the oxidation of the excited photocatalyst by aerial oxygen to generate superoxide anion and PC∙+. The superoxide anion (O2·−) then captures the proton from the active methylene center of intermediate IV to generate the malonyl anion V, which undergoes single electron oxidation by PC∙+ generating the malonyl radical VI [30,31]. Meanwhile, the hydroperoxy radical (∙OOH) formed, reacts with AcOH produced in cycle-1 to give the acetoxy radical (∙OAc) and H2O2. Then, the radical recombination between AcO∙ and radical VI furnishes the desired product 4. In the absence of the acetoxy radical (∙OAc), the hydroperoxy radical (∙OOH) may combine with radical VI to produce VII, which then easily converts into hydroxymalonated product 3 [31].
Conclusion
Thus, we have reported the successful C-3 acetoxymalonylation of imidazo[1,2-a]pyridines and related heterocycles by an organophotocatalytic relay C–H functionalization strategy with Zn(OAc)2 in the triple role of an activator, bromide scavenger, and acetylating agent. The developed method is heavy-metal free, as shown by the use of inexpensive PTH, as well as a base-free approach, and involves aerial oxygen to generate exciting derivatives, which may prove to be valuable in the field of radical chemistry research in future.
Supporting Information
| Supporting Information File 1: Experimental section and characterization of synthesized compounds. | ||
| Format: PDF | Size: 3.8 MB | Download |
References
-
Enguehard-Gueiffier, C.; Gueiffier, A. Mini-Rev. Med. Chem. 2007, 7, 888–899. doi:10.2174/138955707781662645
Return to citation in text: [1] -
Devi, N.; Singh, D.; Rawal, R. K.; Bariwal, J.; Singh, V. Curr. Top. Med. Chem. 2016, 16, 2963–2994. doi:10.2174/1568026616666160506145539
Return to citation in text: [1] -
Kishbaugh, T. L. S. Curr. Top. Med. Chem. 2016, 16, 3274–3302. doi:10.2174/1568026616666160506145141
Return to citation in text: [1] -
Sanapalli, B. K. R.; Ashames, A.; Sigalapalli, D. K.; Shaik, A. B.; Bhandare, R. R.; Yele, V. Antibiotics (Basel, Switz.) 2022, 11, 1680. doi:10.3390/antibiotics11121680
Return to citation in text: [1] -
Mutai, T.; Tomoda, H.; Ohkawa, T.; Yabe, Y.; Araki, K. Angew. Chem., Int. Ed. 2008, 47, 9522–9524. doi:10.1002/anie.200803975
Return to citation in text: [1] -
Padalkar, V. S.; Seki, S. Chem. Soc. Rev. 2016, 45, 169–202. doi:10.1039/c5cs00543d
Return to citation in text: [1] -
Deep, A.; Bhatia, R. K.; Kaur, R.; Kumar, S.; Jain, U. K.; Singh, H.; Batra, S.; Kaushik, D.; Deb, P. K. Curr. Top. Med. Chem. 2016, 17, 238–250. doi:10.2174/1568026616666160530153233
Return to citation in text: [1] -
Goel, R.; Luxami, V.; Paul, K. Curr. Top. Med. Chem. 2016, 16, 3590–3616. doi:10.2174/1568026616666160414122644
Return to citation in text: [1] -
Xi, J.-B.; Fang, Y.-F.; Frett, B.; Zhu, M.-L.; Zhu, T.; Kong, Y.-N.; Guan, F.-J.; Zhao, Y.; Zhang, X.-W.; Li, H.-y.; Ma, M.-L.; Hu, W. Eur. J. Med. Chem. 2017, 126, 1083–1106. doi:10.1016/j.ejmech.2016.12.026
Return to citation in text: [1] -
Shukla, N. M.; Salunke, D. B.; Yoo, E.; Mutz, C. A.; Balakrishna, R.; David, S. A. Bioorg. Med. Chem. 2012, 20, 5850–5863. doi:10.1016/j.bmc.2012.07.052
Return to citation in text: [1] -
Chernyak, N.; Gevorgyan, V. Angew. Chem., Int. Ed. 2010, 49, 2743–2746. doi:10.1002/anie.200907291
Return to citation in text: [1] -
Patel, O. P. S.; Nandwana, N. K.; Legoabe, L. J.; Das, B. C.; Kumar, A. Adv. Synth. Catal. 2020, 362, 4226–4255. doi:10.1002/adsc.202000633
Return to citation in text: [1] -
Ghosh, D.; Ghosh, S.; Hajra, A. Adv. Synth. Catal. 2021, 363, 5047–5071. doi:10.1002/adsc.202100981
Return to citation in text: [1] -
Tran, C.; Hamze, A. Molecules 2022, 27, 3461. doi:10.3390/molecules27113461
Return to citation in text: [1] -
Ravi, C.; Chandra Mohan, D.; Adimurthy, S. Org. Biomol. Chem. 2016, 14, 2282–2290. doi:10.1039/c5ob02475g
Return to citation in text: [1] -
Tashrifi, Z.; Mohammadi-Khanaposhtani, M.; Larijani, B.; Mahdavi, M. Eur. J. Org. Chem. 2020, 269–284. doi:10.1002/ejoc.201901491
Return to citation in text: [1] -
Li, K.; Zhao, X.-M.; Yang, F.-L.; Hou, X.-H.; Xu, Y.; Guo, Y.-C.; Hao, X.-Q.; Song, M.-P. RSC Adv. 2015, 5, 90478–90481. doi:10.1039/c5ra15678e
Return to citation in text: [1] -
Reddy, K. N.; Chary, D. Y.; Sridhar, B.; Reddy, B. V. S. Org. Lett. 2019, 21, 8548–8552. doi:10.1021/acs.orglett.9b03041
Return to citation in text: [1] -
Xiao, Y.; Yu, L.; Yu, Y.; Tan, Z.; Deng, W. Tetrahedron Lett. 2020, 61, 152606. doi:10.1016/j.tetlet.2020.152606
Return to citation in text: [1] -
Chaubey, N. R.; Kapdi, A. R.; Maity, B. Synthesis 2021, 53, 1524–1530. doi:10.1055/s-0040-1706103
Return to citation in text: [1] [2] -
Singsardar, M.; Mondal, S.; Laru, S.; Hajra, A. Org. Lett. 2019, 21, 5606–5610. doi:10.1021/acs.orglett.9b01954
Return to citation in text: [1] [2] -
Huang, M.; Wang, L.; Yang, X.; Kim, J. K.; Gong, M.; Zhang, J.; Li, Y.; Wu, Y. Tetrahedron 2022, 126, 132988. doi:10.1016/j.tet.2022.132988
Return to citation in text: [1] [2] -
Roy Chowdhury, S.; Singh, D.; Hoque, I. U.; Maity, S. J. Org. Chem. 2020, 85, 13939–13950. doi:10.1021/acs.joc.0c01985
Return to citation in text: [1] -
Sakakibara, Y.; Murakami, K. ACS Catal. 2022, 12, 1857–1878. doi:10.1021/acscatal.1c05318
Return to citation in text: [1] -
Holmberg-Douglas, N.; Nicewicz, D. A. Chem. Rev. 2022, 122, 1925–2016. doi:10.1021/acs.chemrev.1c00311
Return to citation in text: [1] -
Samanta, A.; Pramanik, S.; Mondal, S.; Maity, S. Chem. Commun. 2022, 58, 8400–8403. doi:10.1039/d2cc02574d
Return to citation in text: [1] -
Ritu; Sharma, C.; Kumar, S.; Jain, N. Org. Biomol. Chem. 2020, 18, 2921–2928. doi:10.1039/d0ob00563k
With these catalytic systems (entries 4–6) no desired products (3a, 4a, 5, 6) were identified; instead a new product N-(pyridin-2-yl)benzamide was isolated in 8–14% yield. Jain et al. recently reported that aerobic oxygen under photoredox conditions oxidatively cleave the imidazo ring of 1a to benzamide derivatives.
Return to citation in text: [1] -
Nguyen, J. D.; Tucker, J. W.; Konieczynska, M. D.; Stephenson, C. R. J. J. Am. Chem. Soc. 2011, 133, 4160–4163. doi:10.1021/ja108560e
See for details for photoredox ATRA reaction between diethyl bromomalonate 2a and 5-hexen-1-ol.
Return to citation in text: [1] -
Dam, J.; Ismail, Z.; Kurebwa, T.; Gangat, N.; Harmse, L.; Marques, H. M.; Lemmerer, A.; Bode, M. L.; de Koning, C. B. Eur. J. Med. Chem. 2017, 126, 353–368. doi:10.1016/j.ejmech.2016.10.041
Return to citation in text: [1] -
Katta, N.; Zhao, Q.-Q.; Mandal, T.; Reiser, O. ACS Catal. 2022, 12, 14398–14407. doi:10.1021/acscatal.2c04736
Return to citation in text: [1] -
Xia, X.-D.; Ren, Y.-L.; Chen, J.-R.; Yu, X.-L.; Lu, L.-Q.; Zou, Y.-Q.; Wan, J.; Xiao, W.-J. Chem. – Asian J. 2015, 10, 124–128. doi:10.1002/asia.201402990
Return to citation in text: [1] [2]
| 31. | Xia, X.-D.; Ren, Y.-L.; Chen, J.-R.; Yu, X.-L.; Lu, L.-Q.; Zou, Y.-Q.; Wan, J.; Xiao, W.-J. Chem. – Asian J. 2015, 10, 124–128. doi:10.1002/asia.201402990 |
| 1. | Enguehard-Gueiffier, C.; Gueiffier, A. Mini-Rev. Med. Chem. 2007, 7, 888–899. doi:10.2174/138955707781662645 |
| 2. | Devi, N.; Singh, D.; Rawal, R. K.; Bariwal, J.; Singh, V. Curr. Top. Med. Chem. 2016, 16, 2963–2994. doi:10.2174/1568026616666160506145539 |
| 3. | Kishbaugh, T. L. S. Curr. Top. Med. Chem. 2016, 16, 3274–3302. doi:10.2174/1568026616666160506145141 |
| 4. | Sanapalli, B. K. R.; Ashames, A.; Sigalapalli, D. K.; Shaik, A. B.; Bhandare, R. R.; Yele, V. Antibiotics (Basel, Switz.) 2022, 11, 1680. doi:10.3390/antibiotics11121680 |
| 17. | Li, K.; Zhao, X.-M.; Yang, F.-L.; Hou, X.-H.; Xu, Y.; Guo, Y.-C.; Hao, X.-Q.; Song, M.-P. RSC Adv. 2015, 5, 90478–90481. doi:10.1039/c5ra15678e |
| 18. | Reddy, K. N.; Chary, D. Y.; Sridhar, B.; Reddy, B. V. S. Org. Lett. 2019, 21, 8548–8552. doi:10.1021/acs.orglett.9b03041 |
| 19. | Xiao, Y.; Yu, L.; Yu, Y.; Tan, Z.; Deng, W. Tetrahedron Lett. 2020, 61, 152606. doi:10.1016/j.tetlet.2020.152606 |
| 20. | Chaubey, N. R.; Kapdi, A. R.; Maity, B. Synthesis 2021, 53, 1524–1530. doi:10.1055/s-0040-1706103 |
| 29. | Dam, J.; Ismail, Z.; Kurebwa, T.; Gangat, N.; Harmse, L.; Marques, H. M.; Lemmerer, A.; Bode, M. L.; de Koning, C. B. Eur. J. Med. Chem. 2017, 126, 353–368. doi:10.1016/j.ejmech.2016.10.041 |
| 12. | Patel, O. P. S.; Nandwana, N. K.; Legoabe, L. J.; Das, B. C.; Kumar, A. Adv. Synth. Catal. 2020, 362, 4226–4255. doi:10.1002/adsc.202000633 |
| 13. | Ghosh, D.; Ghosh, S.; Hajra, A. Adv. Synth. Catal. 2021, 363, 5047–5071. doi:10.1002/adsc.202100981 |
| 14. | Tran, C.; Hamze, A. Molecules 2022, 27, 3461. doi:10.3390/molecules27113461 |
| 15. | Ravi, C.; Chandra Mohan, D.; Adimurthy, S. Org. Biomol. Chem. 2016, 14, 2282–2290. doi:10.1039/c5ob02475g |
| 16. | Tashrifi, Z.; Mohammadi-Khanaposhtani, M.; Larijani, B.; Mahdavi, M. Eur. J. Org. Chem. 2020, 269–284. doi:10.1002/ejoc.201901491 |
| 30. | Katta, N.; Zhao, Q.-Q.; Mandal, T.; Reiser, O. ACS Catal. 2022, 12, 14398–14407. doi:10.1021/acscatal.2c04736 |
| 31. | Xia, X.-D.; Ren, Y.-L.; Chen, J.-R.; Yu, X.-L.; Lu, L.-Q.; Zou, Y.-Q.; Wan, J.; Xiao, W.-J. Chem. – Asian J. 2015, 10, 124–128. doi:10.1002/asia.201402990 |
| 7. | Deep, A.; Bhatia, R. K.; Kaur, R.; Kumar, S.; Jain, U. K.; Singh, H.; Batra, S.; Kaushik, D.; Deb, P. K. Curr. Top. Med. Chem. 2016, 17, 238–250. doi:10.2174/1568026616666160530153233 |
| 8. | Goel, R.; Luxami, V.; Paul, K. Curr. Top. Med. Chem. 2016, 16, 3590–3616. doi:10.2174/1568026616666160414122644 |
| 9. | Xi, J.-B.; Fang, Y.-F.; Frett, B.; Zhu, M.-L.; Zhu, T.; Kong, Y.-N.; Guan, F.-J.; Zhao, Y.; Zhang, X.-W.; Li, H.-y.; Ma, M.-L.; Hu, W. Eur. J. Med. Chem. 2017, 126, 1083–1106. doi:10.1016/j.ejmech.2016.12.026 |
| 10. | Shukla, N. M.; Salunke, D. B.; Yoo, E.; Mutz, C. A.; Balakrishna, R.; David, S. A. Bioorg. Med. Chem. 2012, 20, 5850–5863. doi:10.1016/j.bmc.2012.07.052 |
| 11. | Chernyak, N.; Gevorgyan, V. Angew. Chem., Int. Ed. 2010, 49, 2743–2746. doi:10.1002/anie.200907291 |
| 28. |
Nguyen, J. D.; Tucker, J. W.; Konieczynska, M. D.; Stephenson, C. R. J. J. Am. Chem. Soc. 2011, 133, 4160–4163. doi:10.1021/ja108560e
See for details for photoredox ATRA reaction between diethyl bromomalonate 2a and 5-hexen-1-ol. |
| 5. | Mutai, T.; Tomoda, H.; Ohkawa, T.; Yabe, Y.; Araki, K. Angew. Chem., Int. Ed. 2008, 47, 9522–9524. doi:10.1002/anie.200803975 |
| 6. | Padalkar, V. S.; Seki, S. Chem. Soc. Rev. 2016, 45, 169–202. doi:10.1039/c5cs00543d |
| 20. | Chaubey, N. R.; Kapdi, A. R.; Maity, B. Synthesis 2021, 53, 1524–1530. doi:10.1055/s-0040-1706103 |
| 21. | Singsardar, M.; Mondal, S.; Laru, S.; Hajra, A. Org. Lett. 2019, 21, 5606–5610. doi:10.1021/acs.orglett.9b01954 |
| 24. | Sakakibara, Y.; Murakami, K. ACS Catal. 2022, 12, 1857–1878. doi:10.1021/acscatal.1c05318 |
| 26. | Samanta, A.; Pramanik, S.; Mondal, S.; Maity, S. Chem. Commun. 2022, 58, 8400–8403. doi:10.1039/d2cc02574d |
| 23. | Roy Chowdhury, S.; Singh, D.; Hoque, I. U.; Maity, S. J. Org. Chem. 2020, 85, 13939–13950. doi:10.1021/acs.joc.0c01985 |
| 27. |
Ritu; Sharma, C.; Kumar, S.; Jain, N. Org. Biomol. Chem. 2020, 18, 2921–2928. doi:10.1039/d0ob00563k
With these catalytic systems (entries 4–6) no desired products (3a, 4a, 5, 6) were identified; instead a new product N-(pyridin-2-yl)benzamide was isolated in 8–14% yield. Jain et al. recently reported that aerobic oxygen under photoredox conditions oxidatively cleave the imidazo ring of 1a to benzamide derivatives. |
| 22. | Huang, M.; Wang, L.; Yang, X.; Kim, J. K.; Gong, M.; Zhang, J.; Li, Y.; Wu, Y. Tetrahedron 2022, 126, 132988. doi:10.1016/j.tet.2022.132988 |
| 21. | Singsardar, M.; Mondal, S.; Laru, S.; Hajra, A. Org. Lett. 2019, 21, 5606–5610. doi:10.1021/acs.orglett.9b01954 |
| 22. | Huang, M.; Wang, L.; Yang, X.; Kim, J. K.; Gong, M.; Zhang, J.; Li, Y.; Wu, Y. Tetrahedron 2022, 126, 132988. doi:10.1016/j.tet.2022.132988 |
| 25. | Holmberg-Douglas, N.; Nicewicz, D. A. Chem. Rev. 2022, 122, 1925–2016. doi:10.1021/acs.chemrev.1c00311 |
© 2023 Singh et al.; licensee Beilstein-Institut.
This is an open access article licensed under the terms of the Beilstein-Institut Open Access License Agreement (https://www.beilstein-journals.org/bjoc/terms), which is identical to the Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0). The reuse of material under this license requires that the author(s), source and license are credited. Third-party material in this article could be subject to other licenses (typically indicated in the credit line), and in this case, users are required to obtain permission from the license holder to reuse the material.