Abstract
The new chiral ligands I–III based on derivatives of imidazolidin-4-one were synthesised and characterised. The catalytic activity and enantioselectivity of their corresponding copper(II) complexes were studied in asymmetric Henry reactions. It was found that the enantioselectivity of these catalysts is overall very high and depends on the relative configuration of the ligand used; cis-configuration of ligand affords the nitroaldols with major enantiomer S- (up to 97% ee), whereas the application of ligands with trans-configuration led to nitroaldols with major R-enantiomer (up to 96% ee). The “proline-type” ligand IV was also tested in asymmetric aldol reactions. Under the optimised reaction conditions, aldol products with enantioselectivities of up to 91% ee were obtained.
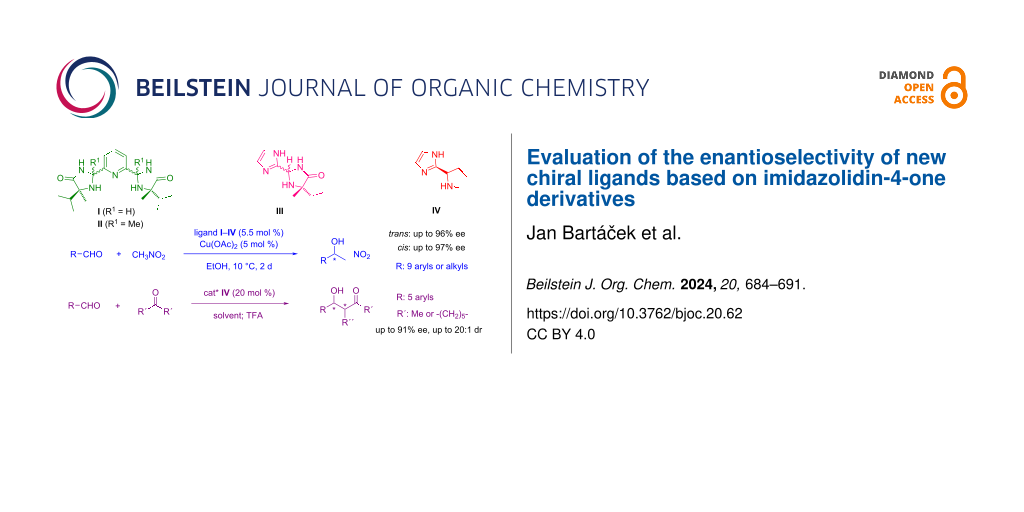
Graphical Abstract
Introduction
The application of chiral metal complexes as enantioselective catalysts is among the fundamental strategies for preparing compounds in non-racemic forms [1-4]. These complexes typically comprise a chelating chiral ligand capable of coordinating with a metal ion; otherwise, a metal atom itself constitutes a stereocentre [4]. The specific pairing of a chiral ligand and a metal ion is essential for the catalytic characteristics and its effectiveness of the complex in asymmetric syntheses [1-3]. In recent years, our research group has synthesised a series of chiral ligands based on 2-(pyridin-2-yl)imidazolidin-4-one, differentiated by various substitutions at the imidazolidine ring [5-7]. Their copper(II) complexes were evaluated as efficient enantioselective catalysts, particularly in asymmetric Henry reactions (Scheme 1). Subsequent research has led to the development of various catalytic arrangements of the most enantioselective catalysts, including anchoring on polystyrene beads [8,9], magnetic nanoparticles [10], or block copolymers composed of PEG-poly(Glu) [11]. These modifications have facilitated the recycling of the catalysts, offering numerous advantages, including enhanced economic efficiency, eco-friendly practices, and reduced waste. Catalysts with the highest enantioselectivity have been employed in the synthesis of essential chiral intermediates for drug production, such as amprenavir [12], rivaroxaban [13,14], linezolid [13] and salmeterol [7]. Therefore, chiral 2-(pyridin-2-yl)imidazolidin-4-one derivatives stand out as a prominent class of chiral nitrogen ligands in enantioselective catalysis.
Scheme 1: The preparation of 5-isopropyl-5-methyl-2-(pyridin-2-yl)imidazolidin-4-one derivatives and their application in asymmetric Henry reaction [5].
Scheme 1: The preparation of 5-isopropyl-5-methyl-2-(pyridin-2-yl)imidazolidin-4-one derivatives and their ap...
In this work, we focused on structurally modifying 2-(pyridin-2-yl)imidazolidin-4-one ligands to potentially expand the range of efficient catalysts. Our goal was to synthesise ligands featuring two chiral imidazolidin-4-one units linked to the pyridine ring at the 2- and 6- positions (Figure 1 – ligands I and II). These newly designed ligands represent tridentate entities. Such structure modification provides the ligands structurally conformable to well-known tridentate chiral ligands: PyBOX [15] and PyBidine [16]. Additionally, we explored further modifications by substituting the pyridine moiety with an imidazole ring in ligand III (Figure 1), motivated by DFT calculations (see Supporting Information File 1, part S4) which confirmed that imidazole has a similar donor ability to pyridine. This change reduces the ring size within the ligand structure, potentially increasing the space available for coordinating reacting species, thereby possibly enhancing catalytic activity and enantioselectivity. We also aimed to assess the impact of alkyl groups at the 5-position of the imidazolidine ring on the enantioselectivity of the reaction. Hence, we developed a ligand incorporating an unsubstituted pyrrolidine ring instead of the imidazolidine unit (Figure 1 – ligand IV). This structure characterises a 'proline-type' derivative, enabling its use not only in a chiral metal complex catalyst but also as an enantioselective organocatalyst [17]. Accordingly, its application in enantioselective organocatalysis, particularly in asymmetric reactions through “enamine activation”, warrants further investigation.
Figure 1: The structure of newly designed ligands I–IV.
Figure 1: The structure of newly designed ligands I–IV.
Results and Discussion
The corresponding copper(II) acetate complexes of ligands I–IV were screened as enantioselective catalysts for asymmetric Henry reactions. The series of nine different aldehydes (aliphatic, aromatic bearing an electron-withdrawing or an electron-donating group, heteroaromatic) was chosen for these studies. The reaction conditions (i.e., temperature, reaction time, amount of catalyst, solvent) were adopted from the pilot study [5] for relevant comparison of catalyst characteristics.
From Table 1 and Table 2, which summarise results obtained using tridentate ligands Ia–c and IIa–c, it is evident that the catalytic activity their copper(II) complexes is comparable with analogous complexes of bidentate ligands [5] (for TON and TOF parameters see Supporting Information File 1, part S5). The conversions were very high in almost all cases under set reaction conditions, except for pivalaldehyde. Here, the low conversions could be explained by the sterical demand of the aldehyde (R = t-Bu), leading to suppressing its coordination with the complex. The ee values achieved with the complex of ligand Ia were variable (29–83%), whereas better enantioselectivity was found for less reactive aldehydes (aliphatic and bearing electron-donating group). The copper complex bearing a ligand with trans-trans configuration (Ia) is less enantioselective than those bearing a bidentate ligand analogue (87–96% ee, see [5]). Interestingly, the introduction of a methyl group at position 2 of the imidazolidin-4-one ring, i.e., ligand IIa, led to an improvement of enantioselectivity and the ee values obtained by its copper(II) complex were satisfactory (75–96%). This phenomenon was more distinct in the case of the application complexes of ligands with cis-trans configuration (Ib vs IIb). Whilst the complex of Ib exhibited only poor enantioselectivity, the other afforded the nitroaldols with ee values of 60–90%. Finally, the complexes of ligands with cis-cis configuration (Ic and IIc) were evaluated. These catalyst are the most efficient catalysts, producing nitroaldols with very high enantiomeric purity (approx. 90–95% ee). This finding contrasts with the results obtained in the previous study [5], where the complex of the analogous bidentate ligand with cis configuration was found unsuccessful (≈25% ee; see Supporting Information File 1, part S5).
Table 1: Asymmetric Henry reactions of various aldehydes with nitromethane catalysed by copper(II) complexes of ligands Ia–c.
|
|
||||||
| Aldehyde |
Ligand Ia
(trans-trans) |
Ligand Ib
(cis-trans) |
Ligand Ic
(cis-cis) |
|||
| R | Conva [%] | eeb [%] | Conva [%] | eeb [%] | Conva [%] | eeb [%] |
| Ph | 99 | 60 (R) | 99 | 38 (R) | 99 | 94 (S) |
| 4-NO2C6H4 | 99 | 29 (R) | 99 | 8 (R) | 99 | 91 (S) |
| 2-CH3OC6H4 | 99 | 80 (R) | 99 | rac | 99 | 94 (S) |
| 4-ClC6H4 | 99 | 52 (R) | 99 | 7 (R) | 99 | 95 (S) |
| 2-thienyl | 94 | 66 (R) | 99 | 20 (R) | 86 | 95 (S) |
| naphth-2-yl | 96 | 69 (R) | 94 | 25 (R) | 94 | 93 (S) |
| PhCH2CH2 | 98 | 73 (R) | 91 | 38 (R) | 95 | 89 (S) |
| t-Bu | 35 | 80 (R) | 30 | 41 (R) | 32 | 96 (S) |
| iPr | 98 | 83 (R) | 94 | 43 (R) | 99 | 91 (S) |
aThe conversion was determined by 1H NMR analysis of the crude product. bThe enantiomeric excess was determined by chiral HPLC.
Table 2: Asymmetric Henry reactions of various aldehydes with nitromethane catalysed by copper(II) complexes of ligands IIa–c.
|
|
||||||
| Aldehyde |
Ligand IIa
(trans-trans) |
Ligand IIb
(cis-trans) |
Ligand IIc
(cis-cis) |
|||
| R | Conva [%] | eeb [%] | Conva [%] | eeb [%] | Conva [%] | eeb [%] |
| Ph | 99 | 80 (R) | 99 | 70 (R) | 99 | 90 (S) |
| 4-NO2C6H4 | 99 | 75 (R) | 99 | 60 (R) | 99 | 82 (S) |
| 2-CH3OC6H4 | 99 | 96 (R) | 99 | 77 (R) | 99 | 86 (S) |
| 4-ClC6H4 | 99 | 88 (R) | 99 | 85 (R) | 99 | 96 (S) |
| 2-thienyl | 95 | 90 (R) | 98 | 86 (R) | 97 | 97 (S) |
| naphth-2-yl | 97 | 93 (R) | 97 | 86 (R) | 96 | 96 (S) |
| PhCH2CH2 | 92 | 75 (R) | 95 | 87 (R) | 97 | 91 (S) |
| t-Bu | 31 | 92 (R) | 42 | 93 (R) | 48 | 96 (S) |
| iPr | 98 | 96 (R) | 99 | 90 (R) | 93 | 96 (S) |
aThe conversion was determined by 1H NMR analysis of the crude product. bThe enantiomeric excess was determined by chiral HPLC.
According to expectations resulted from previous studies [5-14], the complexes of the ligands Ia and IIa afforded the nitroaldols with the prevailing configuration R, while the complexes of Ic and IIc with prevailing configuration S. The application of the complexes of the ligands Ib and IIb (cis-trans form) also led in all cases to nitroaldols with some excess of the R-enantiomer. A possible explanation of these results is illustrated in Figure 2, which shows plausible transition structures for the Henry reaction. Due to the Jahn–Teller effect, i.e., distortion of the octahedral Cu(II) complex forming four equatorial and two perpendicular coordination sites [18], the effective transition state includes the electrophile positioned in the equatorial site (strongly coordinated) and the nucleophile in the perpendicular site (weakly coordinated) [19]. The most favourable orientation of aldehyde should be out of the ligand’s molecular parts, thus forming E-configuration at the C=O bond. In this manner, the asymmetric induction results in the restricted addition of nitromethane influenced by imidazolidin-4-one moieties. The higher enantioselectivity of complexes of the ligands with cis-cis configuration could be explained by the fact that the larger (RL) alkyl group is located toward the reactants. Thus, it blocks unfavourable Si-attack more effectively. Similarly, for the complexes bearing cis-trans ligands, Si-attack is preferred due to the higher restriction of imidazolidin-4-one with cis-configuration, i.e., by more significant steric demand of the RL group.
Figure 2: Plausible transition structures for the Henry reaction.
Figure 2: Plausible transition structures for the Henry reaction.
Next, Table 3 summarises the results of Henry reactions obtained by catalysis with copper(II) complexes of ligands IIIa,b. The mutual comparison of these catalysts with those composed of analogous 2-(pyridin-2-yl)imidazolidin-4-ones [5] follows several significant conclusions. For instance, the catalyst of ligand IIIa possesses lower catalytic activity than the pyridine derivative (for TON and TOF parameters see Supporting Information File 1, part S5). The conversions were poor in the case of less reactive aldehydes, e.g., thiophene-2-carbaldehyde, and 3-phenylpropionaldehyde. To achieve satisfactory yields, the reaction time could be extended. Nevertheless, the ee values obtained by catalysts of IIIa vary in the range of 84–96%; hence its enantioselectivity is comparable with the pyridine analogue [5]. Notably, the complex of ligand IIIb having cis-configuration is more enantioselective than the pyridine derivative [5] (see Supporting Information File 1, part S5); the ee values were overall high (74–92%), however, a little bit lower than those achieved by the complex of ligand IIIa (Δ 1–15% ee).
Table 3: Asymmetric Henry reactions of various aldehydes with nitromethane catalysed by copper(II) complexes of ligands IIIa and IIIb.
|
|
||||
| Aldehyde |
Ligand IIIa
(trans) |
Ligand IIIb
(cis) |
||
| R | Conva [%] | eeb [%] | Conva [%] | eeb [%] |
| Ph | 63 | 89 (R) | 57 | 86 (S) |
| 4-NO2C6H4 | 97 | 84 (R) | 89 | 76 (S) |
| 2-CH3OC6H4 | 98 | 94 (R) | 89 | 93 (S) |
| 4-ClC6H4 | 82 | 89 (R) | 76 | 85 (S) |
| 2-thienyl | 40 | 88 (R) | 36 | 80 (S) |
| naphth-2-yl | 63 | 89 (R) | 63 | 84 (S) |
| PhCH2CH2 | 45 | 89 (R) | 44 | 74 (S) |
| t-Bu | 62 | 96 (R) | 49 | 92 (S) |
| iPr | 90 | 94 (R) | 64 | 88 (S) |
aThe conversion was determined by 1H NMR analysis of the crude product. bThe enantiomeric excess was determined by chiral HPLC.
Further, the copper(II) complex of ligand IV were also tested for asymmetric Henry reaction. The ligand IV structure arose from the ligand IIIb structure – the imidazolidin-4-one ring present at ligand IIIb was formally replaced by a pyrrolidine one. Here, we presume the comparable coordinating ability of both species of heterocycles. However, the pyrrolidine cycle of ligand IV does not contain alkyl groups at position 5. Therefore, the ee values obtained by the complex of ligand IV in the Henry reaction could be used to estimate the impact of this substitution on the resulting enantioselectivity of the studied ligands. The results summarised in Table 4 show that the complex of ligand IV is significantly less enantioselective (37–55% ee) than the complex of ligand IIIb. Thus, the alkyl substitution at position 5 of the imidazolidin-4-one or pyrrolidine ring of ligands I–IV can be considered as a fundamental part of the ligand’s structure, which enables them to possess high enantioselectivity. All nitroaldols were obtained with an excess of S-enantiomer, likewise in catalysis by copper(II) complexes of ligands Ic, IIc and IIIb, as well as ligands based on pyridine-(imidazolidin-4-one) [5-7] owned the S-configuration at the imidazolidin-4-one ring (see Supporting Information File 1, part S5). Hence, the configuration of a ligand at position 2 determines the type of enantiomer of nitroaldol in excess and the environment around the stereogenic centre at position 5 affects the resulting value of ee.
Table 4: Asymmetric Henry reactions of various aldehydes with nitromethane catalysed by copper(II) complex of ligand IV.
|
|
||
| Aldehyde | Ligand IV | |
| R | Conva [%] | eeb [%] |
| Ph | 97 | 50 (S) |
| 4-NO2C6H4 | 99 | 37 (S) |
| 2-CH3OC6H4 | 99 | 55 (S) |
| 4-ClC6H4 | 91 | 50 (S) |
| 2-thienyl | 99 | 36 (S) |
| naphth-2-yl | 98 | 46 (S) |
| PhCH2CH2 | 92 | 50 (S) |
| t-Bu | 40 | 52 (S) |
| iPr | 75 | 49 (S) |
aThe conversion was determined by 1H NMR analysis of the crude product. bThe enantiomeric excess was determined by chiral HPLC.
The structure of compound IV is similar to the well-known organocatalyst 5-(S)-pyrrolidin-2-yl-1H-tetrazole, which was successfully used in many asymmetric reactions via “enamine activation”, especially in asymmetric aldol reactions [20-23]. Moreover, compound IV was previously included in a study dealing with asymmetric cascade reactions (based on aldol reactions) of aldehydes with α-keto acids producing chiral isotetronic acids. However, the application of compound IV as the organocatalyst in these reactions proceeded sluggishly, and the corresponding products were obtained in only moderate ees [24]. Herein, the aldol reaction was chosen as a standard asymmetric reaction to explore the catalytic features of the proline derivate IV in detail.
Although various proline derivatives have been described for the use in asymmetric aldol reactions [25], the optimal reaction conditions for achieving maximal enantioselectivity and conversions vary and must be tailored to the specific catalyst used. Thus, early attempts at the aldol reaction of 4-nitrobenzaldehyde with cyclohexanone were performed to evaluate the reaction parameters, i.e., solvent, reaction temperature, and amount of acidic additive (Table 5). Hence, using DMF at −25 °C were the most convenient conditions regarding diastereo- and enantioselectivity and chemical yield. Next, the series of aromatic aldehydes with different substitutions were tested under the set reaction conditions. The yields decreased expectably in the range from aldehydes with electron-withdrawing substituents to that with electron-donating substituents. Thus, 4-tolualdehyde afforded the aldol product only with a moderate yield of 30%; however, the diastereo- and enantioselectivity was satisfactory. A similar result was obtained in the reaction of 4-nitrobenzaldehyde with acetone. The comparison of IV with 5-(S)-pyrrolidin-2-yl-1H-tetrazole shows that both proline derivatives possess similar enantioselectivity. However, compound IV is less catalytically active, probably due to the lower acidity of protonated 1H-imidazole than 1H-tetrazole [17].
Table 5: Asymmetric aldol reactions of various aldehydes with ketones catalysed by compound IV.
|
|
||||||||
| Aldehyde R | Ketone R´-CO-R´ | Temp [°C] | Solvent | TFA [mol %] | Time [d] | Yield [%] | dra (anti/syn) | eeb (anti) [%] |
| 4-NO2C6H4 | -(CH2)5- | 20 | MeOH | 20 | 5 | 88 | 2.25:1 | 73 |
| 4-NO2C6H4 | -(CH2)5- | 3 | MeOH | 20 | 5 | 82 | 2.70:1 | 78 |
| 4-NO2C6H4 | -(CH2)5- | 3 | MeOH | 40 | 5 | 89 | 2.60:1 | 82 |
| 4-NO2C6H4 | -(CH2)5- | 3 | DMF | 20 | 3 | 96 | 2.70:1 | 83 |
| 4-NO2C6H4 | -(CH2)5- | –25 | DMF | 20 | 5 | 82 | 7.70:1 | 90 |
| 4-NO2C6H4 | -(CH2)5- | –25 | DMF | 40 | 5 | 95 | 20.0:1 | 89 |
| 4-NO2C6H4 | -(CH2)5- | –25 | DMSO | 40 | 5 | 40 | 2.50:1 | 81 |
| 4-NO2C6H4 | -(CH2)5- | –25 | MeCN | 40 | 14 | 26 | 6.70:1 | 91 |
| 4-CNC6H4 | -(CH2)5- | –25 | DMF | 40 | 5 | 85 | 2.35:1 | 85 |
| 4-ClC6H4 | -(CH2)5- | –25 | DMF | 40 | 5 | 88 | 4.00:1 | 82 |
| Ph | -(CH2)5- | –25 | DMF | 40 | 5 | 56 | 11.0:1 | 86 |
| 4-CH3C6H4 | -(CH2)5- | –25 | DMF | 40 | 5 | 30 | 20.0:1 | 85 |
| 4-NO2C6H4 | CH3- | –25 | DMF | 40 | 5 | 36 | – | 84 |
aThe dr was determined by 1H NMR analysis of the crude product. bThe enantiomeric excess was determined by chiral HPLC.
Conclusion
In this study, we successfully synthesised enantiomerically pure stereoisomers of 2,2'-(pyridin-2,6-diyl)-bis(imidazolidin-4-one) derivatives, Ia–c and IIa–c, analogous to well-known tridentate chiral ligands such as PyBOX [15] and PyBidine [16]. The copper(II) complexes of these ligands demonstrated remarkable enantioselectivity in asymmetric Henry reactions, achieving enantiomeric excess values exceeding 90% in numerous instances, categorising them among the most efficient catalysts for this reaction type. A key finding of our research was the identification of position 2 on the imidazolidine-4-one unit as the crucial site for asymmetric induction. While the nature of the substituent at this chiral centre (i.e., hydrogen or methyl) influenced the catalytic results, it was always part of a more complex interplay involving the ligand–substrate combination, suggesting that the type of the substituent was not the sole determining factor.
Our investigation also revealed that the configuration of the chiral centre at position 5 significantly influences the catalyst's activity. While the bidentate 2-(pyridin-2-yl)imidazolidin-4-one ligands with a trans configuration at this position outperformed their cis counterparts (see Supporting Information File 1, part S5), the tridentate ligands (Ia–c and IIa–c) exhibited higher enantioselectivity with a cis configuration at position 5 relative to position 2. Additionally, when mixed configurations were present at position 2 in tridentate ligands (Ib and IIb), the 2R configuration facilitated more effective chirality transfer.
Substituting the pyridine moiety with an imidazole in ligands IIIa,b led to a lower reaction rate compared to their pyridine counterparts. However, the cis diastereomer (IIIb) showed significantly enhanced enantioselectivity compared to its equivalent pyridine-based ligand with the same configuration (see Supporting Information File 1, part S5). This change from pyridine to imidazole lowered the reaction rate but did not alter the critical role of configuration at position 2 in determining the enantiomeric purity of the final product.
Furthermore, the study of “proline-type” derivative IV underscored the importance of position 2 in chirality transfer, although with reduced efficiency in the absence of a second stereogenic centre at position 5. Nonetheless, IV proved to be an effective enantioselective organocatalyst in the asymmetric aldol reaction, matching the enantioselectivity levels of other proline-heterocycle derivatives [25].
Overall, our findings underscore the paramount importance of the stereochemistry at position 2 in chiral ligands dictating enantioselectivity. This study provides essential insights for the design of asymmetric catalysts, especially for Henry and aldol reactions.
Supporting Information
| Supporting Information File 1: General information and experimental data of prepared compounds, copies of 1H and 13C NMR spectra and DFT calculations. | ||
| Format: PDF | Size: 2.8 MB | Download |
Acknowledgements
The parts of this work were published in the diploma thesis by Karel Chlumský (“Synthesis and catalytic application of chiral 2,2'-(pyridine-2,6-diyl)-bis(imidazolidine-4-ones”) and in the diploma thesis by Jan Mrkvička (“Synthesis and catalytic application of 2-(proline-2-yl)imidazole”).
Data Availability Statement
All data that supports the findings of this study is available in the published article and/or the supporting information to this article.
References
-
Mikami, K.; Lautens, M., Eds. New Frontiers in Asymmetric Catalysis; John Wiley & Sons: New Jersey, 2007. doi:10.1002/0470098007
Return to citation in text: [1] [2] -
Caprio, V.; Williams, J. M. J. Catalysis in Asymmetric Synthesis; John Wiley & Sons: Oxford, UK, 2009.
Return to citation in text: [1] [2] -
Zhou, Q.-L., Ed. Privileged Chiral Ligands and Catalysts; Wiley-VCH: Weinheim, Germany, 2011. doi:10.1002/9783527635207
Return to citation in text: [1] [2] -
Steinlandt, P. S.; Zhang, L.; Meggers, E. Chem. Rev. 2023, 123, 4764–4794. doi:10.1021/acs.chemrev.2c00724
Return to citation in text: [1] [2] -
Panov, I.; Drabina, P.; Padělková, Z.; Šimůnek, P.; Sedlák, M. J. Org. Chem. 2011, 76, 4787–4793. doi:10.1021/jo200703j
Return to citation in text: [1] [2] [3] [4] [5] [6] [7] [8] [9] [10] [11] -
Drabina, P.; Karel, S.; Panov, I.; Sedlák, M. Tetrahedron: Asymmetry 2013, 24, 334–339. doi:10.1016/j.tetasy.2013.02.007
Return to citation in text: [1] [2] [3] -
Drabina, P.; Horáková, E.; Růžičková, Z.; Sedlák, M. Tetrahedron: Asymmetry 2015, 26, 141–147. doi:10.1016/j.tetasy.2015.01.001
Return to citation in text: [1] [2] [3] [4] -
Harmand, L.; Drabina, P.; Pejchal, V.; Husáková, L.; Sedlák, M. Tetrahedron Lett. 2015, 56, 6240–6243. doi:10.1016/j.tetlet.2015.09.112
Return to citation in text: [1] [2] -
Nováková, G.; Drabina, P.; Frumarová, B.; Sedlák, M. Adv. Synth. Catal. 2016, 358, 2541–2552. doi:10.1002/adsc.201600198
Return to citation in text: [1] [2] -
Bhosale, D. S.; Drabina, P.; Kincl, M.; Vlček, M.; Sedlák, M. Tetrahedron: Asymmetry 2015, 26, 1300–1306. doi:10.1016/j.tetasy.2015.10.003
Return to citation in text: [1] [2] -
Bhosale, D. S.; Drabina, P.; Palarčík, J.; Hanusek, J.; Sedlák, M. Tetrahedron: Asymmetry 2014, 25, 334–339. doi:10.1016/j.tetasy.2014.01.001
Return to citation in text: [1] [2] -
Panov, I.; Drabina, P.; Hanusek, J.; Sedlák, M. Synlett 2013, 24, 1280–1282. doi:10.1055/s-0033-1338803
Return to citation in text: [1] [2] -
Drabina, P.; Feixová, V.; Sedlák, M. Tetrahedron Lett. 2019, 60, 99–101. doi:10.1016/j.tetlet.2018.11.067
Return to citation in text: [1] [2] [3] -
Vrbický, M.; Macek, K.; Pochobradský, J.; Svoboda, J.; Sedlák, M.; Drabina, P. Beilstein J. Org. Chem. 2022, 18, 438–445. doi:10.3762/bjoc.18.46
Return to citation in text: [1] [2] -
Desimoni, G.; Faita, G.; Quadrelli, P. Chem. Rev. 2003, 103, 3119–3154. doi:10.1021/cr020004h
Return to citation in text: [1] [2] -
Arai, T.; Mishiro, A.; Yokoyama, N.; Suzuki, K.; Sato, H. J. Am. Chem. Soc. 2010, 132, 5338–5339. doi:10.1021/ja100265j
Return to citation in text: [1] [2] -
Dalko, P. I., Ed. Enantioselective Organocatalysis: Reaction and Experimental Procedures; Wiley-VCH: Weinheim, Germany, 2007. doi:10.1002/9783527610945
Return to citation in text: [1] [2] -
Hathaway, B. J. Copper. In Comprehensive Coordination Chemistry; Wilkinson, G., Ed.; Pergamon Press: New York, 1987; Vol. 5, Chapter 53, pp 533–774.
Return to citation in text: [1] -
Evans, D. A.; Seidel, D.; Rueping, M.; Lam, H. W.; Shaw, J. T.; Downey, C. W. J. Am. Chem. Soc. 2003, 125, 12692–12693. doi:10.1021/ja0373871
Return to citation in text: [1] -
Hartikka, A.; Arvidsson, P. I. Tetrahedron: Asymmetry 2004, 15, 1831–1834. doi:10.1016/j.tetasy.2004.04.029
Return to citation in text: [1] -
Torii, H.; Nakadai, M.; Ishihara, K.; Saito, S.; Yamamoto, H. Angew. Chem., Int. Ed. 2004, 43, 1983–1986. doi:10.1002/anie.200352724
Return to citation in text: [1] -
Grondal, C.; Enders, D. Tetrahedron 2006, 62, 329–337. doi:10.1016/j.tet.2005.09.060
Return to citation in text: [1] -
Thayumanavan, R.; Tanaka, F.; Barbas, C. F. Org. Lett. 2004, 6, 3541–3544. doi:10.1021/ol0485417
Return to citation in text: [1] -
Zhang, B.; Jiang, Z.; Zhou, X.; Lu, S.; Li, J.; Liu, Y.; Li, C. Angew. Chem., Int. Ed. 2012, 51, 13159–13162. doi:10.1002/anie.201206438
Return to citation in text: [1] -
Yamashita, Y.; Yasukawa, T.; Yoo, W.-J.; Kitanosono, T.; Kobayashi, S. Chem. Soc. Rev. 2018, 47, 4388–4480. doi:10.1039/c7cs00824d
Return to citation in text: [1] [2]
| 24. | Zhang, B.; Jiang, Z.; Zhou, X.; Lu, S.; Li, J.; Liu, Y.; Li, C. Angew. Chem., Int. Ed. 2012, 51, 13159–13162. doi:10.1002/anie.201206438 |
| 25. | Yamashita, Y.; Yasukawa, T.; Yoo, W.-J.; Kitanosono, T.; Kobayashi, S. Chem. Soc. Rev. 2018, 47, 4388–4480. doi:10.1039/c7cs00824d |
| 17. | Dalko, P. I., Ed. Enantioselective Organocatalysis: Reaction and Experimental Procedures; Wiley-VCH: Weinheim, Germany, 2007. doi:10.1002/9783527610945 |
| 1. | Mikami, K.; Lautens, M., Eds. New Frontiers in Asymmetric Catalysis; John Wiley & Sons: New Jersey, 2007. doi:10.1002/0470098007 |
| 2. | Caprio, V.; Williams, J. M. J. Catalysis in Asymmetric Synthesis; John Wiley & Sons: Oxford, UK, 2009. |
| 3. | Zhou, Q.-L., Ed. Privileged Chiral Ligands and Catalysts; Wiley-VCH: Weinheim, Germany, 2011. doi:10.1002/9783527635207 |
| 4. | Steinlandt, P. S.; Zhang, L.; Meggers, E. Chem. Rev. 2023, 123, 4764–4794. doi:10.1021/acs.chemrev.2c00724 |
| 8. | Harmand, L.; Drabina, P.; Pejchal, V.; Husáková, L.; Sedlák, M. Tetrahedron Lett. 2015, 56, 6240–6243. doi:10.1016/j.tetlet.2015.09.112 |
| 9. | Nováková, G.; Drabina, P.; Frumarová, B.; Sedlák, M. Adv. Synth. Catal. 2016, 358, 2541–2552. doi:10.1002/adsc.201600198 |
| 17. | Dalko, P. I., Ed. Enantioselective Organocatalysis: Reaction and Experimental Procedures; Wiley-VCH: Weinheim, Germany, 2007. doi:10.1002/9783527610945 |
| 5. | Panov, I.; Drabina, P.; Padělková, Z.; Šimůnek, P.; Sedlák, M. J. Org. Chem. 2011, 76, 4787–4793. doi:10.1021/jo200703j |
| 6. | Drabina, P.; Karel, S.; Panov, I.; Sedlák, M. Tetrahedron: Asymmetry 2013, 24, 334–339. doi:10.1016/j.tetasy.2013.02.007 |
| 7. | Drabina, P.; Horáková, E.; Růžičková, Z.; Sedlák, M. Tetrahedron: Asymmetry 2015, 26, 141–147. doi:10.1016/j.tetasy.2015.01.001 |
| 5. | Panov, I.; Drabina, P.; Padělková, Z.; Šimůnek, P.; Sedlák, M. J. Org. Chem. 2011, 76, 4787–4793. doi:10.1021/jo200703j |
| 1. | Mikami, K.; Lautens, M., Eds. New Frontiers in Asymmetric Catalysis; John Wiley & Sons: New Jersey, 2007. doi:10.1002/0470098007 |
| 2. | Caprio, V.; Williams, J. M. J. Catalysis in Asymmetric Synthesis; John Wiley & Sons: Oxford, UK, 2009. |
| 3. | Zhou, Q.-L., Ed. Privileged Chiral Ligands and Catalysts; Wiley-VCH: Weinheim, Germany, 2011. doi:10.1002/9783527635207 |
| 15. | Desimoni, G.; Faita, G.; Quadrelli, P. Chem. Rev. 2003, 103, 3119–3154. doi:10.1021/cr020004h |
| 4. | Steinlandt, P. S.; Zhang, L.; Meggers, E. Chem. Rev. 2023, 123, 4764–4794. doi:10.1021/acs.chemrev.2c00724 |
| 16. | Arai, T.; Mishiro, A.; Yokoyama, N.; Suzuki, K.; Sato, H. J. Am. Chem. Soc. 2010, 132, 5338–5339. doi:10.1021/ja100265j |
| 13. | Drabina, P.; Feixová, V.; Sedlák, M. Tetrahedron Lett. 2019, 60, 99–101. doi:10.1016/j.tetlet.2018.11.067 |
| 14. | Vrbický, M.; Macek, K.; Pochobradský, J.; Svoboda, J.; Sedlák, M.; Drabina, P. Beilstein J. Org. Chem. 2022, 18, 438–445. doi:10.3762/bjoc.18.46 |
| 7. | Drabina, P.; Horáková, E.; Růžičková, Z.; Sedlák, M. Tetrahedron: Asymmetry 2015, 26, 141–147. doi:10.1016/j.tetasy.2015.01.001 |
| 25. | Yamashita, Y.; Yasukawa, T.; Yoo, W.-J.; Kitanosono, T.; Kobayashi, S. Chem. Soc. Rev. 2018, 47, 4388–4480. doi:10.1039/c7cs00824d |
| 12. | Panov, I.; Drabina, P.; Hanusek, J.; Sedlák, M. Synlett 2013, 24, 1280–1282. doi:10.1055/s-0033-1338803 |
| 5. | Panov, I.; Drabina, P.; Padělková, Z.; Šimůnek, P.; Sedlák, M. J. Org. Chem. 2011, 76, 4787–4793. doi:10.1021/jo200703j |
| 11. | Bhosale, D. S.; Drabina, P.; Palarčík, J.; Hanusek, J.; Sedlák, M. Tetrahedron: Asymmetry 2014, 25, 334–339. doi:10.1016/j.tetasy.2014.01.001 |
| 15. | Desimoni, G.; Faita, G.; Quadrelli, P. Chem. Rev. 2003, 103, 3119–3154. doi:10.1021/cr020004h |
| 10. | Bhosale, D. S.; Drabina, P.; Kincl, M.; Vlček, M.; Sedlák, M. Tetrahedron: Asymmetry 2015, 26, 1300–1306. doi:10.1016/j.tetasy.2015.10.003 |
| 13. | Drabina, P.; Feixová, V.; Sedlák, M. Tetrahedron Lett. 2019, 60, 99–101. doi:10.1016/j.tetlet.2018.11.067 |
| 16. | Arai, T.; Mishiro, A.; Yokoyama, N.; Suzuki, K.; Sato, H. J. Am. Chem. Soc. 2010, 132, 5338–5339. doi:10.1021/ja100265j |
| 5. | Panov, I.; Drabina, P.; Padělková, Z.; Šimůnek, P.; Sedlák, M. J. Org. Chem. 2011, 76, 4787–4793. doi:10.1021/jo200703j |
| 5. | Panov, I.; Drabina, P.; Padělková, Z.; Šimůnek, P.; Sedlák, M. J. Org. Chem. 2011, 76, 4787–4793. doi:10.1021/jo200703j |
| 5. | Panov, I.; Drabina, P.; Padělková, Z.; Šimůnek, P.; Sedlák, M. J. Org. Chem. 2011, 76, 4787–4793. doi:10.1021/jo200703j |
| 5. | Panov, I.; Drabina, P.; Padělková, Z.; Šimůnek, P.; Sedlák, M. J. Org. Chem. 2011, 76, 4787–4793. doi:10.1021/jo200703j |
| 6. | Drabina, P.; Karel, S.; Panov, I.; Sedlák, M. Tetrahedron: Asymmetry 2013, 24, 334–339. doi:10.1016/j.tetasy.2013.02.007 |
| 7. | Drabina, P.; Horáková, E.; Růžičková, Z.; Sedlák, M. Tetrahedron: Asymmetry 2015, 26, 141–147. doi:10.1016/j.tetasy.2015.01.001 |
| 20. | Hartikka, A.; Arvidsson, P. I. Tetrahedron: Asymmetry 2004, 15, 1831–1834. doi:10.1016/j.tetasy.2004.04.029 |
| 21. | Torii, H.; Nakadai, M.; Ishihara, K.; Saito, S.; Yamamoto, H. Angew. Chem., Int. Ed. 2004, 43, 1983–1986. doi:10.1002/anie.200352724 |
| 22. | Grondal, C.; Enders, D. Tetrahedron 2006, 62, 329–337. doi:10.1016/j.tet.2005.09.060 |
| 23. | Thayumanavan, R.; Tanaka, F.; Barbas, C. F. Org. Lett. 2004, 6, 3541–3544. doi:10.1021/ol0485417 |
| 5. | Panov, I.; Drabina, P.; Padělková, Z.; Šimůnek, P.; Sedlák, M. J. Org. Chem. 2011, 76, 4787–4793. doi:10.1021/jo200703j |
| 5. | Panov, I.; Drabina, P.; Padělková, Z.; Šimůnek, P.; Sedlák, M. J. Org. Chem. 2011, 76, 4787–4793. doi:10.1021/jo200703j |
| 19. | Evans, D. A.; Seidel, D.; Rueping, M.; Lam, H. W.; Shaw, J. T.; Downey, C. W. J. Am. Chem. Soc. 2003, 125, 12692–12693. doi:10.1021/ja0373871 |
| 5. | Panov, I.; Drabina, P.; Padělková, Z.; Šimůnek, P.; Sedlák, M. J. Org. Chem. 2011, 76, 4787–4793. doi:10.1021/jo200703j |
| 5. | Panov, I.; Drabina, P.; Padělková, Z.; Šimůnek, P.; Sedlák, M. J. Org. Chem. 2011, 76, 4787–4793. doi:10.1021/jo200703j |
| 6. | Drabina, P.; Karel, S.; Panov, I.; Sedlák, M. Tetrahedron: Asymmetry 2013, 24, 334–339. doi:10.1016/j.tetasy.2013.02.007 |
| 7. | Drabina, P.; Horáková, E.; Růžičková, Z.; Sedlák, M. Tetrahedron: Asymmetry 2015, 26, 141–147. doi:10.1016/j.tetasy.2015.01.001 |
| 8. | Harmand, L.; Drabina, P.; Pejchal, V.; Husáková, L.; Sedlák, M. Tetrahedron Lett. 2015, 56, 6240–6243. doi:10.1016/j.tetlet.2015.09.112 |
| 9. | Nováková, G.; Drabina, P.; Frumarová, B.; Sedlák, M. Adv. Synth. Catal. 2016, 358, 2541–2552. doi:10.1002/adsc.201600198 |
| 10. | Bhosale, D. S.; Drabina, P.; Kincl, M.; Vlček, M.; Sedlák, M. Tetrahedron: Asymmetry 2015, 26, 1300–1306. doi:10.1016/j.tetasy.2015.10.003 |
| 11. | Bhosale, D. S.; Drabina, P.; Palarčík, J.; Hanusek, J.; Sedlák, M. Tetrahedron: Asymmetry 2014, 25, 334–339. doi:10.1016/j.tetasy.2014.01.001 |
| 12. | Panov, I.; Drabina, P.; Hanusek, J.; Sedlák, M. Synlett 2013, 24, 1280–1282. doi:10.1055/s-0033-1338803 |
| 13. | Drabina, P.; Feixová, V.; Sedlák, M. Tetrahedron Lett. 2019, 60, 99–101. doi:10.1016/j.tetlet.2018.11.067 |
| 14. | Vrbický, M.; Macek, K.; Pochobradský, J.; Svoboda, J.; Sedlák, M.; Drabina, P. Beilstein J. Org. Chem. 2022, 18, 438–445. doi:10.3762/bjoc.18.46 |
| 18. | Hathaway, B. J. Copper. In Comprehensive Coordination Chemistry; Wilkinson, G., Ed.; Pergamon Press: New York, 1987; Vol. 5, Chapter 53, pp 533–774. |
© 2024 Bartáček et al.; licensee Beilstein-Institut.
This is an open access article licensed under the terms of the Beilstein-Institut Open Access License Agreement (https://www.beilstein-journals.org/bjoc/terms), which is identical to the Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0). The reuse of material under this license requires that the author(s), source and license are credited. Third-party material in this article could be subject to other licenses (typically indicated in the credit line), and in this case, users are required to obtain permission from the license holder to reuse the material.