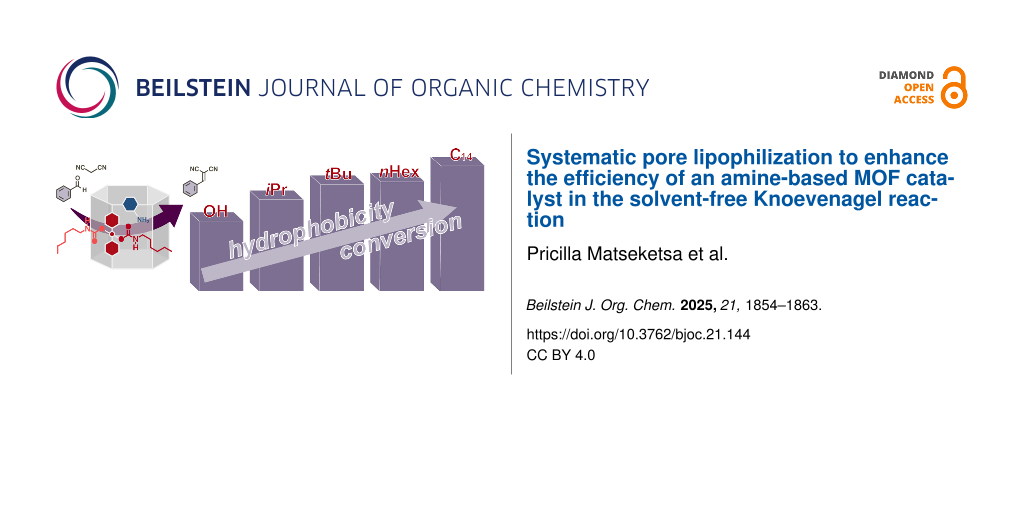
Abstract
We systematically lipophilized an amine-based metal-organic framework (MOF) catalyst and applied the functionalized MOFs to the Knoevenagel condensation reaction. A well-defined MOF material composed of both amine- and hydroxy-bearing linkers was reacted with a series of aliphatic isocyanates (isopropyl, tert-butyl, n-hexyl, and tetradecyl) and, incongruously, was found to preferentially react at the hydroxy groups. This selective functionalization yielded MOFs in which the catalytically active amines are confined within highly lipophilic pores, reminiscent of many enzyme active sites. We determined that systematically increasing the lipophilicity of the pores results in a commensurate increase of catalyst efficiency.

Graphical Abstract
Introduction
Most enzymatic reactions take place in multifunctional cavities in which multiple amino acid residues work cooperatively to orient and activate reactants [1-3]. These residues may also enhance covalent and/or acid–base catalysis via any combination of non-covalent interactions (hydrogen bonding, π–π stacking, lipophilic interactions, etc) [4-6]. Inspired by enzymes, Nature's most efficient catalysts, chemists have long endeavored to synthesize catalytic materials in which multiple functional groups are isolated together in confined space [7-9]. In the solid state, the generation of such multifunctional cavities has been pursued upon nanoporous scaffolds that include polymers of intrinsic microporosity (PIMs) [10-13], mesoporous silica materials (MSMs) [14-17], and metal-organic-frameworks (MOFs), among others [18-20]. Within this group of porous materials, MOFs boast the advantages of their crystallinity, the uniformity of their pores that are typically in the microporous range (5–20 Å), and the ability to fine-tune their pore chemical environment [21,22]. These attributes allow us to construct MOF-based catalysts with active sites that are isolated within cavities of the same size range as small molecules and whose walls are decorated with precisely located functional groups. We can rationally elaborate these functional groups to modulate catalytic performance and/or systematically investigate the influence of a particular chemical or structural property on catalyst efficiency [23].
For examples of tailoring the pore environment in MOF-based catalysts to modulate catalytic performance, we can refer to the elegant work of Telfer and co-workers. In two separate reports, they synthesized well-defined MOFs composed of three different linkers: a proline-functionalized linker acted as the catalytic unit, while two auxiliary linkers were varied to alter catalyst activity and enantioselectivity [24], or product selectivity [25]. In those works, the researchers tailored their catalyst via de novo solvothermal synthesis of their MOFs using differently substituted auxiliary linkers; a non-trivial effort which involves the synthesis of several new organic linkers and their subsequent assembly into completely new frameworks (Figure 1A). In this report we describe, how a similar tailoring of a MOF’s pore environment, with consequent activity modulation, can be realized more efficiently using covalent post-synthetic modification (PSM) strategies [26,27]. Starting with a single MOF material that has both catalytic linkers and auxiliary linkers that bear reactive “tags”, we can graft additional functionalities onto the auxiliaries to adjust the steric and electronic environment of the catalytic units (Figure 1B).
![[1860-5397-21-144-1]](/bjoc/content/figures/1860-5397-21-144-1.png?scale=2.0&max-width=1024&background=FFFFFF)
Figure 1: Schematic representation of the modulation of MOF pore environments. A) de novo synthesis of several MOFs requiring the multistep synthesis of different organic linkers. B) Synthesis of several frameworks from a single parent MOF using post-synthesis modification (PSM).
Figure 1: Schematic representation of the modulation of MOF pore environments. A) de novo synthesis of severa...
The advantages of PSM as a strategy for generating MOF-based catalysts are that we can efficiently generate several MOF catalysts from a single parent framework. Additionally, we can introduce new functionality into a MOF without changing the framework topology, thus minimizing the number of variables to consider as we study the influence of a particular property on catalytic performance. Perhaps more importantly for enzyme-inspired materials, PSM allows us to incorporate functionalities that are pertinent to catalysis but that would normally interfere with MOF assembly, e.g. hydrogen bonding groups like –OH and –COOH that are difficult to obtain as free uncoordinated moieties within MOF pores [28,29]. Given these benefits, it is no surprise that PSM is a prevalent method for synthesizing MOF-based catalysts [30].
On this topic, our current work was inspired by Canivet et al. who previously reported the lipophilization of a MOF by grafting long alkyl chains to the external surfaces of its crystals. The active sites are believed to be coordinatively unsaturated zincs at the MOF surfaces, and their lipophilization resulted in a greater than ten-fold increase in the initial rate of the reaction [31]. The promotion of this reaction was attributed to the repulsion of the water by-product by the lipophilic surface, thereby preventing its interference with the Lewis acidic catalyst surface sites, but we wondered, if similar reaction acceleration of a condensation reaction could be achieved by the lipophilization of the internal surfaces of an amine-based MOF catalyst.
The majority of studies of lipophilic MOFs applied to catalysis have focused on lipophilization to prevent water-based catalyst decomposition, with only a few investigating how lipophilic pores surfaces can increase catalyst efficiency [32,33], despite enzymes employing such a strategy. The lipophilicity of enzyme active sites tends to improve reaction rates by increasing the binding affinity for the lipophilic reactants and by decreasing the energy required to desolvate acid/base amino acid catalysts [34,35]. Lipophilicity has also been found to be beneficial in condensation reactions as the removed water molecules are repelled by the hydrophobic environment, suppressing the hydrolysis reaction that would return the starting materials [36]. Thus, in this work, we investigate the influence of pore lipophilicity on the amine-catalyzed Knoevenagel condensation.
The Knoevenagel condensation reaction is a vital organic reaction involving the condensation of carbonyl compounds, such as aldehydes or ketones, with active methylene compounds [37]. The resulting α,β-unsaturated carbonyl products can then be further elaborated to form natural products, therapeutic agents, polymers, pesticides, and insecticides [38], which have important applications in the pharmaceutical and agrochemical industries [38,39]. Various types of catalysts are used to enhance the efficiency and selectivity of the Knoevenagel reaction, including Lewis acids, ureas/ thioureas, amino acids, and bases such as alkali metal hydroxides, alkali metal alkoxide, amines, etc. [37,40-44].
Results and Discussion
We opted for amine-based catalysis using our modifiable framework KSU-1, a pillared paddlewheel MOF assembled using zinc, 2-aminobenzene-1,4-dicarboxylic acid (H2BDC-NH2), and meso-α,β-di(4-pyridyl)glycol (DPG), as our parent MOF (Figure 2A). As this is a well-defined mixed-linker MOF with molecular formula Zn2(BDC-NH2)2(DPG), each unit cell has a 2:1 ratio of dicarboxylate to dipyridyl and, therefore, a 1:1 ratio of amine (–NH2) to hydroxy (–OH) groups. The Zn atoms in the paddlewheel metal clusters are coordinatively saturated [45], thus we anticipated that only the amine group would function as the catalytic unit for the Knoevenagel reaction, while the hydroxy group would serve as a handle through which we would tune the lipophilicity of the catalyst.
![[1860-5397-21-144-2]](/bjoc/content/figures/1860-5397-21-144-2.png?scale=2.0&max-width=1024&background=FFFFFF)
Figure 2: A) Schematic representation of the reaction of KSU-1 with aliphatic isocyanates and the estimated conversions at –OH and –NH2. B) The corresponding 1H NMR spectra of the MOF reaction products digested in a solution of D2SO4 in DMSO-d6.
Figure 2: A) Schematic representation of the reaction of KSU-1 with aliphatic isocyanates and the estimated c...
Recently, we found that isopropyl isocyanate reacts preferentially at the DPG hydroxy groups of KSU-1 [46]; this, despite an earlier demonstration of the superior nucleophilicity of BDC-NH2 [45]. Interestingly, this apparent reversal in reactivity was most significant with aliphatic isocyanates, while the reactivity reverted to what is expected with the use of more activated isocyanates. Subsequently, we determined that, when incubated with secondary or tertiary isocyanates, KSU-1 reacts exclusively at the hydroxy groups of the DPG linker before proceeding to react at the amines of BDC-NH2 (Table 1, entries 1 and 2). Thus, we had a method to generate, from a single framework, a series of amine-based MOFs whose pores are uniformly decorated with different lipophilic groups (Figure 2A). Using this strategy, we quantitatively functionalized the –OH groups of KSU-1 with isopropyl and tert-butyl isocyanate. While primary isocyanates were less selective, starting to react at the amines before the hydroxy reaction was complete, we reacted KSU-1 with n-hexyl and tetradecyl isocyanate as well (Table 1, entries 3 and 4) because we wanted to use longer alkyl chains to further increase the lipophilicity.
Table 1: Estimated conversions of the reactions of isocyanates with the –OH and –NH2 groups of KSU-1 to form carbamates and ureas, respectively.a
| Entry | Isocyanate |
–OH % conv.
(stdev) |
–NH2 % conv.
(stdev) |
|---|---|---|---|
| 1 | isopropylb | 100 (0) | 0 |
| 2 | tert-butylc | 94 (5) | 0 |
| 3 | n-hexyld | 73 (2) | 11 (3) |
| 4 | tetradecyld | 64 (5) | 8 (3) |
a0.2 M in acetonitrile, 80 °C; b3 h; c4 h; d2 h.
To obtain our bifunctional amine-based KSU-1 MOF catalysts, we incubated KSU-1 in a 0.2 M solution of the respective isocyanate in acetonitrile at 80 °C; KSU-1 reacted with isopropyl, tert-butyl, n-hexyl, and tetradecyl isocyanate to generate KSU-1iPr and KSU-1t-Bu, KSU-1n-Hex, and KSU-1C14, respectively. Successful post synthetic reaction was observed by proton nuclear magnetic resonance (1H NMR) spectroscopy of the MOF product digested in D2SO4/DMSO-d6 (Figure 2B). Conversions were estimated by selecting the “same” proton in the starting material and in the products and integrating the corresponding peaks and setting the total to 100% (see Supporting Information File 1). We observed that the reactions with isopropyl isocyanate and tert-butyl isocyanate required 3 and 4 hours respectively to achieve complete conversion at the hydroxy group without any amine reaction. With n-hexyl isocyanate and tetradecyl isocyanate, reaction at the amine was observed after just one hour, before complete conversion at the hydroxy. To prevent excessive reaction at the amine, both reactions were stopped at 2 hours.
Aside from 1H NMR, the independent functionalization of KSU-1 with isocyanates was also confirmed by conducting electrospray ionization mass spectrometry (ESI–MS) on samples of the MOF products digested by 1,4-diazabicyclo[2.2.2]octane (DABCO) (Figures S4–S7, Supporting Information File 1). For KSU-1iPr and KSU-1t-Bu, the mass spectra in negative mode had [M − H]− peaks corresponding to deprotonated BDC-NH2, while in the positive mode, the [M + H]+ peaks indicated the presence of the protonated DPG dicarbamates, along with their various fragmentation products. The mass spectra for KSU-1n-Hex and KSU-1C14, show evidence of urea products in negative mode, and protonated DPG carbamates along with their fragmentation products in positive mode (Figures S4–S7, Supporting Information File 1). Powder X-ray diffraction (PXRD) confirmed that crystallinity was preserved even after complete functionalization of DPG (Figure S8, Supporting Information File 1).
To test the catalytic behavior of our amine-based lipophilic MOF catalysts, we chose the Knoevenagel condensation between benzaldehyde and malononitrile to form benzylidenemalononitrile (BMN). In a typical primary amine-catalyzed Knoevenagel condensation, the amine would undergo imine condensation with benzaldehyde. The imine would then deprotonate malononitrile, and the resulting carbanion would react with the imine, releasing the final product and the amine catalyst (Scheme 1A) [47,48]. However, there is another possible mechanism where malononitrile is deprotonated by the amine catalyst. Here, the resulting carbanion would attack benzaldehyde to form 2-(hydroxy(phenyl)methyl)malononitrile (HPMM) as an intermediate that then loses a water molecule, yielding BMN as the final product (Scheme 1B) [49].
Scheme 1: Probable mechanisms for the Knoevenegel condensation reaction between benzaldehyde and malononitrile catalyzed by a MOF-immobilized amine to form benzylidenemalononitrile (BMN). A) Mechanism in which the amine catalyst first undergoes imine condensation with benzaldehyde. B) Mechanism in which the amine acts as a base, deprotonating malononitrile.
Scheme 1: Probable mechanisms for the Knoevenegel condensation reaction between benzaldehyde and malononitril...
In the initial trial, 12 mol % of the MOF catalyst was added to a vial containing benzaldehyde, malononitrile, toluene as solvent, and dodecane as internal standard, and the reaction was shaken at 50 °C. Aliquots were collected at 30 minutes, diluted in CDCl3 and conversions were determined by analyzing the 1H NMR specta of the samples (Figure 3). The results showed a general increase in catalytic efficiency as we increased the lipophilicity of the MOF pores. However, the observed differences in conversion between the lipophilicized catalysts was marginal, with a variation of ≈8% (Table 2A). We were concerned that dodecane, our aliphatic internal standard, was negatively affecting the influence of lipophilization. Our working hypothesis was that increasing the lipophilicity of the MOF promotes the exclusion of water from the pores, which increases the rate of the condensation reaction. Thus, we initially supposed that, though dodecane was only 5.6 vol % to toluene, its addition was decreasing the difference in lipophilicity between the MOF and the bulk solution, reducing the driving force to remove water from the MOF pores.
Figure 3: A) Schematic representation of the reaction between benzaldehyde and malononitrile to form benzylidenemalononitrile (BMN). B) Representative 1H NMR spectra for the reaction of benzaldehyde and malononitrile in toluene with dodecane as internal standard, analyzed after 30 minutes.
Figure 3: A) Schematic representation of the reaction between benzaldehyde and malononitrile to form benzylid...
Table 2: Comparison of Knoevenagel catalysis results under different conditions.a
| Entry | Catalyst | % Conversion | |||
|---|---|---|---|---|---|
| A. toluene + dodecaneb | B. toluenec | C. toluened | D. neate | ||
| 1 | nonef | 0 (0) | 0 (0) | 0 (0) | 3.5 (1) |
| 2 | KSU-1 | 36 (2) | 8 (1) | 3 (0) | 43 (2) |
| 3 | KSU-1iPr | 48 (1) | 19 (2) | 3 (0.5) | 56 (3) |
| 4 | KSU-1t-Bu | 51 (1) | 23 (4) | 2.5 (0.5) | 65 (5) |
| 5 | KSU-1n-Hex | 51 (1) | 15 (3) | 5 (1) | 67 (5) |
| 6 | KSU-1C14 | 56 (3) | 16 (5) | 8 (0) | 77 (5) |
a0.0625 mmol benzaldehyde, 0.068 mmol malononitrile, 50 °C, 30 min; b12 mol % catalyst, 0.083 mmol dodecane, 250 μL toluene; c12 mol % catalyst, 250 μL toluene; d1.5 mol % catalyst, 250 μL toluene; e1.5 mol % catalyst; f0 mol % catalyst in all cases.
When we compared the conversions we obtained using the dodecane calibration curve with those determined by directly comparing the integrations of the benzaldehyde and BMN protons in the 1H NMR spectra, we found little difference (Table S2, Supporting Information File 1). Thus, we decided to discontinue the addition of internal standard to the catalysis reactions. Interestingly, performing the reaction without dodecane revealed significantly lower conversions (Table 2B). This led us to a modified hypothesis; namely that in a lipophilic environment, such as a lipophilicized pore or one containing a hydrophobic solvent, water molecules prefer to self-assemble to minimize interactions with the nonpolar solvent or pore walls. These clusters could be in the MOF channels or could conceivably be driven out of the hydrophobic frameworks into the bulk solvent where they can agglomerate with other water clusters to reduce overall surface tension.
Along with reduced conversions, we also found that our anticipated trend in catalyst efficiency, i.e. increasing conversions with the increasing lipophilicity of the carbamate substituents, was not consistently followed (Table 2B). While conversions increased going from no substitution, to isopropyl, then tert-butyl, subsequent increases in lipophilicity with n-hexyl and tetradecyl resulted in decreased conversions; a trend that can be more clearly perceived in Figure 4A. The combined dodecane-free results led us to speculate that it would be better to remove solvent as a variable and rely solely on MOF modification to create a lipophilic environment in the pores. However, because solvents improve the solubility of the reactants and products, making it easier for the reagents to reach the active sites, we were concerned that performing the reaction under neat conditions would be detrimental: malononitrile is a solid and the rapid formation of solid product BMN often results in a thick sludge that hinders the flow of the reaction solution. Additionally, we were concerned that the lack of solvent, would mean that the MOF catalyst would not be completely submerged in the reaction mixture.
Figure 4: Graphical representation of the Knoevenagel catalysis results. A) Comparison of the reaction in toluene with and without internal standard using 12 mol % catalyst. B) Catalysis performed under neat conditions and in toluene using 1.5 mol % catalyst.
Figure 4: Graphical representation of the Knoevenagel catalysis results. A) Comparison of the reaction in tol...
To address both concerns of performing the neat Knoevenagel reaction – product precipitation and the incomplete submersion of the MOF catalyst – we reduced the catalyst amount to 1.5 mol %. Surprisingly, the reaction conversions were higher than those in toluene with 12 mol % catalyst, and significantly so (ca. 10-fold) with a more appropriate comparison at the same catalyst loading of 1.5 mol% (Table 2C and D). Further, to our delight, KSU-1C14 was the most active catalyst achieving 77% conversion vs 43% for KSU-1 after 30 min. In addition, a remarkably clear and gradual increase in the rate of reaction was observed from KSU-1 < KSU-1iPr < KSU-1t-Bu < KSU-1n-Hex < KSU-1C14 (Figure 4B) even though roughly 10% of the –NH2 groups in both KSU-1n-Hex and KSU-1C14 had been converted to the less catalytically active alkyl ureas [50].
We should also point out that, despite the lower extent of alkyl grafting, the –CH2– content in the pores of KSU-1n-Hex and KSU-1C14 is still greater than for KSU-1iPr and KSU-1t-Bu, assuming a uniform distribution of alkyl chains. Additionally, although the introduction of large alkyl substituents in a MOF is associated with a reduction in pore accessibility, the lower percentage of alkyl grafting for both KSU-1n-Hex and KSU-1C14 results in similar solvent accessible volumes for all the modified MOFs, as shown by thermogravimetric analysis (TGA; Figure S9 in Supporting Information File 1). Thus, our results indicate that catalytic efficiency improves with the increasing lipophilicity of the alkyl chains, which is a result of the increase in surface area of the alkyl groups [51].
Of further interest are the results of the catalysis under the same neat conditions using the dimethyl ester of our catalytically active linker, Me2-BDC-NH2 (Table S3, Supporting Information File 1). We found that the conversions were significantly better than those reported for H2BDC-NH2 under more forcing conditions (60 °C, 6 h) [52], which makes sense given that the methyl substituents on the carboxylate increase the basicity of –NH2, which should increase the catalytic capability. What is surprising, however, is that the conversions for Me2BDC-NH2 are much lower than those observed for all our KSU-1 derivatives despite the presumably lower basicity of the MOF-immobilized amino groups due to the dicarboxylates coordinating to Zn2+ clusters. This unexpected result indicates that the reaction experiences a positive confinement effect which is enhanced further by increasing the lipophilicity of the pore environment (Figure S3, Supporting Information File 1).
Finally, we emphasize that our study was not geared toward obtaining particular conversions with our catalysts, but rather at investigating the effect that systematic pore environment modulation has on catalyst reactivity; specifically, how lipophilization affects a condensation process like the Knoevenagel reaction. As such, it was also interesting to observe differences in the relative amount of reaction intermediate depending on the lipophilicity of the pores. Under solvent-free conditions, we observed the formation of 2-(hydroxy(phenyl)methyl)malononitrile (HPMM, Figure 5), an intermediate which is subsequently dehydrated to yield the main product (BMN) as the reaction progresses [53]. Looking at the ratio of final product to intermediate (BMN:HPMM), we observed that relatively more of the hydroxy intermediate was observed with the unfunctionalized KSU-1 catalyst (Figure 5) when compared to the lipophilicized catalysts, with the amount of HPMM decreasing with increasing aliphatic chain surface area. This trend suggests that the lipophilic surfaces may destabilize hydrophilic intermediates, promoting faster conversion of HPMM to BMN, similar to the ground-state destabilization of polar substrates observed in enzymes with lipophilic pockets [54,55]. It should be noted that this result is in contrast to a previous reportthat observed an increase in the intermediate when a non-polar solvent is used in the grinding-assisted Knoevenagel reaction of the same reactants [56].
![[1860-5397-21-144-5]](/bjoc/content/figures/1860-5397-21-144-5.png?scale=2.0&max-width=1024&background=FFFFFF)
Figure 5: Left: comparison of BMN and HPMM protons in 1H NMR spectra. Note that the peaks corresponding to HPMM are under ×20 magnification. Right: ratios of BMN:HPMM products for each catalyst.
Figure 5: Left: comparison of BMN and HPMM protons in 1H NMR spectra. Note that the peaks corresponding to HP...
Conclusion
By employing a covalent post synthesis modification strategy that selectively introduces lipophilic functionality into MOFs, we confined catalytically active amines within MOF pores of systematically increasing lipophilicity. Our results reveal a clear correlation between increased pore lipophilicity and enhanced catalytic activity. Additionally, increasing lipophilicity resulted in congruent changes in the distribution of intermediate versus product during the reaction. Both these behaviors, increased efficiency and different intermediate:product distributions, call to mind the effect of lipophilic pockets in enzyme catalysis, and they offer a view to the possibilities that can be achieved in enzyme-inspired catalysis via the rational functionalization of MOF pores.
Supporting Information
| Supporting Information File 1: Experimental and characterization data. | ||
| Format: PDF | Size: 1.1 MB | Download |
Funding
We thank the National Science Foundation for support of this work. Specifically, we acknowledge support to our lab (Grant CHE-2240021), as well as an NSF-MRI grant to Kansas State University for the acquisition of the NMR spectrometer (Grant CHE-1826982) used in this study. We thank the Ping Li Lab for high-resolution mass spectrometry, and the NIH for the grant (R01GM117259-01S1) which partially funded the purchase of the instrument.
Data Availability Statement
Data generated and analyzed during this study is available from the corresponding author upon reasonable request.
References
-
Pravda, L.; Berka, K.; Svobodová Vařeková, R.; Sehnal, D.; Banáš, P.; Laskowski, R. A.; Koča, J.; Otyepka, M. BMC Bioinf. 2014, 15, 379. doi:10.1186/s12859-014-0379-x
Return to citation in text: [1] -
Marques, S. M.; Brezovsky, J.; Damborsky, J. Role of Tunnels and Gates in Enzymatic Catalysis. Understanding Enzymes; Jenny Standford Publishing: Singapore, 2016; pp 421–442.
Return to citation in text: [1] -
Zhang, Z.; Cai, Y.; Zheng, N.; Deng, Y.; Gao, L.; Wang, Q.; Xia, X. Biotechnol. Adv. 2024, 72, 108346. doi:10.1016/j.biotechadv.2024.108346
Return to citation in text: [1] -
Knowles, J. R. Nature 1991, 350, 121–124. doi:10.1038/350121a0
Return to citation in text: [1] -
Zhang, X.; Houk, K. N. Acc. Chem. Res. 2005, 38, 379–385. doi:10.1021/ar040257s
Return to citation in text: [1] -
Klinman, J. P.; Offenbacher, A. R.; Hu, S. J. Am. Chem. Soc. 2017, 139, 18409–18427. doi:10.1021/jacs.7b08418
Return to citation in text: [1] -
Cragg, P. J. Supramolecular Enzyme Mimics. In Supramolecular Chemistry: From Biological Inspiration to Biomedical Applications; Cragg, P. J., Ed.; Springer: Dordrecht, Netherlands, 2010; pp 113–151. doi:10.1007/978-90-481-2582-1_4
Return to citation in text: [1] -
Raynal, M.; Ballester, P.; Vidal-Ferran, A.; van Leeuwen, P. W. N. M. Chem. Soc. Rev. 2014, 43, 1734–1787. doi:10.1039/c3cs60037h
Return to citation in text: [1] -
Kuah, E.; Toh, S.; Yee, J.; Ma, Q.; Gao, Z. Chem. – Eur. J. 2016, 22, 8404–8430. doi:10.1002/chem.201504394
Return to citation in text: [1] -
Wulff, G. Chem. Rev. 2002, 102, 1–28. doi:10.1021/cr980039a
Return to citation in text: [1] -
Li, S.; Zhu, M.; Whitcombe, M. J.; Piletsky, S. A.; Turner, A. P. F. Molecularly Imprinted Polymers for Enzyme-like Catalysis: Principle, Design, and Applications. Molecularly Imprinted Catalysts; Elsevier: Amsterdam, 2016; pp 1–17. doi:10.1016/b978-0-12-801301-4.00001-3
Return to citation in text: [1] -
Bahrami, F.; Zhao, Y. Org. Lett. 2024, 26, 73–77. doi:10.1021/acs.orglett.3c03666
Return to citation in text: [1] -
Bhaskaran, A.; Aitken, H. M.; Xiao, Z.; Blyth, M.; Nothling, M. D.; Kamdar, S.; O’Mara, M. L.; Connal, L. A. Polymer 2021, 225, 123735. doi:10.1016/j.polymer.2021.123735
Return to citation in text: [1] -
Margelefsky, E. L.; Zeidan, R. K.; Davis, M. E. Chem. Soc. Rev. 2008, 37, 1118–1126. doi:10.1039/b710334b
Return to citation in text: [1] -
Shylesh, S.; Wagner, A.; Seifert, A.; Ernst, S.; Thiel, W. R. Chem. – Eur. J. 2009, 15, 7052–7062. doi:10.1002/chem.200900851
Return to citation in text: [1] -
Chandra, P.; Jonas, A. M.; Fernandes, A. E. J. Am. Chem. Soc. 2018, 140, 5179–5184. doi:10.1021/jacs.8b00872
Return to citation in text: [1] -
Fernandes, A. E.; Jonas, A. M. Catal. Today 2019, 334, 173–186. doi:10.1016/j.cattod.2018.11.040
Return to citation in text: [1] -
Zhang, M.; Gu, Z.-Y.; Bosch, M.; Perry, Z.; Zhou, H.-C. Coord. Chem. Rev. 2015, 293-294, 327–356. doi:10.1016/j.ccr.2014.05.031
Return to citation in text: [1] -
Fracaroli, A. M.; Siman, P.; Nagib, D. A.; Suzuki, M.; Furukawa, H.; Toste, F. D.; Yaghi, O. M. J. Am. Chem. Soc. 2016, 138, 8352–8355. doi:10.1021/jacs.6b04204
Return to citation in text: [1] -
Zhang, Y.; Gui, B.; Chen, R.; Hu, G.; Meng, Y.; Yuan, D.; Zeller, M.; Wang, C. Inorg. Chem. 2018, 57, 2288–2295. doi:10.1021/acs.inorgchem.7b03123
Return to citation in text: [1] -
Liu, J.; Chen, L.; Cui, H.; Zhang, J.; Zhang, L.; Su, C.-Y. Chem. Soc. Rev. 2014, 43, 6011–6061. doi:10.1039/c4cs00094c
Return to citation in text: [1] -
Alhumaimess, M. S. J. Saudi Chem. Soc. 2020, 24, 461–473. doi:10.1016/j.jscs.2020.04.002
Return to citation in text: [1] -
Garibay, S. J.; Wang, Z.; Cohen, S. M. Inorg. Chem. 2010, 49, 8086–8091. doi:10.1021/ic1011549
Return to citation in text: [1] -
Liu, L.; Zhou, T.-Y.; Telfer, S. G. J. Am. Chem. Soc. 2017, 139, 13936–13943. doi:10.1021/jacs.7b07921
Return to citation in text: [1] -
Zhou, T.-Y.; Auer, B.; Lee, S. J.; Telfer, S. G. J. Am. Chem. Soc. 2019, 141, 1577–1582. doi:10.1021/jacs.8b11221
Return to citation in text: [1] -
Cohen, S. M. Chem. Rev. 2012, 112, 970–1000. doi:10.1021/cr200179u
Return to citation in text: [1] -
Kalaj, M.; Cohen, S. M. ACS Cent. Sci. 2020, 6, 1046–1057. doi:10.1021/acscentsci.0c00690
Return to citation in text: [1] -
Chui, S. S.-Y.; Lo, S. M.-F.; Charmant, J. P. H.; Orpen, A. G.; Williams, I. D. Science 1999, 283, 1148–1150. doi:10.1126/science.283.5405.1148
Return to citation in text: [1] -
Rosi, N. L.; Kim, J.; Eddaoudi, M.; Chen, B.; O’Keeffe, M.; Yaghi, O. M. J. Am. Chem. Soc. 2005, 127, 1504–1518. doi:10.1021/ja045123o
Return to citation in text: [1] -
Gadzikwa, T.; Matseketsa, P. Dalton Trans. 2024, 53, 7659–7668. doi:10.1039/d4dt00514g
Return to citation in text: [1] -
Canivet, J.; Aguado, S.; Daniel, C.; Farrusseng, D. ChemCatChem 2011, 3, 675–678. doi:10.1002/cctc.201000386
Return to citation in text: [1] -
Liu, L.; Tao, Z.-P.; Chi, H.-R.; Wang, B.; Wang, S.-M.; Han, Z.-B. Dalton Trans. 2021, 50, 39–58. doi:10.1039/d0dt03635h
Return to citation in text: [1] -
Joharian, M.; Morsali, A.; Azhdari Tehrani, A.; Carlucci, L.; Proserpio, D. M. Green Chem. 2018, 20, 5336–5345. doi:10.1039/c8gc02367k
Return to citation in text: [1] -
Breslow, R. J. Phys. Org. Chem. 2006, 19, 813–822. doi:10.1002/poc.1037
Return to citation in text: [1] -
Blaha-Nelson, D.; Krüger, D. M.; Szeler, K.; Ben-David, M.; Kamerlin, S. C. L. J. Am. Chem. Soc. 2017, 139, 1155–1167. doi:10.1021/jacs.6b10801
Return to citation in text: [1] -
Zieniuk, B.; Białecka-Florjańczyk, E.; Wierzchowska, K.; Fabiszewska, A. World J. Microbiol. Biotechnol. 2022, 38, 11. doi:10.1007/s11274-021-03200-5
Return to citation in text: [1] -
Appaturi, J. N.; Ratti, R.; Phoon, B. L.; Batagarawa, S. M.; Din, I. U.; Selvaraj, M.; Ramalingam, R. J. Dalton Trans. 2021, 50, 4445–4469. doi:10.1039/d1dt00456e
Return to citation in text: [1] [2] -
Khare, R.; Pandey, J.; Smriti, S.; Ruchi, R. Orient. J. Chem. 2019, 35, 423–429. doi:10.13005/ojc/350154
Return to citation in text: [1] [2] -
Freeman, F. Chem. Rev. 1980, 80, 329–350. doi:10.1021/cr60326a004
Return to citation in text: [1] -
Prout, F. S. J. Org. Chem. 1953, 18, 928–933. doi:10.1021/jo50014a005
Return to citation in text: [1] -
Saghian, M.; Dehghanpour, S.; bayatani, Z. Sci. Rep. 2023, 13, 15563. doi:10.1038/s41598-023-42832-5
Return to citation in text: [1] -
Zarei, N.; Yarie, M.; Torabi, M.; Zolfigol, M. A. RSC Adv. 2024, 14, 1094–1105. doi:10.1039/d3ra08354c
Return to citation in text: [1] -
Calvino-Casilda, V.; Martín-Aranda, R. M.; López-Peinado, A. J.; Sobczak, I.; Ziolek, M. Catal. Today 2009, 142, 278–282. doi:10.1016/j.cattod.2008.08.023
Return to citation in text: [1] -
Qi, S.; Lan‐Xiang, S.; Ze‐Mei, G.; Tie‐Ming, C.; Run‐Tao, L. Chin. J. Chem. 2005, 23, 745–748. doi:10.1002/cjoc.200590745
Return to citation in text: [1] -
Samarakoon, K. P.; Satterfield, C. S.; McCoy, M. C.; Pivaral-Urbina, D. A.; Islamoglu, T.; Day, V. W.; Gadzikwa, T. Inorg. Chem. 2019, 58, 8906–8909. doi:10.1021/acs.inorgchem.9b00838
Return to citation in text: [1] [2] -
Matseketsa, P.; Mafukidze, D.; Pothupitiya, L.; Otuonye, U. P.; Mutlu, Y. Ç.; Averkiev, B. B.; Gadzikwa, T. Mol. Syst. Des. Eng. 2024, 9, 445–448. doi:10.1039/d3me00185g
Return to citation in text: [1] -
van Beurden, K.; de Koning, S.; Molendijk, D.; van Schijndel, J. Green Chem. Lett. Rev. 2020, 13, 349–364. doi:10.1080/17518253.2020.1851398
Return to citation in text: [1] -
Cortese, R.; Duca, D. Phys. Chem. Chem. Phys. 2011, 13, 15995–16004. doi:10.1039/c1cp21301f
Return to citation in text: [1] -
List, B. Angew. Chem., Int. Ed. 2010, 49, 1730–1734. doi:10.1002/anie.200906900
Return to citation in text: [1] -
van Schijndel, J.; Canalle, L. A.; Molendijk, D.; Meuldijk, J. Green Chem. Lett. Rev. 2017, 10, 404–411. doi:10.1080/17518253.2017.1391881
Return to citation in text: [1] -
Lee, A.; Mirica, K. A.; Whitesides, G. M. J. Phys. Chem. B 2011, 115, 1199–1210. doi:10.1021/jp107765h
Return to citation in text: [1] -
Zhai, Z.-W.; Yang, S.-H.; Lv, Y.-R.; Du, C.-X.; Li, L.-K.; Zang, S.-Q. Dalton Trans. 2019, 48, 4007–4014. doi:10.1039/c9dt00391f
Return to citation in text: [1] -
Antonangelo, A. R.; Hawkins, N.; Tocci, E.; Muzzi, C.; Fuoco, A.; Carta, M. J. Am. Chem. Soc. 2022, 144, 15581–15594. doi:10.1021/jacs.2c04739
Return to citation in text: [1] -
Biler, M.; Crean, R. M.; Schweiger, A. K.; Kourist, R.; Kamerlin, S. C. L. J. Am. Chem. Soc. 2020, 142, 20216–20231. doi:10.1021/jacs.0c10701
Return to citation in text: [1] -
Chen, D.; Li, Y.; Li, X.; Hong, X.; Fan, X.; Savidge, T. Chem. Sci. 2022, 13, 8193–8202. doi:10.1039/d2sc01994a
Return to citation in text: [1] -
Scheurrell, K.; Martins, I. C. B.; Murray, C.; Emmerling, F. Phys. Chem. Chem. Phys. 2023, 25, 23637–23644. doi:10.1039/d3cp02883f
Return to citation in text: [1]
| 51. | Lee, A.; Mirica, K. A.; Whitesides, G. M. J. Phys. Chem. B 2011, 115, 1199–1210. doi:10.1021/jp107765h |
| 52. | Zhai, Z.-W.; Yang, S.-H.; Lv, Y.-R.; Du, C.-X.; Li, L.-K.; Zang, S.-Q. Dalton Trans. 2019, 48, 4007–4014. doi:10.1039/c9dt00391f |
| 53. | Antonangelo, A. R.; Hawkins, N.; Tocci, E.; Muzzi, C.; Fuoco, A.; Carta, M. J. Am. Chem. Soc. 2022, 144, 15581–15594. doi:10.1021/jacs.2c04739 |
| 1. | Pravda, L.; Berka, K.; Svobodová Vařeková, R.; Sehnal, D.; Banáš, P.; Laskowski, R. A.; Koča, J.; Otyepka, M. BMC Bioinf. 2014, 15, 379. doi:10.1186/s12859-014-0379-x |
| 2. | Marques, S. M.; Brezovsky, J.; Damborsky, J. Role of Tunnels and Gates in Enzymatic Catalysis. Understanding Enzymes; Jenny Standford Publishing: Singapore, 2016; pp 421–442. |
| 3. | Zhang, Z.; Cai, Y.; Zheng, N.; Deng, Y.; Gao, L.; Wang, Q.; Xia, X. Biotechnol. Adv. 2024, 72, 108346. doi:10.1016/j.biotechadv.2024.108346 |
| 14. | Margelefsky, E. L.; Zeidan, R. K.; Davis, M. E. Chem. Soc. Rev. 2008, 37, 1118–1126. doi:10.1039/b710334b |
| 15. | Shylesh, S.; Wagner, A.; Seifert, A.; Ernst, S.; Thiel, W. R. Chem. – Eur. J. 2009, 15, 7052–7062. doi:10.1002/chem.200900851 |
| 16. | Chandra, P.; Jonas, A. M.; Fernandes, A. E. J. Am. Chem. Soc. 2018, 140, 5179–5184. doi:10.1021/jacs.8b00872 |
| 17. | Fernandes, A. E.; Jonas, A. M. Catal. Today 2019, 334, 173–186. doi:10.1016/j.cattod.2018.11.040 |
| 32. | Liu, L.; Tao, Z.-P.; Chi, H.-R.; Wang, B.; Wang, S.-M.; Han, Z.-B. Dalton Trans. 2021, 50, 39–58. doi:10.1039/d0dt03635h |
| 33. | Joharian, M.; Morsali, A.; Azhdari Tehrani, A.; Carlucci, L.; Proserpio, D. M. Green Chem. 2018, 20, 5336–5345. doi:10.1039/c8gc02367k |
| 10. | Wulff, G. Chem. Rev. 2002, 102, 1–28. doi:10.1021/cr980039a |
| 11. | Li, S.; Zhu, M.; Whitcombe, M. J.; Piletsky, S. A.; Turner, A. P. F. Molecularly Imprinted Polymers for Enzyme-like Catalysis: Principle, Design, and Applications. Molecularly Imprinted Catalysts; Elsevier: Amsterdam, 2016; pp 1–17. doi:10.1016/b978-0-12-801301-4.00001-3 |
| 12. | Bahrami, F.; Zhao, Y. Org. Lett. 2024, 26, 73–77. doi:10.1021/acs.orglett.3c03666 |
| 13. | Bhaskaran, A.; Aitken, H. M.; Xiao, Z.; Blyth, M.; Nothling, M. D.; Kamdar, S.; O’Mara, M. L.; Connal, L. A. Polymer 2021, 225, 123735. doi:10.1016/j.polymer.2021.123735 |
| 34. | Breslow, R. J. Phys. Org. Chem. 2006, 19, 813–822. doi:10.1002/poc.1037 |
| 35. | Blaha-Nelson, D.; Krüger, D. M.; Szeler, K.; Ben-David, M.; Kamerlin, S. C. L. J. Am. Chem. Soc. 2017, 139, 1155–1167. doi:10.1021/jacs.6b10801 |
| 7. | Cragg, P. J. Supramolecular Enzyme Mimics. In Supramolecular Chemistry: From Biological Inspiration to Biomedical Applications; Cragg, P. J., Ed.; Springer: Dordrecht, Netherlands, 2010; pp 113–151. doi:10.1007/978-90-481-2582-1_4 |
| 8. | Raynal, M.; Ballester, P.; Vidal-Ferran, A.; van Leeuwen, P. W. N. M. Chem. Soc. Rev. 2014, 43, 1734–1787. doi:10.1039/c3cs60037h |
| 9. | Kuah, E.; Toh, S.; Yee, J.; Ma, Q.; Gao, Z. Chem. – Eur. J. 2016, 22, 8404–8430. doi:10.1002/chem.201504394 |
| 30. | Gadzikwa, T.; Matseketsa, P. Dalton Trans. 2024, 53, 7659–7668. doi:10.1039/d4dt00514g |
| 4. | Knowles, J. R. Nature 1991, 350, 121–124. doi:10.1038/350121a0 |
| 5. | Zhang, X.; Houk, K. N. Acc. Chem. Res. 2005, 38, 379–385. doi:10.1021/ar040257s |
| 6. | Klinman, J. P.; Offenbacher, A. R.; Hu, S. J. Am. Chem. Soc. 2017, 139, 18409–18427. doi:10.1021/jacs.7b08418 |
| 31. | Canivet, J.; Aguado, S.; Daniel, C.; Farrusseng, D. ChemCatChem 2011, 3, 675–678. doi:10.1002/cctc.201000386 |
| 24. | Liu, L.; Zhou, T.-Y.; Telfer, S. G. J. Am. Chem. Soc. 2017, 139, 13936–13943. doi:10.1021/jacs.7b07921 |
| 26. | Cohen, S. M. Chem. Rev. 2012, 112, 970–1000. doi:10.1021/cr200179u |
| 27. | Kalaj, M.; Cohen, S. M. ACS Cent. Sci. 2020, 6, 1046–1057. doi:10.1021/acscentsci.0c00690 |
| 23. | Garibay, S. J.; Wang, Z.; Cohen, S. M. Inorg. Chem. 2010, 49, 8086–8091. doi:10.1021/ic1011549 |
| 28. | Chui, S. S.-Y.; Lo, S. M.-F.; Charmant, J. P. H.; Orpen, A. G.; Williams, I. D. Science 1999, 283, 1148–1150. doi:10.1126/science.283.5405.1148 |
| 29. | Rosi, N. L.; Kim, J.; Eddaoudi, M.; Chen, B.; O’Keeffe, M.; Yaghi, O. M. J. Am. Chem. Soc. 2005, 127, 1504–1518. doi:10.1021/ja045123o |
| 21. | Liu, J.; Chen, L.; Cui, H.; Zhang, J.; Zhang, L.; Su, C.-Y. Chem. Soc. Rev. 2014, 43, 6011–6061. doi:10.1039/c4cs00094c |
| 22. | Alhumaimess, M. S. J. Saudi Chem. Soc. 2020, 24, 461–473. doi:10.1016/j.jscs.2020.04.002 |
| 54. | Biler, M.; Crean, R. M.; Schweiger, A. K.; Kourist, R.; Kamerlin, S. C. L. J. Am. Chem. Soc. 2020, 142, 20216–20231. doi:10.1021/jacs.0c10701 |
| 55. | Chen, D.; Li, Y.; Li, X.; Hong, X.; Fan, X.; Savidge, T. Chem. Sci. 2022, 13, 8193–8202. doi:10.1039/d2sc01994a |
| 18. | Zhang, M.; Gu, Z.-Y.; Bosch, M.; Perry, Z.; Zhou, H.-C. Coord. Chem. Rev. 2015, 293-294, 327–356. doi:10.1016/j.ccr.2014.05.031 |
| 19. | Fracaroli, A. M.; Siman, P.; Nagib, D. A.; Suzuki, M.; Furukawa, H.; Toste, F. D.; Yaghi, O. M. J. Am. Chem. Soc. 2016, 138, 8352–8355. doi:10.1021/jacs.6b04204 |
| 20. | Zhang, Y.; Gui, B.; Chen, R.; Hu, G.; Meng, Y.; Yuan, D.; Zeller, M.; Wang, C. Inorg. Chem. 2018, 57, 2288–2295. doi:10.1021/acs.inorgchem.7b03123 |
| 25. | Zhou, T.-Y.; Auer, B.; Lee, S. J.; Telfer, S. G. J. Am. Chem. Soc. 2019, 141, 1577–1582. doi:10.1021/jacs.8b11221 |
| 56. | Scheurrell, K.; Martins, I. C. B.; Murray, C.; Emmerling, F. Phys. Chem. Chem. Phys. 2023, 25, 23637–23644. doi:10.1039/d3cp02883f |
| 38. | Khare, R.; Pandey, J.; Smriti, S.; Ruchi, R. Orient. J. Chem. 2019, 35, 423–429. doi:10.13005/ojc/350154 |
| 36. | Zieniuk, B.; Białecka-Florjańczyk, E.; Wierzchowska, K.; Fabiszewska, A. World J. Microbiol. Biotechnol. 2022, 38, 11. doi:10.1007/s11274-021-03200-5 |
| 37. | Appaturi, J. N.; Ratti, R.; Phoon, B. L.; Batagarawa, S. M.; Din, I. U.; Selvaraj, M.; Ramalingam, R. J. Dalton Trans. 2021, 50, 4445–4469. doi:10.1039/d1dt00456e |
| 49. | List, B. Angew. Chem., Int. Ed. 2010, 49, 1730–1734. doi:10.1002/anie.200906900 |
| 50. | van Schijndel, J.; Canalle, L. A.; Molendijk, D.; Meuldijk, J. Green Chem. Lett. Rev. 2017, 10, 404–411. doi:10.1080/17518253.2017.1391881 |
| 45. | Samarakoon, K. P.; Satterfield, C. S.; McCoy, M. C.; Pivaral-Urbina, D. A.; Islamoglu, T.; Day, V. W.; Gadzikwa, T. Inorg. Chem. 2019, 58, 8906–8909. doi:10.1021/acs.inorgchem.9b00838 |
| 47. | van Beurden, K.; de Koning, S.; Molendijk, D.; van Schijndel, J. Green Chem. Lett. Rev. 2020, 13, 349–364. doi:10.1080/17518253.2020.1851398 |
| 48. | Cortese, R.; Duca, D. Phys. Chem. Chem. Phys. 2011, 13, 15995–16004. doi:10.1039/c1cp21301f |
| 45. | Samarakoon, K. P.; Satterfield, C. S.; McCoy, M. C.; Pivaral-Urbina, D. A.; Islamoglu, T.; Day, V. W.; Gadzikwa, T. Inorg. Chem. 2019, 58, 8906–8909. doi:10.1021/acs.inorgchem.9b00838 |
| 46. | Matseketsa, P.; Mafukidze, D.; Pothupitiya, L.; Otuonye, U. P.; Mutlu, Y. Ç.; Averkiev, B. B.; Gadzikwa, T. Mol. Syst. Des. Eng. 2024, 9, 445–448. doi:10.1039/d3me00185g |
| 38. | Khare, R.; Pandey, J.; Smriti, S.; Ruchi, R. Orient. J. Chem. 2019, 35, 423–429. doi:10.13005/ojc/350154 |
| 39. | Freeman, F. Chem. Rev. 1980, 80, 329–350. doi:10.1021/cr60326a004 |
| 37. | Appaturi, J. N.; Ratti, R.; Phoon, B. L.; Batagarawa, S. M.; Din, I. U.; Selvaraj, M.; Ramalingam, R. J. Dalton Trans. 2021, 50, 4445–4469. doi:10.1039/d1dt00456e |
| 40. | Prout, F. S. J. Org. Chem. 1953, 18, 928–933. doi:10.1021/jo50014a005 |
| 41. | Saghian, M.; Dehghanpour, S.; bayatani, Z. Sci. Rep. 2023, 13, 15563. doi:10.1038/s41598-023-42832-5 |
| 42. | Zarei, N.; Yarie, M.; Torabi, M.; Zolfigol, M. A. RSC Adv. 2024, 14, 1094–1105. doi:10.1039/d3ra08354c |
| 43. | Calvino-Casilda, V.; Martín-Aranda, R. M.; López-Peinado, A. J.; Sobczak, I.; Ziolek, M. Catal. Today 2009, 142, 278–282. doi:10.1016/j.cattod.2008.08.023 |
| 44. | Qi, S.; Lan‐Xiang, S.; Ze‐Mei, G.; Tie‐Ming, C.; Run‐Tao, L. Chin. J. Chem. 2005, 23, 745–748. doi:10.1002/cjoc.200590745 |
© 2025 Matseketsa et al.; licensee Beilstein-Institut.
This is an open access article licensed under the terms of the Beilstein-Institut Open Access License Agreement (https://www.beilstein-journals.org/bjoc/terms), which is identical to the Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0). The reuse of material under this license requires that the author(s), source and license are credited. Third-party material in this article could be subject to other licenses (typically indicated in the credit line), and in this case, users are required to obtain permission from the license holder to reuse the material.