Abstract
Background
The formation of novel N-substituted-1,2,3,4-tetrahydro[1,3]-dioxolo-[6,7]-5H-[1]benzopyrano [3,4-c]pyridines were observed unexpectedly during the acid-mediated ketal removal of ethylenedioxy ketal protected 4-piperidones. The literature revealed that benzopyranopyridine derivatives are of scientific interest and some exhibit interesting biological activities. Diastereomeric resolution was utilized to isolate optically pure chiral molecules.
Results
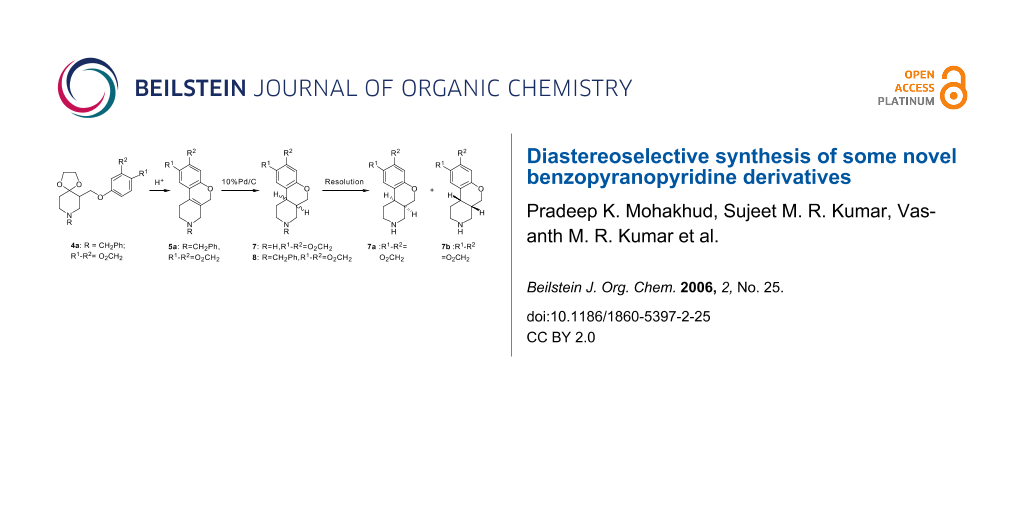
The acid catalyzed deprotection of N-substituted-4,4-ethylenedioxy-3- [(1,3-benzodioxol-5-yloxy)methyl]piperidines, prepared by condensation of the corresponding phenols and mesylate derivatives, unexpectedly resulted in cyclodehydration leading to new benzopyrano derivatives, N-substituted-1,2,3,4-tetrahydro[1,3]-dioxolo-[6,7]-5H-[1]benzopyrano [3,4-c]pyridines. The process involves the deprotection of the carbonyl protecting group, and then the cyclization reaction occurs followed by dehydration to give the final product.
These N-substituted-1,2,3,4-tetrahydro[1,3]-dioxolo-[6,7]-5H-[1]benzopyrano [3,4-c] pyridines were dealkylated giving the corresponding N-unsubstituted derivatives. The cis-1,3,4,4a,5,10b-hexahydro-[6,7]-2H-[1]benzopyrano [3,4-c]pyridine derivative was also obtained from the N-benzylated-1,2,3,4-tetrahydro[1,3]-dioxolo-[6,7]-5H-[1]benzopyrano [3,4-c]pyridine via catalytic hydrogenation. The resolution of the enantiomers was carried out using D-(-)-mandelic acid as chiral reagent. The absolute configuration of the S,S-mandelate salt derivative was determined by X-ray crystallographic analysis.
Conclusion
The approach led to the construction of N-substituted-1,2,3,4-tetrahydro[1,3]-dioxolo-[6,7]-5H-[1]benzopyrano [3,4-c] pyridines ring systems involving the one-pot deprotection, cyclization and dehydration of N-substituted-4,4-ethylenedioxy-3- [(1,3-benzodioxol-5-yloxy)methyl]piperidines. The hydrogenation of the N-benzylated benzopyrano [3,4-c]pyridine derivative followed by resolution led to the formation of a new compound.
Graphical Abstract
Introduction
The present paper describes the study of a novel synthesis of benzopyranopyridine derivatives obtained by the unexpected cyclization of 3-substituted aryloxy methyl-4,4-ethylenedioxy-N-substituted piperidine derivatives (4a, 4b). This was discovered whilst attempting the deprotection of the ethylenedioxy ketal at the 4-position to form intermediate 4c for the preparation of the antipsychotic drug Paroxetine. [1] This compound was characterized and the literature search revealed that such a compound has not been synthesized by this strategy.
It was also found in the literature that various nitrogenous analogs of tetrahydro cannabinole derivatives like 1,2,3,4-tetrahydro-5H-[1]-benzopyrano[3,4]pyridine-5-one (A) were synthesized by the Pechman condensation [2] and are potent bronchodilators, [3] which have the potential to be useful in the treatment of asthma and bronchitis. Furthermore, it is reported that benzopyrano derivatives of type B, which exhibit significant CNS depressant and hypotensive activity [4] were prepared from derivatives of compound A.
Figure 1: General structures A & B, Tetrahydro cannabinole derivatives.
Figure 1: General structures A & B, Tetrahydro cannabinole derivatives.
A recent patent [5] relates to a compound of general structure C, and the pharmaceutically accepted salts, which exhibited α-1 adrenergic antagonist behavior and are useful in the treatment of benign prostatic hyperplasia (BPH) and other urological diseases. Taking in to account the available medicinal chemistry data, molecules of types C and D are interesting synthetic targets, especially as they have a wide spectrum of chemical reactivity as well as diverse and marked biological activities, which are useful for drug discovery research.
Figure 2: General structures C & D, Benzopyrano pyridine derivatives.
Figure 2: General structures C & D, Benzopyrano pyridine derivatives.
To the best of our knowledge, compounds with the general structure D (with no substitution at C-5) have not been reported. Compounds of type C have been reported with the "trans" configuration, [5] while we report the synthesis of compounds with the cis configuration by a different synthetic approach. The reported synthesis [5] for the trans compound involved reaction of ethyl 2-methoxy-6-methoxymethyl cinnamate with ethyl N-benzylamidomalonate followed by reduction with LiAlH4. Subsequent conversion of the hydroxymethyl to a leaving group and then intramolecular cyclization, followed by debenzylation, furnished the racemic trans 10-methoxy-1,3,4,4a,5,10b-hexahydro-2H-[1]-benzopyrano [3,4-c]pyridine.
Results and discussion
The present study relates to the synthesis of new molecules of type C with cis configuration (7a, 7b, 8) as shown in Scheme 2 and new molecules of type D (5a, 5b, 6) as shown in Scheme 1. Also the intermediates 3a, 3b, 4a, 4b involved during the synthesis of these new molecules are not reported in the literature but are explained below (For full experimental data, see also: Supporting Information File 1).
Serendipitous synthesis of 1,2,3,4-tetrahydro[1,3]-dioxolo-[6,7]-5H-[1] benzopyrano [3,4-c] pyridine skeleton
Scheme 1: Synthetic sequence to tetrahydropyridine compounds.
Scheme 1: Synthetic sequence to tetrahydropyridine compounds.
Scheme 2: Resolution of enantiomers using D(-) mandelic acid.
Scheme 2: Resolution of enantiomers using D(-) mandelic acid.
The synthesis of these compounds involved the reduction of N-substituted 3-carbethoxy-4,4-ethylenedioxy piperidines 1a and 1b, [6,7] using lithium aluminum hydride to form 2a and 2b. The ketones in 2a and 2b were deprotected in acidic conditions, but the required N-substituted-3-hydroxymethyl-4-piperidones were not isolated. From the literature, [6] it was found that heating 2a with 20% hydrochloric acid resulted in dimerization leading to 2,8-dibenzyldecahydro-2H,5aH-4a,9a-epoxydipyrido [4,3-b:3'4'-f]oxepin-5a-ol. Accordingly, alcohols 2a and 2b were treated with methanesulfonylchloride, in the presence of base, which provided the required 3-mesylate derivatives 3a and 3b. Mesylates 3a and 3b were condensed with 3,4-methylenedioxyphenol in the presence of base and solvent to give ethers 4a and 4b. These ethers, on treatment with acid, underwent unexpected cyclization to give rise to 5a and 5b, instead of the desired ketone 4c. A proposed mechanism for the cyclodehydration leading to the cyclized product is discussed below. The cyclized product 5b, was dealkylated using chloroformates by two different methods to yield 6 as a free base. Also compound 6 was obtained by hydrogenolysis of 5a (Scheme 1).
The reaction mechanism is suggested to follow a ketal deprotection sequence to arrive at the 4-piperidone derivative, which is protonated and reacts with the aromatic ring to afford the six member heterocycle. Deprotonation restores the aromatic ring and subsequent elimination of water results in the stabilized cyclized product 5b. The structural elucidation of 5b confirmed the benzopyranopyridine skeleton (see below).
Figure 3: Proposed mechanism of tetrahydropyridine formation, Proposed mechanism.
Figure 3: Proposed mechanism of tetrahydropyridine formation, Proposed mechanism.
The saturated product cis-1,3,4,4a,5,10b-hexahydro-[6,7]-2H-[1]-benzopyrano- [3,4-c]pyridine 7 (skeleton C) was made by hydrogenation of N-benzylated benzopyranopyridine derivative 5a using 10% palladium on charcoal in a methanol-HCl mixture (Scheme 2). Initial hydrogenation experiments provided N-benzyl saturated pyridine derivative 8 and N-unsubstituted unsaturated product 6. After extended reaction time, debenzylated saturated product 7 was formed with the cis configuration. Compound 6 can be obtained by terminating the reaction after 4–6 hours followed by chromatographic purification of the crude. The enantiomers were separated by diastereomeric resolution using D-(-)-mandelic acid as the chiral agent. The absolute configuration of the mandelate salt of 7b was determined as S,S by X-ray diffraction analysis (Figure 4) (For details see also: Figure 4 and Supporting Information File 1).
Figure 4: Molecular structure of the mandalate salt.
Figure 4: Molecular structure of the mandalate salt.
Structure elucidation of 5b
The mass spectrum of 5b displayed a molecular ion at m/z 245 corresponding to the molecular formula C14H15NO3. Interestingly the molecular ion is 18 amu less than the expected structure 4c (R = Me). The 1H NMR data in the aromatic region showed only two sharp singlets at 6.4 and 6.6 ppm contrary to the three expected signals i.e. one singlet and two doublet signals for the structure 4c (R = Me). The missing aromatic signal in structure 5b could have been involved in the bond formation. It is also interesting to note that in the DEPT experiment, the only aliphatic methine signal expected for the structure 4c (R = Me) is not present. The absence of characteristic ketonic absorption in IR and absence of quaternary signal beyond 150 ppm in 13C NMR indicated the absence of a ketonic functionality in structure 5b, which is further confirmed by the NOESY experiment, and the through space interactions (nOe's) are shown in 5b-nOe. The formation of this new skeletal system has been confirmed beyond doubt by the single crystal X-ray studies of 7b. The compound 7 is formed from the hydrogenation of 5a and 6.
Conclusion
In summary, the synthesis of novel cis-1,3,4,4a,5,10b-hexahydro-[6,7]-2H-[1]benzopyrano [3,4-c]pyridine 7 is achieved using a different synthetic route than that reported for a similar ring structure with a trans configuration. This paper reports the formation of 7 via key intermediates 5a and 5b. It is hypothesized that the presence of the activated aromatic ring facilitates the cyclodehydration in acidic medium leading to the benzopyranopyridine derivative. The saturated cis derivative 7 is successfully resolved to get the individual enantiomers 7a and 7b. The absolute configuration is determined by single crystal X-ray analysis of the mandelate salt.
Supporting Information
| Supporting Information File 1: Supplementary experimental data. The file contains all experimental procedure and analytical data belonging to the compounds described in the article. | ||
| Format: DOC | Size: 43.0 KB | Download |
References
-
Christensen, J. A.; Squires, R. F. 4-Phenylpiperidine Compounds. U.S. Patent 3,912,743, Oct 14, 1975.
Return to citation in text: [1] -
Connor, D. T.; Unangst, P. C.; Schwender, C. F.; Sorenson, R. J.; Carethers, M. E.; Puchalski, C.; Brown, R. E. J. Heterocycl. Chem. 1984, 21, 1557–1559.
Return to citation in text: [1] -
Connor, D. T.; Unangst, P. C.; Schwender, C. F.; Sorenson, R. J.; Carethers, M. E.; Puchalski, C.; Brown, R. E. J. Heterocycl. Chem. 1984, 21, 1561–1564.
Return to citation in text: [1] -
Pars, H. G.; Granchelli, F. E.; Razdan, R. K.; Keller, J. K.; Teiger, D. G.; Rosenberg, F. J.; Harris, L. S. J. Med. Chem. 1976, 19, 445–454. doi:10.1021/jm00226a001
Return to citation in text: [1] -
Meyer, M. D.; Altenbach, R. J.; Basha, F. Z.; Carroll, W. A.; Drizin, I.; Kerwin, J. F., Jr.; Wendt, M. D. Benzopyranopyrrole and Benzopyranopyridine α-1 Adenergic Compounds. U.S. Patent 5,891,882, April 6, 1999.
Return to citation in text: [1] [2] [3] -
Nagai, Y.; Uno, H.; Umemoto, S. Chem. Pharm. Bull. 1977, 25, 1911–1922.
Return to citation in text: [1] [2] -
Alam, M.; Baty, J. D.; Jones, G.; Moore, C. J. Chem. Soc. C 1969, 1520–1528.
Return to citation in text: [1]
| 1. | Christensen, J. A.; Squires, R. F. 4-Phenylpiperidine Compounds. U.S. Patent 3,912,743, Oct 14, 1975. |
| 5. | Meyer, M. D.; Altenbach, R. J.; Basha, F. Z.; Carroll, W. A.; Drizin, I.; Kerwin, J. F., Jr.; Wendt, M. D. Benzopyranopyrrole and Benzopyranopyridine α-1 Adenergic Compounds. U.S. Patent 5,891,882, April 6, 1999. |
| 4. | Pars, H. G.; Granchelli, F. E.; Razdan, R. K.; Keller, J. K.; Teiger, D. G.; Rosenberg, F. J.; Harris, L. S. J. Med. Chem. 1976, 19, 445–454. doi:10.1021/jm00226a001 |
| 3. | Connor, D. T.; Unangst, P. C.; Schwender, C. F.; Sorenson, R. J.; Carethers, M. E.; Puchalski, C.; Brown, R. E. J. Heterocycl. Chem. 1984, 21, 1561–1564. |
| 2. | Connor, D. T.; Unangst, P. C.; Schwender, C. F.; Sorenson, R. J.; Carethers, M. E.; Puchalski, C.; Brown, R. E. J. Heterocycl. Chem. 1984, 21, 1557–1559. |
| 6. | Nagai, Y.; Uno, H.; Umemoto, S. Chem. Pharm. Bull. 1977, 25, 1911–1922. |
| 7. | Alam, M.; Baty, J. D.; Jones, G.; Moore, C. J. Chem. Soc. C 1969, 1520–1528. |
| 5. | Meyer, M. D.; Altenbach, R. J.; Basha, F. Z.; Carroll, W. A.; Drizin, I.; Kerwin, J. F., Jr.; Wendt, M. D. Benzopyranopyrrole and Benzopyranopyridine α-1 Adenergic Compounds. U.S. Patent 5,891,882, April 6, 1999. |
| 5. | Meyer, M. D.; Altenbach, R. J.; Basha, F. Z.; Carroll, W. A.; Drizin, I.; Kerwin, J. F., Jr.; Wendt, M. D. Benzopyranopyrrole and Benzopyranopyridine α-1 Adenergic Compounds. U.S. Patent 5,891,882, April 6, 1999. |
© 2006 Mohakhud et al; licensee Beilstein-Institut.
This is an Open Access article under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
The license is subject to the Beilstein Journal of Organic Chemistry terms and conditions: (http://www.beilstein-journals.org/bjoc)