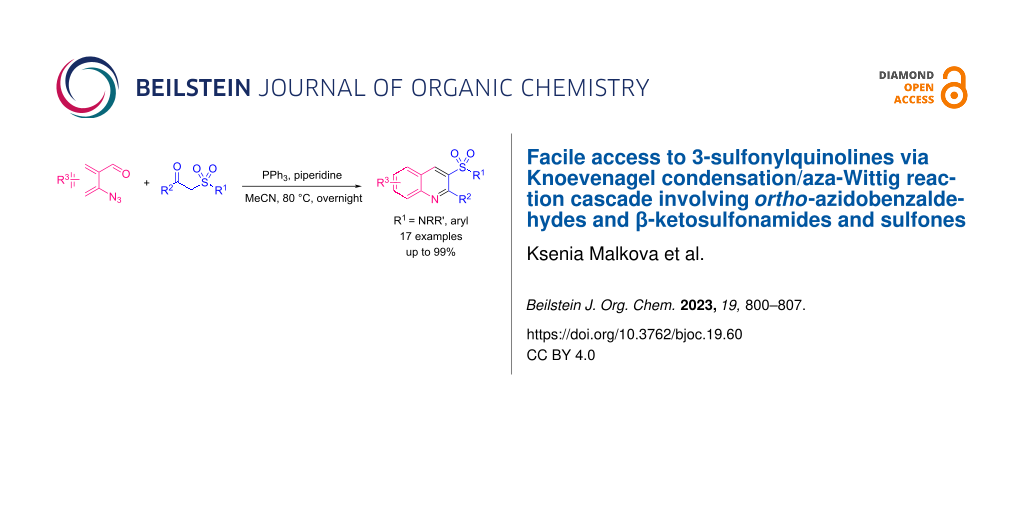
Abstract
Quinoline-based sulfonyl derivatives, and especially sulfonamides, are relevant and promising structures for drug design. We have developed a new convenient protocol for the synthesis of 3-sulfonyl-substituted quinolines (sulfonamides and sulfones). The approach is based on a Knoevenagel condensation/aza-Wittig reaction cascade involving o-azidobenzaldehydes and ketosulfonamides or ketosulfones as key building blocks. The protocol is appropriate for both ketosulfonyl reagents and α-sulfonyl-substituted alkyl acetates providing the target quinoline derivatives in good to excellent yields.
Graphical Abstract
Introduction
The quinoline scaffold has a wide occurrence among natural products [1] and is a key structural component of several pharmaceuticals, agrochemicals, dyestuffs, and materials. Particularly, the well-known antimalarial alkaloid quinine isolated from Cinchona bark comprises a quinoline core (Figure 1a) [2]. Moreover, numerous quinoline derivatives have been recently reported to possess intriguing pharmacological activities [3] including antiprotozoal [4-7], antitubercular [8,9], anticancer [10,11], anti-inflammatory [12], antioxidant [13], anti-HIV [14], antifungal [15], and an antineurodegenerative effect [16]. Hence, designing novel quinoline construction and functionalization techniques resulting in new or rare derivatives [17-26] is an important mission in the field of drug discovery and medicinal chemistry.
Figure 1: a) Conventional drugs containing either a sulfonamide fragment or a quinoline core; b) biologically active quinoline sulfonamides.
Figure 1: a) Conventional drugs containing either a sulfonamide fragment or a quinoline core; b) biologically...
The sulfonamide group is a known privileged motif in drug design often serving as a linker or pharmacophore group. In fact, more than one hundred FDA-approved drugs are sulfonamide-bearing small molecules. Screening libraries of aromatic and heteroaromatic sulfonamides gave rise to the discovery of multiple physiologically active compounds [27-30] including important pharmaceuticals, such as sulfamethoxazole and sulfasalazine (Figure 1a). In this context, combining sulfonamide and quinoline fragments promises to be a fruitful strategy to identify diverse types of therapeutically relevant compounds. The effectiveness of this approach is demonstrated by a series of bioactive structures developed recently, and, significantly, quinoline-3-sulfonamides are frequently encountered among such pharmacologically active hybrids (Figure 1b) [31-34].
Despite these facts, the diversity of quinoline-3-sulfonamides reported in the literature is limited due to obstacles in the synthesis of quinoline-3-sulfonyl chlorides which are the most common reagents for their preparation. As a possible solution, the approach to the heterocyclic core construction from a sulfonamide-containing building block may be considered. In turn, diversely substituted quinoline-3-sulfones are available through a range of synthetic methodologies suggested recently. Cyclization strategies [35-45] as well as cycloaddition/cyclocondensation techniques [46-51] represent those with hetero-ring construction. Alternative approaches rely on a peripheral modification of various substrates, such as 3-bromoquinolines [52-55], quinoline-3-boronic acids [56], and diazonium salts [57].
When considering general methods for the quinoline core formation, aromatic ortho-substituted carbonyl compounds attract attention as decent and easily available reagents. While the ortho-amino carbonyl reagents are not always easily accessible and sometimes unstable (e.g., aminoaldehydes), both o-azidoaldehydes [58-65] and o-azidoketones [66-69] have been proved to be appropriate substrates for quinoline derivatives synthesis. Recently, the method for the synthesis of 3-acyl-substituted quinolines from o-azidobenzaldehydes and 1,3-dicarbonyl compounds was reported [70,71] (Figure 2a). A combination of Knoevenagel condensation and aza-Wittig reaction allowed to build up target products in high yields. In case of [70], the procedure was predominantly applied for the preparation of the corresponding esters.
Figure 2: Knoevenagel condensation/aza-Wittig reaction cascade for the quinoline core formation.
Figure 2: Knoevenagel condensation/aza-Wittig reaction cascade for the quinoline core formation.
Inspired by this study, we became interested to utilize o-azidobenzaldehydes 1 in combination with ketosulfonamides/ketosulfones 2 as precursors in a new convenient synthetic procedure leading towards 3-sulfonyl-substituted quinolines (sulfonamides and sulfones) (Figure 2b). Herein, we report the successful implementation of this approach.
Results and Discussion
The Knoevenagel condensation/aza-Wittig reaction cascade was used for the preparation of 3-sulfonyl-substituted quinolines. The process proceeds in a domino fashion including the following steps: the formation of iminophosphorane 3 from o-azidobenzaldehyde (1) and PPh3 followed by the base-mediated Knoevenagel condensation results in compound 4; a subsequent intramolecular aza-Wittig reaction leads to the desired product 5 (Scheme 1).
Scheme 1: Key reaction steps during the synthesis of 3-sulfonyl-substituted quinolines.
Scheme 1: Key reaction steps during the synthesis of 3-sulfonyl-substituted quinolines.
Starting from the reaction conditions reported previously, we began our investigation using o-azidobenzaldehyde (1a), 2-oxopropanesulfonamide 2a, triphenylphosphine, and diethylamine as reagents for the quinoline-3-sulfonamide assembly (Table 1). The reaction mixture was stirred in MeCN at 95 °C for 6 h which led to a mediocre yield of the target compound 5a estimated by NMR (Table 1, entry 1). Different organic bases were tested, with piperidine performing most efficiently (Table 1, entry 4). Next it was found out that using o-azidobenzaldehyde (1a), PPh3, and an excess of piperidine in relation to ketosulfonamide 2a resulted in higher yields of quinoline 5a. The optimal solvent volume (the concentration of 2a) was chosen considering both reaction yields and practical reasons. Subsequent tuning of temperature and reaction time ensured quantitative NMR yield in the model reaction (Table 1, entry 13).
Table 1: Optimization of reaction conditions.a
|
|
|||||||
| Entry | Base (equiv) | 1a, equiv | 2a, equiv | PPh3, equiv | c2a, M | Δ, °C | NMR yield, % |
| 1 | Et2NH (1.0) | 1.0 | 1.2 | 1.2 | 0.147 | 95 | 31 |
| 2 | Et3N (1.0) | 1.0 | 1.2 | 1.2 | 0.147 | 95 | 43 |
| 3 | pyrrolidine (1.0) | 1.0 | 1.2 | 1.2 | 0.147 | 95 | 45 |
| 4 | piperidine (1.0) | 1.0 | 1.2 | 1.2 | 0.147 | 95 | 63 |
| 5 | piperidine (1.0) | 1.0 | 1.0 | 1.2 | 0.147 | 95 | 63 |
| 6 | piperidine (1.1) | 1.1 | 1.0 | 1.3 | 0.147 | 95 | 69 |
| 7 | piperidine (1.25) | 1.25 | 1.0 | 1.5 | 0.147 | 95 | 82 |
| 8 | piperidine (0.5) | 1.25 | 1.0 | 1.5 | 0.147 | 95 | 73 |
| 9 | piperidine (1.5) | 1.25 | 1.0 | 1.5 | 0.147 | 95 | 75 |
| 10 | piperidine (1.25) | 1.25 | 1.0 | 1.5 | 0.294 | 95 | 58 |
| 11 | piperidine (1.25) | 1.25 | 1.0 | 1.5 | 0.074 | 95 | 91 |
| 12 | piperidine (1.25) | 1.25 | 1.0 | 1.5 | 0.037 | 95 | 95 |
| 13 | piperidine (1.25) | 1.25 | 1.0 | 1.5 | 0.074 | 80 | 99b |
| 14 | piperidine (1.25) | 1.25 | 1.0 | 1.5 | 0.074 | 65 | 91 |
aReaction scale ‒ 0.1 mmol, reaction time ‒ 6 h. bReaction was run overnight (16 h).
A remarkable advantage of the approach devised is that all starting materials including sulfonyl compounds are easily accessible. Furthermore, the preparation techniques are flexible concerning the variations of substituents, which is of high importance for the potential medicinal chemistry applications. Scheme 2 illustrates unobstructed synthetic routes [72-74] to sulfonamides and sulfones 2, and the diversity of reagents used to prepare the target products 5.
Scheme 2: Synthetic routes to sulfonamides and sulfones 2 and the set of reagents for the preparation of compounds 5.
Scheme 2: Synthetic routes to sulfonamides and sulfones 2 and the set of reagents for the preparation of comp...
With the reaction conditions optimized, a series of novel tertiary quinoline-3-sulfonamides and quinoline-3-sulfones was successfully generated. In case of the secondary quinoline-3-sulfonamide synthesis, the increase of reagent excesses in relation to ketosulfonamide resulted in the conversion and an increase of the yield as observed by TLC (see GP2 in Supporting Information File 1).
Chromatographic purification afforded compounds 5a–q mostly in good to excellent yields (Scheme 3). The product structures were confirmed by the standard set of characterization data as well as the single-crystal X-ray structure of the representative compound 5a.
Scheme 3: Preparation of 3-sulfonyl substituted quinolines 5a–q.
Scheme 3: Preparation of 3-sulfonyl substituted quinolines 5a–q.
The presence of an electron-withdrawing group in the o-azidobenzaldehyde leads to decreased yields of target products (Scheme 3, 5e and 5g). The drop was especially dramatic for the nitro group containing reagent 1f. Indeed, the transformation was accompanied by a number of side reactions according to TLC. To our delight, the protocol turned out to be suitable for α-sulfonyl-substituted alkyl acetates leading to 2-alkoxyquinolines. Compounds 5l an 5q were obtained in 63 and 51% yield, respectively (Scheme 3). It is worth noticing that chromenopyridine-3-sulfonamide 5h derived from heterocyclic azidoaldehyde 1h was also successfully synthesized following the methodology designed.
Some limitations on the substrate scope for the protocol proposed were found out during the course of the study. Indole- and pyrazole-based azidoaldehydes 1r and 1s failed to provide the desired compounds 5r and 5s (Scheme 4). The reaction stopped on the iminophosphorane formation and did not progress further likely due to carbonyl group deactivation. Furthermore, while implementing the protocol for 2-azidoquinoline-3-carbaldehyde (1t), a low conversion of this reagent was detected, which can be explained by the fact that 1t tends to exist in the inactive tetrazole form. In addition, our attempt to involve Boc-protected ketosulfonamide 2u in the transformation resulted in the N-deprotected product. Finally, N,N-diethyl-2-tosylacetamide (2v) appeared to be incapable of entering the Knoevenagel condensation in the suggested conditions.
Scheme 4: 3-Sulfonyl-substituted quinolines 5r–v that failed to be synthesized.
Scheme 4: 3-Sulfonyl-substituted quinolines 5r–v that failed to be synthesized.
Conclusion
In summary, we have successfully developed a new straightforward protocol for the synthesis of 3-sulfonyl-substituted quinolines (sulfonamides and sulfones). The approach is based on a Knoevenagel condensation/aza-Wittig reaction cascade for the quinoline core assembly. Hence, o-azidobenzaldehyde, ketosulfonamide or ketosulfone were utilized as key building blocks. The method devised proved to be a convenient approach to the preparation of 3-sulfonyl-substituted quinolines. The desired compounds were obtained in good to excellent yields. Importantly, the protocol was found suitable not only for ketosulfonyl reagents but also for α-sulfonyl-substituted alkyl acetates providing a pathway to 2-alkoxyquinolines.
Supporting Information
Deposition number 2242072 (for 5a) contain the supplementary crystallographic data for this paper. These data are provided free of charge by the joint Cambridge Crystallographic Data Centre and Fachinformationszentrum Karlsruhe Access Structures service https://www.ccdc.cam.ac.uk/structures.
| Supporting Information File 1: General experimental information, X-ray crystallographic data, synthetic procedures, analytical data and NMR spectra for the reported compounds. | ||
| Format: PDF | Size: 3.6 MB | Download |
References
-
Michael, J. P. Nat. Prod. Rep. 2008, 25, 166–187. doi:10.1039/b612168n
Return to citation in text: [1] -
Achan, J.; Talisuna, A. O.; Erhart, A.; Yeka, A.; Tibenderana, J. K.; Baliraine, F. N.; Rosenthal, P. J.; D'Alessandro, U. Malar. J. 2011, 10, 144. doi:10.1186/1475-2875-10-144
Return to citation in text: [1] -
Ajani, O. O.; Iyaye, K. T.; Ademosun, O. T. RSC Adv. 2022, 12, 18594–18614. doi:10.1039/d2ra02896d
Return to citation in text: [1] -
Dorababu, A. ChemistrySelect 2021, 6, 2164–2177. doi:10.1002/slct.202100115
Return to citation in text: [1] -
Gryzło, B.; Kulig, K. Mini-Rev. Med. Chem. 2014, 14, 332–344. doi:10.2174/1389557514666140220123226
Return to citation in text: [1] -
Gorka, A. P.; de Dios, A.; Roepe, P. D. J. Med. Chem. 2013, 56, 5231–5246. doi:10.1021/jm400282d
Return to citation in text: [1] -
Reynolds, K. A.; Loughlin, W. A.; Young, D. J. Mini-Rev. Med. Chem. 2013, 13, 730–743. doi:10.2174/1389557511313050010
Return to citation in text: [1] -
Vaitilingam, B.; Nayyar, A.; Palde, P. B.; Monga, V.; Jain, R.; Kaur, S.; Singh, P. P. Bioorg. Med. Chem. 2004, 12, 4179–4188. doi:10.1016/j.bmc.2004.05.018
Return to citation in text: [1] -
Keri, R. S.; Patil, S. A. Biomed. Pharmacother. 2014, 68, 1161–1175. doi:10.1016/j.biopha.2014.10.007
Return to citation in text: [1] -
Solomon, V. R.; Lee, H. Curr. Med. Chem. 2011, 18, 1488–1508. doi:10.2174/092986711795328382
Return to citation in text: [1] -
Afzal, O.; Kumar, S.; Haider, M. R.; Ali, M. R.; Kumar, R.; Jaggi, M.; Bawa, S. Eur. J. Med. Chem. 2015, 97, 871–910. doi:10.1016/j.ejmech.2014.07.044
Return to citation in text: [1] -
Mukherjee, S.; Pal, M. Drug Discovery Today 2013, 18, 389–398. doi:10.1016/j.drudis.2012.11.003
Return to citation in text: [1] -
Orhan Puskullu, M.; Tekiner, B.; Suzen, S. Mini-Rev. Med. Chem. 2013, 13, 365–372. doi:10.2174/1389557511313030005
Return to citation in text: [1] -
Musiol, R. Curr. Pharm. Des. 2013, 19, 1835–1849. doi:10.2174/1381612811319100008
Return to citation in text: [1] -
Musiol, R.; Serda, M.; Hensel-Bielowka, S.; Polanski, J. Curr. Med. Chem. 2010, 17, 1960–1973. doi:10.2174/092986710791163966
Return to citation in text: [1] -
Bongarzone, S.; Bolognesi, M. L. Expert Opin. Drug Discovery 2011, 6, 251–268. doi:10.1517/17460441.2011.550914
Return to citation in text: [1] -
Wakade, S. B.; Tiwari, D. K.; Ganesh, P. S. K. P.; Phanindrudu, M.; Likhar, P. R.; Tiwari, D. K. Org. Lett. 2017, 19, 4948–4951. doi:10.1021/acs.orglett.7b02429
Return to citation in text: [1] -
Phanindrudu, M.; Wakade, S. B.; Tiwari, D. K.; Likhar, P. R.; Tiwari, D. K. J. Org. Chem. 2018, 83, 9137–9143. doi:10.1021/acs.joc.8b01204
Return to citation in text: [1] -
Jiao, J.; Wang, P.; Xiao, F.; Zhang, Z. Synlett 2022, 33, 569–574. doi:10.1055/a-1735-6250
Return to citation in text: [1] -
Jiao, J.; Xiao, F.; Wang, C.; Zhang, Z. J. Org. Chem. 2022, 87, 4965–4970. doi:10.1021/acs.joc.1c03030
Return to citation in text: [1] -
Li, S.; Wu, X.; Ke, Y.; Ma, C. Eur. J. Org. Chem. 2023, 26, e202201473. doi:10.1002/ejoc.202201473
Return to citation in text: [1] -
Liu, J.-Y.; Wang, Z.-Y.; Li, Q.; Li, D.; Dou, J. New J. Chem. 2023, 47, 5155–5159. doi:10.1039/d2nj04119g
Return to citation in text: [1] -
Ma, J.-T.; Chen, T.; Tang, B.-C.; Chen, X.-L.; Yu, Z.-C.; Zhou, Y.; Zhuang, S.-Y.; Wu, Y.-D.; Xiang, J.-C.; Wu, A.-X. J. Org. Chem. 2023, 88, 3760–3771. doi:10.1021/acs.joc.2c03034
Return to citation in text: [1] -
Mitrofanov, A. Y.; Beletskaya, I. P. J. Org. Chem. 2023, 88, 2367–2376. doi:10.1021/acs.joc.2c02780
Return to citation in text: [1] -
Sherborne, G. J.; Kemmitt, P.; Prentice, C.; Zysman-Colman, E.; Smith, A. D.; Fallan, C. Angew. Chem., Int. Ed. 2023, 62, e202207829. doi:10.1002/anie.202207829
Return to citation in text: [1] -
Wei, G.; Sun, Y.; Zheng, D.; Qiu, S.; Chen, Z.; Wu, X.-F. Eur. J. Org. Chem. 2023, 26, e202300090. doi:10.1002/ejoc.202300090
Return to citation in text: [1] -
Moskalik, M. Y. Molecules 2023, 28, 51. doi:10.3390/molecules28010051
Return to citation in text: [1] -
Azevedo-Barbosa, H.; Dias, D. F.; Franco, L. L.; Hawkes, J. A.; Carvalho, D. T. Mini-Rev. Med. Chem. 2020, 20, 2052–2066. doi:10.2174/1389557520666200905125738
Return to citation in text: [1] -
Ajeet, A.; Mishra, A. K.; Kumar, A. A. J. Pharmacol. Sci. 2015, 3, 18–24.
Return to citation in text: [1] -
Ovung, A.; Bhattacharyya, J. Biophys. Rev. 2021, 13, 259–272. doi:10.1007/s12551-021-00795-9
Return to citation in text: [1] -
Marciniec, K.; Pawełczak, B.; Latocha, M.; Skrzypek, L.; Maciążek-Jurczyk, M.; Boryczka, S. Molecules 2017, 22, 300. doi:10.3390/molecules22020300
Return to citation in text: [1] -
Harvey, C. M.; Sargent, C.; Siegl, P. K. S. Endothelin receptor antagonists for the treatment of emesis. WO Patent WO1996009818, April 4, 1996.
Return to citation in text: [1] -
Chen, Y.; Cushing, T. D.; Hao, X.; He, X.; Reuchelt, A.; Rzasa, R. M.; Seganish, J.; Shon, Y.; Zhang, D. δ3-Substituted quinoline or quinoxaline derivatives and their use as phosphatidylinositol 3-kinase (PI3K) inhibitors. WO Patent WO2008118455, Oct 2, 2008.
Return to citation in text: [1] -
Marciniec, K.; Rzepka, Z.; Chrobak, E.; Boryczka, S.; Latocha, M.; Wrześniok, D.; Beberok, A. Molecules 2023, 28, 2509. doi:10.3390/molecules28062509
Return to citation in text: [1] -
Lee, K. Y.; Kim, J. M.; Kim, J. N. Tetrahedron 2003, 59, 385–390. doi:10.1016/s0040-4020(02)01518-1
Return to citation in text: [1] -
Zhang, L.; Chen, S.; Gao, Y.; Zhang, P.; Wu, Y.; Tang, G.; Zhao, Y. Org. Lett. 2016, 18, 1286–1289. doi:10.1021/acs.orglett.6b00198
Return to citation in text: [1] -
Sun, D.; Yin, K.; Zhang, R. Chem. Commun. 2018, 54, 1335–1338. doi:10.1039/c7cc09410h
Return to citation in text: [1] -
Wang, B.; Jin, S.; Sun, S.; Cheng, J. Org. Chem. Front. 2018, 5, 958–961. doi:10.1039/c7qo01048f
Return to citation in text: [1] -
Li, L.; Zhang, X.-G.; Hu, B.-L.; Zhang, X.-H. Chem. – Asian J. 2019, 14, 4358–4364. doi:10.1002/asia.201901298
Return to citation in text: [1] -
Zhang, Y.; Chen, W.; Jia, X.; Wang, L.; Li, P. Chem. Commun. 2019, 55, 2785–2788. doi:10.1039/c8cc10235j
Return to citation in text: [1] -
Yuan, J.-M.; Li, J.; Zhou, H.; Xu, J.; Zhu, F.; Liang, Q.; Liu, Z.; Huang, G.; Huang, J. New J. Chem. 2020, 44, 3189–3193. doi:10.1039/c9nj05248h
Return to citation in text: [1] -
Liu, J.; Wang, M.; Li, L.; Wang, L. Green Chem. 2021, 23, 4733–4740. doi:10.1039/d1gc00171j
Return to citation in text: [1] -
Zhou, N.; Xia, Z.; Wu, S.; Kuang, K.; Xu, Q.; Zhang, M. J. Org. Chem. 2021, 86, 15253–15262. doi:10.1021/acs.joc.1c01866
Return to citation in text: [1] -
Ma, Q.; Li, M.; Chen, Z.; Ni, S.-F.; Wright, J. S.; Wen, L.-R.; Zhang, L.-B. Green Chem. 2022, 24, 4425–4431. doi:10.1039/d2gc00151a
Return to citation in text: [1] -
Ye, H.; Zhou, L.; Chen, Y.; Tong, H. Org. Biomol. Chem. 2023, 21, 846–850. doi:10.1039/d2ob02069f
Return to citation in text: [1] -
Kang, S.; Yoon, H.; Lee, Y. Chem. Lett. 2016, 45, 1356–1358. doi:10.1246/cl.160772
Return to citation in text: [1] -
Wang, F.; Xu, P.; Wang, S.-Y.; Ji, S.-J. Org. Lett. 2018, 20, 2204–2207. doi:10.1021/acs.orglett.8b00525
Return to citation in text: [1] -
Chan, C.-K.; Lai, C.-Y.; Lo, W.-C.; Cheng, Y.-T.; Chang, M.-Y.; Wang, C.-C. Org. Biomol. Chem. 2020, 18, 305–315. doi:10.1039/c9ob02445j
Return to citation in text: [1] -
Kim, A. R.; Lim, H. N. RSC Adv. 2020, 10, 7855–7866. doi:10.1039/d0ra01352h
Return to citation in text: [1] -
Baek, J.; Si, T.; Kim, H. Y.; Oh, K. Org. Lett. 2022, 24, 4982–4986. doi:10.1021/acs.orglett.2c02037
Return to citation in text: [1] -
Fobi, K.; Bunce, R. A. Molecules 2022, 27, 4123. doi:10.3390/molecules27134123
Return to citation in text: [1] -
Liu, N.-W.; Liang, S.; Margraf, N.; Shaaban, S.; Luciano, V.; Drost, M.; Manolikakes, G. Eur. J. Org. Chem. 2018, 1208–1210. doi:10.1002/ejoc.201701478
Return to citation in text: [1] -
Chen, L.; Liang, J.; Chen, Z.-y.; Chen, J.; Yan, M.; Zhang, X.-j. Adv. Synth. Catal. 2019, 361, 956–960. doi:10.1002/adsc.201900370
Return to citation in text: [1] -
Phanindrudu, M.; Jaya, P.; Likhar, P. R.; Tiwari, D. K. Tetrahedron 2020, 76, 131263. doi:10.1016/j.tet.2020.131263
Return to citation in text: [1] -
Jiang, S.; Zhang, Z.-T.; Young, D. J.; Chai, L.-L.; Wu, Q.; Li, H.-X. Org. Chem. Front. 2022, 9, 1437–1444. doi:10.1039/d1qo01850g
Return to citation in text: [1] -
Luo, Y.; Ding, H.; Zhen, J.-S.; Du, X.; Xu, X.-H.; Yuan, H.; Li, Y.-H.; Qi, W.-Y.; Liu, B.-Z.; Lu, S.-M.; Xue, C.; Ding, Q. Chem. Sci. 2021, 12, 9556–9560. doi:10.1039/d1sc02266k
Return to citation in text: [1] -
Wang, L.; Zhang, L.-f. Synlett 2022, 33, 1929–1932. doi:10.1055/s-0042-1752344
Return to citation in text: [1] -
Vidyacharan, S.; Sagar, A.; Sharada, D. S. Org. Biomol. Chem. 2015, 13, 7614–7618. doi:10.1039/c5ob01023c
Return to citation in text: [1] -
Gharpure, S. J.; Nanda, S. K.; Adate, P. A.; Shelke, Y. G. J. Org. Chem. 2017, 82, 2067–2080. doi:10.1021/acs.joc.6b02896
Return to citation in text: [1] -
Mao, X.-F.; Zhu, X.-P.; Li, D.-Y.; Liu, P.-N. J. Org. Chem. 2017, 82, 7032–7039. doi:10.1021/acs.joc.7b00937
Return to citation in text: [1] -
Zhang, X.; Dhawan, G.; Muthengi, A.; Liu, S.; Wang, W.; Legris, M.; Zhang, W. Green Chem. 2017, 19, 3851–3855. doi:10.1039/c7gc01380a
Return to citation in text: [1] -
Yi, R.; Li, X.; Wan, B. Org. Chem. Front. 2018, 5, 3488–3493. doi:10.1039/c8qo00984h
Return to citation in text: [1] -
Zheng, L.; Zeng, Z.; Yan, Q.; Jia, F.; Jia, L.; Chen, Y. Adv. Synth. Catal. 2018, 360, 4037–4042. doi:10.1002/adsc.201800773
Return to citation in text: [1] -
Gharpure, S. J.; Nanda, S. K.; Fartade, D. J. Adv. Synth. Catal. 2021, 363, 2562–2567. doi:10.1002/adsc.202100074
Return to citation in text: [1] -
Sun, M.; Yu, Y.-L.; Zhao, L.; Ding, M.-W. Tetrahedron 2021, 80, 131868. doi:10.1016/j.tet.2020.131868
Return to citation in text: [1] -
Amaresh, R. R.; Perumal, P. T. Tetrahedron 1998, 54, 14327–14340. doi:10.1016/s0040-4020(98)00887-4
Return to citation in text: [1] -
Kamal, A.; Reddy, K. S.; Khan, M. N. A.; Shetty, R. V. C. R. N. C.; Ahmed, S. K.; Kumar, K. P.; Murty, U. S. N. Lett. Drug Des. Discovery 2007, 4, 580–586. doi:10.2174/157018007782794536
Return to citation in text: [1] -
Yang, W.; Xu, L.; Chen, Z.; Zhang, L.; Miao, M.; Ren, H. Org. Lett. 2013, 15, 1282–1285. doi:10.1021/ol400223d
Return to citation in text: [1] -
Su, H.; Bao, M.; Huang, J.; Qiu, L.; Xu, X. Adv. Synth. Catal. 2019, 361, 826–831. doi:10.1002/adsc.201801425
Return to citation in text: [1] -
Zhang, X.; Ma, X.; Qiu, W.; Evans, J.; Zhang, W. Green Chem. 2019, 21, 349–354. doi:10.1039/c8gc03180k
Return to citation in text: [1] [2] -
Qu, F.; He, P.; Hu, R.-F.; Cheng, X.-H.; Wang, S.; Wu, J. Synth. Commun. 2015, 45, 2802–2809. doi:10.1080/00397911.2015.1105982
Return to citation in text: [1] -
Klochkova, A.; Bubyrev, A.; Dar’in, D.; Bakulina, O.; Krasavin, M.; Sokolov, V. Synthesis 2021, 53, 1795–1804. doi:10.1055/a-1343-9451
Return to citation in text: [1] -
Swenson, R. E.; Sowin, T. J.; Zhang, H. Q. J. Org. Chem. 2002, 67, 9182–9185. doi:10.1021/jo0203387
Return to citation in text: [1] -
Bubyrev, A.; Dar'in, D.; Kantin, G.; Krasavin, M. Eur. J. Org. Chem. 2020, 4112–4115. doi:10.1002/ejoc.202000446
Return to citation in text: [1]
| 57. | Wang, L.; Zhang, L.-f. Synlett 2022, 33, 1929–1932. doi:10.1055/s-0042-1752344 |
| 52. | Liu, N.-W.; Liang, S.; Margraf, N.; Shaaban, S.; Luciano, V.; Drost, M.; Manolikakes, G. Eur. J. Org. Chem. 2018, 1208–1210. doi:10.1002/ejoc.201701478 |
| 53. | Chen, L.; Liang, J.; Chen, Z.-y.; Chen, J.; Yan, M.; Zhang, X.-j. Adv. Synth. Catal. 2019, 361, 956–960. doi:10.1002/adsc.201900370 |
| 54. | Phanindrudu, M.; Jaya, P.; Likhar, P. R.; Tiwari, D. K. Tetrahedron 2020, 76, 131263. doi:10.1016/j.tet.2020.131263 |
| 55. | Jiang, S.; Zhang, Z.-T.; Young, D. J.; Chai, L.-L.; Wu, Q.; Li, H.-X. Org. Chem. Front. 2022, 9, 1437–1444. doi:10.1039/d1qo01850g |
| 56. | Luo, Y.; Ding, H.; Zhen, J.-S.; Du, X.; Xu, X.-H.; Yuan, H.; Li, Y.-H.; Qi, W.-Y.; Liu, B.-Z.; Lu, S.-M.; Xue, C.; Ding, Q. Chem. Sci. 2021, 12, 9556–9560. doi:10.1039/d1sc02266k |
| 8. | Vaitilingam, B.; Nayyar, A.; Palde, P. B.; Monga, V.; Jain, R.; Kaur, S.; Singh, P. P. Bioorg. Med. Chem. 2004, 12, 4179–4188. doi:10.1016/j.bmc.2004.05.018 |
| 9. | Keri, R. S.; Patil, S. A. Biomed. Pharmacother. 2014, 68, 1161–1175. doi:10.1016/j.biopha.2014.10.007 |
| 35. | Lee, K. Y.; Kim, J. M.; Kim, J. N. Tetrahedron 2003, 59, 385–390. doi:10.1016/s0040-4020(02)01518-1 |
| 36. | Zhang, L.; Chen, S.; Gao, Y.; Zhang, P.; Wu, Y.; Tang, G.; Zhao, Y. Org. Lett. 2016, 18, 1286–1289. doi:10.1021/acs.orglett.6b00198 |
| 37. | Sun, D.; Yin, K.; Zhang, R. Chem. Commun. 2018, 54, 1335–1338. doi:10.1039/c7cc09410h |
| 38. | Wang, B.; Jin, S.; Sun, S.; Cheng, J. Org. Chem. Front. 2018, 5, 958–961. doi:10.1039/c7qo01048f |
| 39. | Li, L.; Zhang, X.-G.; Hu, B.-L.; Zhang, X.-H. Chem. – Asian J. 2019, 14, 4358–4364. doi:10.1002/asia.201901298 |
| 40. | Zhang, Y.; Chen, W.; Jia, X.; Wang, L.; Li, P. Chem. Commun. 2019, 55, 2785–2788. doi:10.1039/c8cc10235j |
| 41. | Yuan, J.-M.; Li, J.; Zhou, H.; Xu, J.; Zhu, F.; Liang, Q.; Liu, Z.; Huang, G.; Huang, J. New J. Chem. 2020, 44, 3189–3193. doi:10.1039/c9nj05248h |
| 42. | Liu, J.; Wang, M.; Li, L.; Wang, L. Green Chem. 2021, 23, 4733–4740. doi:10.1039/d1gc00171j |
| 43. | Zhou, N.; Xia, Z.; Wu, S.; Kuang, K.; Xu, Q.; Zhang, M. J. Org. Chem. 2021, 86, 15253–15262. doi:10.1021/acs.joc.1c01866 |
| 44. | Ma, Q.; Li, M.; Chen, Z.; Ni, S.-F.; Wright, J. S.; Wen, L.-R.; Zhang, L.-B. Green Chem. 2022, 24, 4425–4431. doi:10.1039/d2gc00151a |
| 45. | Ye, H.; Zhou, L.; Chen, Y.; Tong, H. Org. Biomol. Chem. 2023, 21, 846–850. doi:10.1039/d2ob02069f |
| 4. | Dorababu, A. ChemistrySelect 2021, 6, 2164–2177. doi:10.1002/slct.202100115 |
| 5. | Gryzło, B.; Kulig, K. Mini-Rev. Med. Chem. 2014, 14, 332–344. doi:10.2174/1389557514666140220123226 |
| 6. | Gorka, A. P.; de Dios, A.; Roepe, P. D. J. Med. Chem. 2013, 56, 5231–5246. doi:10.1021/jm400282d |
| 7. | Reynolds, K. A.; Loughlin, W. A.; Young, D. J. Mini-Rev. Med. Chem. 2013, 13, 730–743. doi:10.2174/1389557511313050010 |
| 46. | Kang, S.; Yoon, H.; Lee, Y. Chem. Lett. 2016, 45, 1356–1358. doi:10.1246/cl.160772 |
| 47. | Wang, F.; Xu, P.; Wang, S.-Y.; Ji, S.-J. Org. Lett. 2018, 20, 2204–2207. doi:10.1021/acs.orglett.8b00525 |
| 48. | Chan, C.-K.; Lai, C.-Y.; Lo, W.-C.; Cheng, Y.-T.; Chang, M.-Y.; Wang, C.-C. Org. Biomol. Chem. 2020, 18, 305–315. doi:10.1039/c9ob02445j |
| 49. | Kim, A. R.; Lim, H. N. RSC Adv. 2020, 10, 7855–7866. doi:10.1039/d0ra01352h |
| 50. | Baek, J.; Si, T.; Kim, H. Y.; Oh, K. Org. Lett. 2022, 24, 4982–4986. doi:10.1021/acs.orglett.2c02037 |
| 51. | Fobi, K.; Bunce, R. A. Molecules 2022, 27, 4123. doi:10.3390/molecules27134123 |
| 3. | Ajani, O. O.; Iyaye, K. T.; Ademosun, O. T. RSC Adv. 2022, 12, 18594–18614. doi:10.1039/d2ra02896d |
| 27. | Moskalik, M. Y. Molecules 2023, 28, 51. doi:10.3390/molecules28010051 |
| 28. | Azevedo-Barbosa, H.; Dias, D. F.; Franco, L. L.; Hawkes, J. A.; Carvalho, D. T. Mini-Rev. Med. Chem. 2020, 20, 2052–2066. doi:10.2174/1389557520666200905125738 |
| 29. | Ajeet, A.; Mishra, A. K.; Kumar, A. A. J. Pharmacol. Sci. 2015, 3, 18–24. |
| 30. | Ovung, A.; Bhattacharyya, J. Biophys. Rev. 2021, 13, 259–272. doi:10.1007/s12551-021-00795-9 |
| 72. | Klochkova, A.; Bubyrev, A.; Dar’in, D.; Bakulina, O.; Krasavin, M.; Sokolov, V. Synthesis 2021, 53, 1795–1804. doi:10.1055/a-1343-9451 |
| 73. | Swenson, R. E.; Sowin, T. J.; Zhang, H. Q. J. Org. Chem. 2002, 67, 9182–9185. doi:10.1021/jo0203387 |
| 74. | Bubyrev, A.; Dar'in, D.; Kantin, G.; Krasavin, M. Eur. J. Org. Chem. 2020, 4112–4115. doi:10.1002/ejoc.202000446 |
| 2. | Achan, J.; Talisuna, A. O.; Erhart, A.; Yeka, A.; Tibenderana, J. K.; Baliraine, F. N.; Rosenthal, P. J.; D'Alessandro, U. Malar. J. 2011, 10, 144. doi:10.1186/1475-2875-10-144 |
| 31. | Marciniec, K.; Pawełczak, B.; Latocha, M.; Skrzypek, L.; Maciążek-Jurczyk, M.; Boryczka, S. Molecules 2017, 22, 300. doi:10.3390/molecules22020300 |
| 32. | Harvey, C. M.; Sargent, C.; Siegl, P. K. S. Endothelin receptor antagonists for the treatment of emesis. WO Patent WO1996009818, April 4, 1996. |
| 33. | Chen, Y.; Cushing, T. D.; Hao, X.; He, X.; Reuchelt, A.; Rzasa, R. M.; Seganish, J.; Shon, Y.; Zhang, D. δ3-Substituted quinoline or quinoxaline derivatives and their use as phosphatidylinositol 3-kinase (PI3K) inhibitors. WO Patent WO2008118455, Oct 2, 2008. |
| 34. | Marciniec, K.; Rzepka, Z.; Chrobak, E.; Boryczka, S.; Latocha, M.; Wrześniok, D.; Beberok, A. Molecules 2023, 28, 2509. doi:10.3390/molecules28062509 |
| 14. | Musiol, R. Curr. Pharm. Des. 2013, 19, 1835–1849. doi:10.2174/1381612811319100008 |
| 16. | Bongarzone, S.; Bolognesi, M. L. Expert Opin. Drug Discovery 2011, 6, 251–268. doi:10.1517/17460441.2011.550914 |
| 70. | Zhang, X.; Ma, X.; Qiu, W.; Evans, J.; Zhang, W. Green Chem. 2019, 21, 349–354. doi:10.1039/c8gc03180k |
| 71. | Qu, F.; He, P.; Hu, R.-F.; Cheng, X.-H.; Wang, S.; Wu, J. Synth. Commun. 2015, 45, 2802–2809. doi:10.1080/00397911.2015.1105982 |
| 13. | Orhan Puskullu, M.; Tekiner, B.; Suzen, S. Mini-Rev. Med. Chem. 2013, 13, 365–372. doi:10.2174/1389557511313030005 |
| 17. | Wakade, S. B.; Tiwari, D. K.; Ganesh, P. S. K. P.; Phanindrudu, M.; Likhar, P. R.; Tiwari, D. K. Org. Lett. 2017, 19, 4948–4951. doi:10.1021/acs.orglett.7b02429 |
| 18. | Phanindrudu, M.; Wakade, S. B.; Tiwari, D. K.; Likhar, P. R.; Tiwari, D. K. J. Org. Chem. 2018, 83, 9137–9143. doi:10.1021/acs.joc.8b01204 |
| 19. | Jiao, J.; Wang, P.; Xiao, F.; Zhang, Z. Synlett 2022, 33, 569–574. doi:10.1055/a-1735-6250 |
| 20. | Jiao, J.; Xiao, F.; Wang, C.; Zhang, Z. J. Org. Chem. 2022, 87, 4965–4970. doi:10.1021/acs.joc.1c03030 |
| 21. | Li, S.; Wu, X.; Ke, Y.; Ma, C. Eur. J. Org. Chem. 2023, 26, e202201473. doi:10.1002/ejoc.202201473 |
| 22. | Liu, J.-Y.; Wang, Z.-Y.; Li, Q.; Li, D.; Dou, J. New J. Chem. 2023, 47, 5155–5159. doi:10.1039/d2nj04119g |
| 23. | Ma, J.-T.; Chen, T.; Tang, B.-C.; Chen, X.-L.; Yu, Z.-C.; Zhou, Y.; Zhuang, S.-Y.; Wu, Y.-D.; Xiang, J.-C.; Wu, A.-X. J. Org. Chem. 2023, 88, 3760–3771. doi:10.1021/acs.joc.2c03034 |
| 24. | Mitrofanov, A. Y.; Beletskaya, I. P. J. Org. Chem. 2023, 88, 2367–2376. doi:10.1021/acs.joc.2c02780 |
| 25. | Sherborne, G. J.; Kemmitt, P.; Prentice, C.; Zysman-Colman, E.; Smith, A. D.; Fallan, C. Angew. Chem., Int. Ed. 2023, 62, e202207829. doi:10.1002/anie.202207829 |
| 26. | Wei, G.; Sun, Y.; Zheng, D.; Qiu, S.; Chen, Z.; Wu, X.-F. Eur. J. Org. Chem. 2023, 26, e202300090. doi:10.1002/ejoc.202300090 |
| 70. | Zhang, X.; Ma, X.; Qiu, W.; Evans, J.; Zhang, W. Green Chem. 2019, 21, 349–354. doi:10.1039/c8gc03180k |
| 12. | Mukherjee, S.; Pal, M. Drug Discovery Today 2013, 18, 389–398. doi:10.1016/j.drudis.2012.11.003 |
| 58. | Vidyacharan, S.; Sagar, A.; Sharada, D. S. Org. Biomol. Chem. 2015, 13, 7614–7618. doi:10.1039/c5ob01023c |
| 59. | Gharpure, S. J.; Nanda, S. K.; Adate, P. A.; Shelke, Y. G. J. Org. Chem. 2017, 82, 2067–2080. doi:10.1021/acs.joc.6b02896 |
| 60. | Mao, X.-F.; Zhu, X.-P.; Li, D.-Y.; Liu, P.-N. J. Org. Chem. 2017, 82, 7032–7039. doi:10.1021/acs.joc.7b00937 |
| 61. | Zhang, X.; Dhawan, G.; Muthengi, A.; Liu, S.; Wang, W.; Legris, M.; Zhang, W. Green Chem. 2017, 19, 3851–3855. doi:10.1039/c7gc01380a |
| 62. | Yi, R.; Li, X.; Wan, B. Org. Chem. Front. 2018, 5, 3488–3493. doi:10.1039/c8qo00984h |
| 63. | Zheng, L.; Zeng, Z.; Yan, Q.; Jia, F.; Jia, L.; Chen, Y. Adv. Synth. Catal. 2018, 360, 4037–4042. doi:10.1002/adsc.201800773 |
| 64. | Gharpure, S. J.; Nanda, S. K.; Fartade, D. J. Adv. Synth. Catal. 2021, 363, 2562–2567. doi:10.1002/adsc.202100074 |
| 65. | Sun, M.; Yu, Y.-L.; Zhao, L.; Ding, M.-W. Tetrahedron 2021, 80, 131868. doi:10.1016/j.tet.2020.131868 |
| 10. | Solomon, V. R.; Lee, H. Curr. Med. Chem. 2011, 18, 1488–1508. doi:10.2174/092986711795328382 |
| 11. | Afzal, O.; Kumar, S.; Haider, M. R.; Ali, M. R.; Kumar, R.; Jaggi, M.; Bawa, S. Eur. J. Med. Chem. 2015, 97, 871–910. doi:10.1016/j.ejmech.2014.07.044 |
| 15. | Musiol, R.; Serda, M.; Hensel-Bielowka, S.; Polanski, J. Curr. Med. Chem. 2010, 17, 1960–1973. doi:10.2174/092986710791163966 |
| 66. | Amaresh, R. R.; Perumal, P. T. Tetrahedron 1998, 54, 14327–14340. doi:10.1016/s0040-4020(98)00887-4 |
| 67. | Kamal, A.; Reddy, K. S.; Khan, M. N. A.; Shetty, R. V. C. R. N. C.; Ahmed, S. K.; Kumar, K. P.; Murty, U. S. N. Lett. Drug Des. Discovery 2007, 4, 580–586. doi:10.2174/157018007782794536 |
| 68. | Yang, W.; Xu, L.; Chen, Z.; Zhang, L.; Miao, M.; Ren, H. Org. Lett. 2013, 15, 1282–1285. doi:10.1021/ol400223d |
| 69. | Su, H.; Bao, M.; Huang, J.; Qiu, L.; Xu, X. Adv. Synth. Catal. 2019, 361, 826–831. doi:10.1002/adsc.201801425 |
© 2023 Malkova et al.; licensee Beilstein-Institut.
This is an open access article licensed under the terms of the Beilstein-Institut Open Access License Agreement (https://www.beilstein-journals.org/bjoc/terms), which is identical to the Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0). The reuse of material under this license requires that the author(s), source and license are credited. Third-party material in this article could be subject to other licenses (typically indicated in the credit line), and in this case, users are required to obtain permission from the license holder to reuse the material.