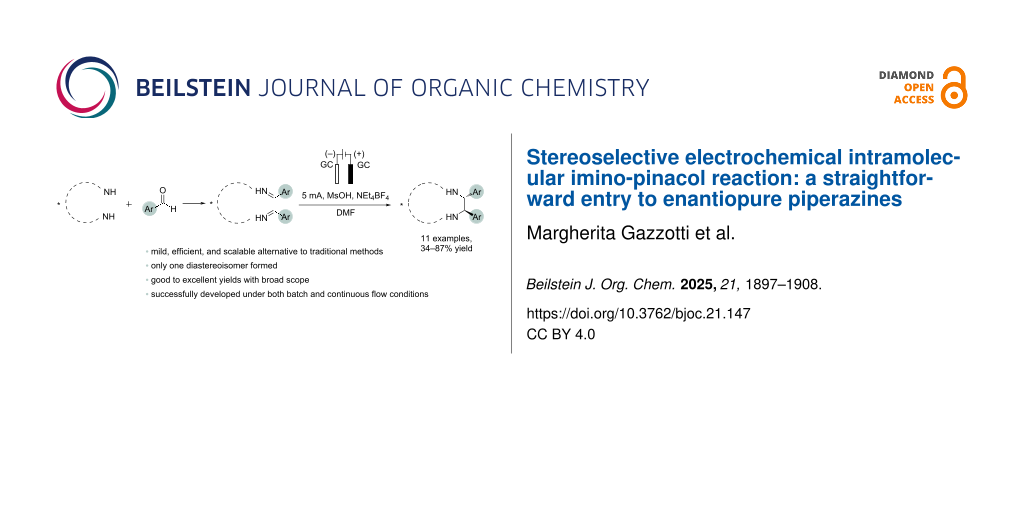
Abstract
The stereoselective electroreductive intramolecular coupling of chiral diimines of aromatic aldehydes with trans-1,2-diaminocyclohexane for the synthesis of enantiopure tetrasubstituted piperazines has been investigated by an electrochemical approach. The methodology was successfully developed under both batch and continuous flow conditions, and afforded enantiomerically pure products with complete stereoselectivity. Substrates bearing electron-donating or electron-withdrawing groups on the aromatic rings provided good to excellent yields, indicating that both types of substituents are well tolerated under the reaction conditions. Although modest yields were obtained under flow conditions, the continuous process afforded higher productivities and space-time yields than the batch reactions due to a short residence time. This work provides a mild, efficient, and scalable alternative to traditional methods for the synthesis of tetrasubstituted enantiopure piperazines, with potential applications in the preparation of chiral ligands.
Graphical Abstract
Introduction
Vicinal diamines represent a highly valuable class of compounds that, over the past decades, have found widespread application in natural products, agrochemicals, and pharmacologically active compounds. Enantiomerically pure 1,2-diamines and their derivatives are also increasingly used in stereoselective synthesis, particularly as chiral auxiliaries or as ligands for metal complexes in asymmetric catalysis [1]. Metal-based reductants represent the most established approach to imino-pinacol coupling (Scheme 1), with zero-valent metals traditionally employed as reductants and various strategies extensively explored [2]. The use of alkali metals [3-5], as lithium and sodium, and alkaline earth metals [6], as magnesium, are characteristic of the earliest versions of this transformation. Several metals from the p- and the d-blocks of the periodic table, such as aluminum [7], indium [8], bismuth [7], zinc [9-17], and manganese [18,19], were later studied. The use of zinc, in particular, has attracted significant attention in this context due to its flexibility, efficiency, and practical applicability. Since the late 1980s, many research groups have investigated the use of in situ-generated low-valent titanium [20-25] and niobium [26] reagents to promote the pinacol-type coupling of imines. Among the metal-based reducing agents explored for this transformation, divalent lanthanide-based reductants derived from samarium and ytterbium have received significant attention since the early 90’s [27-34].
Scheme 1: Synthesis of vicinal diamines via imino-pinacol coupling in the presence of metal-based reductants.
Scheme 1: Synthesis of vicinal diamines via imino-pinacol coupling in the presence of metal-based reductants.
Although traditional well-established procedures for the imino-pinacol coupling reaction are efficient, they often require more than stoichiometric amounts of metal reductants and produce significant quantities of metal waste, which in some cases can present considerable environmental and safety concerns. To mitigate this issue, more sustainable approaches, such as photochemical and electrochemical methods, have been explored. Over the past two decades a variety of light-promoted imino-pinacol coupling reactions have been developed, involving the use of catalytic transition-metal complexes [35,36], organic dyes [37-39], and polyaromatic compounds [40,41] as photocatalysts (Scheme 2). The combination of photoredox catalysis with imine activation enabled the reductive coupling of imines under mild reaction conditions, providing direct access to benzyl and aryl vicinal diamines with good to excellent yields.
Scheme 2: Light-promoted imino-pinacol coupling for the synthesis of vicinal diamines.
Scheme 2: Light-promoted imino-pinacol coupling for the synthesis of vicinal diamines.
Organic electrochemistry represents an attractive and sustainable alternative; however, unfortunately, the electrochemical application is limited to only a few examples (Scheme 3). The electroreductive coupling of imines was first reported in the early 20th century by Law [42]. However, this initial method led to the formation of vicinal diamines with low to moderate yields and required geometrically complex divided cells. In 1989, a more efficient procedure was introduced by Torii et al. [43], who simplified the process by using PbBr2, TFA and THF, in a beaker-type undivided cell equipped with two platinum electrodes. This approach afforded vicinal diamines for different N-benzyl benzaldimines in moderate to good yields. In 1991, Shono’s research group described the electroreductive intramolecular coupling of aromatic diimines, carried out in DMF in the presence of methanesulfonic acid in a divided cell equipped with a lead cathode, a carbon rod anode, and a ceramic diaphragm [44]. This method was found to be effective for synthesizing trans-2,3-diarylpiperazines through the intramolecular reductive coupling of 1,2-diimines and seven- and eight-membered heterocycles which were obtained in moderate to good yields via the cyclization of 1,3- and 1,4-diimines.
Scheme 3: Historical perspective on electrochemical imino-coupling protocols.
Scheme 3: Historical perspective on electrochemical imino-coupling protocols.
Later, in 2001, Yudin and co-workers described the parallel reductive coupling of aldimines using a spatially addressable electrosynthesis platform, which employed a stainless steel cathode, a sacrificial aluminum anode and a procedure adapted from Torii's earlier work including the use of PbBr2, TFA, and Bu4NBr [45]. Under these conditions, parallel electrosynthesis allowed the synthesis of up to 16 vicinal diamines in good yields in 30 minutes. To the best of our knowledge, the most recent example of electroreductive coupling of imines was reported in 2025 by Wang’s research group [46], who described a new electrochemical procedure to provide vicinal diamines involving the use of Sn electrodes as both anode and cathode in a divided cell, Mn(OAc)3·2H2O as additive and n-Bu4NOAc as electrolyte. Under these conditions, using aldehydes and amines as starting materials to form the imines in situ resulted in a wide range of diamines, obtained in moderate to good yields.
Inspired by these works, we aimed to investigate and optimize the stereoselective electrochemical intramolecular imino-pinacol coupling reaction under both batch and continuous flow conditions as a direct approach to enantiopure scaffolds that are commonly used as chiral ligands. In this context we report the development of a more sustainable method – which avoids the use of lead bromide or lead electrodes – employing an undivided cell with two glassy carbon electrodes for the electroreductive intramolecular coupling of aromatic diimines (Scheme 4). This methodology has been successfully applied to the synthesis of enantiopure tetrasubstituted piperazines, featuring both electron-withdrawing and electron-donating groups on the aromatic rings.
Scheme 4: Stereoselective electroreductive intramolecular imino-pinacol reaction.
Scheme 4: Stereoselective electroreductive intramolecular imino-pinacol reaction.
Results and Discussion
The stereoselective electroreductive intramolecular coupling of chiral diimines of aromatic aldehydes with trans-1,2-diaminocyclohexane was initially investigated under traditional batch conditions through a series of screening experiments designed to find the optimal reaction conditions.
Diimine 1a was selected as model substrate for the intramolecular coupling reaction and a screening of reaction parameters such as solvents, electrodes materials, electrolytes, total charges, and concentrations of the reaction mixture was performed. All optimization studies were carried out using 0.5 mmol of 1a in the presence of methanesulfonic acid in an undivided cell (5 mL volume) equipped with two electrodes under galvanostatic conditions (constant current 5 mA, 2.5 mA/cm2) and the results are summarized in Table 1.
Table 1: Screening of the imino-pinacol reaction conditions.
|
|
||||||||
| Entry |
Working
(cathode) |
Counter
(anode) |
Electrolyte | Electrolyte equivalents | Total charge (F/mol) | Solvent | Substrate concentration (M) | Yield 2a (%)a |
| 1 | Pt | Pt | NEt4BF4 | 2.6 | 2.2 | MeCN | 0.125 | 51 |
| 2 | Pt | Pt | NEt4BF4 | 2.6 | 2.2 | DMF | 0.125 | 68 |
| 3b | Pt | Pt | NEt4BF4 | 2.6 | 2.2 | DMF | 0.125 | – |
| 4 | Pt | Gr | NEt4BF4 | 2.6 | 2.2 | DMF | 0.125 | 64 |
| 5 | Pt | Zn | NEt4BF4 | 2.6 | 2.2 | DMF | 0.125 | 64 |
| 6 | Pt | Zn | NEt4BF4 | 2.6 | 2.5 | DMF | 0.125 | 61 |
| 7 | Pt | Zn | NEt4BF4 | 2.6 | 2.5 | DMF | 0.250 | 64 |
| 8 | SS | SS | NEt4BF4 | 2.6 | 2.2 | DMF | 0.125 | 47 |
| 9 | GC | GC | NEt4BF4 | 2.6 | 2.2 | DMF | 0.125 | 85 |
| 10 | GC | GC | NEt4BF4 | 1.3 | 2.2 | DMF | 0.125 | 50 |
| 11 | GC | GC | NBu4BF4 | 2.6 | 2.2 | DMF | 0.125 | 72 |
aIsolated yields. Reactions were performed on a 0.5 mmol scale. bReaction performed without MsOH.
Using platinum electrodes and NEt₄BF₄ as electrolyte, the tetrasubstituted piperazine 2a was obtained as a single stereoisomer in 51% yield in CH3CN, which increased to 68% when DMF was used (Table 1, entry 2). The replacement of the platinum anode with graphite or zinc (Table 1, entries 4 and 5) had no significant effect on the yield. Similarly, increasing the total charge from 2.2 to 2.5 F/mol (Table 1, entries 6 and 7) resulted in only marginal changes. Doubling the substrate concentration (Table 1, entry 7) also failed to provide a significant advantage in terms of yield, leading to the formation of the desired product in 64% yield.
The replacement of both working cathode and counter anode to stainless steel electrodes caused a decrement of the yield to 47% (Table 1, entry 8), while with two glassy carbon electrodes desired product 2a was isolated in 85% yield (Table 1, entry 9).
Reducing the electrolyte loading from 2.6 to 1.3 equivalents significantly affected the reaction outcome, leading to a noticeable decrease in the yield suggesting that sufficient ionic conductivity is crucial to achieve efficient conversion (Table 1, entry 10).
Lastly, replacing tetraethylammonium tetrafluoroborate with tetrabutylammonium tetrafluoroborate resulted in a negligible reduction of the chemical efficiency (Table 1, entry 9 vs 11).
As demonstrated by Shono and co-workers [44], the presence of a strong protic acid, such as methanesulfonic acid, MsOH, is essential to promote the intramolecular coupling. A control experiment revealed that when the electroreduction of 1a was performed in the absence of MsOH (Table 1, entry 3), no formation of the desired product was observed and the starting diimine 1a was quantitatively recovered.
The optimized reaction conditions, consisting in the use of GC electrodes, NEt4BF4 (2.6 equiv) as electrolyte, MsOH (3 equiv) as additive, constant current of 5 mA (2.5 mA/cm2), total charge of 2.2 F/mol, dry DMF as solvent (0.125 M), were then applied to evaluate the scope of the reaction. Under these conditions a small library of chiral enantiopure tetrasubstituted piperazines 2b–j bearing both electron-withdrawing and electron-donating substituents, was synthetized. The results are summarized in Scheme 5.
Scheme 5: Scope of the imino-pinacol coupling reaction. Reaction conditions: GC electrodes, NEt4BF4 (2.6 equiv), MsOH (3 equiv), constant current 5 mA (2.5 mA/cm2), total charge 2.2 F/mol, dry DMF (0.125 M). Yields reported are isolated yields. Reactions were performed on a 0.5 mmol scale.
Scheme 5: Scope of the imino-pinacol coupling reaction. Reaction conditions: GC electrodes, NEt4BF4 (2.6 equi...
The chiral tetrasubstituted piperazines 2b–j were obtained as a single, enantiopure diastereoisomer from diimines 1b–j, prepared from the enantiopure trans-1,2-diaminocyclohexane and aromatic aldehydes, in good to excellent yields. Substrates bearing electron-withdrawing groups such as trifluoromethyl (2b,c) and bromine (2d), as well as electron-donating groups like methoxy (2f), aryl/alkyl (2h,i), and alkenyl substituents (2e), were well tolerated. Notably, the sterically hindered derivative 2h was isolated with the highest yield of 87% indicating that bulky ortho-substituents on the aromatic rings do not influence the electrochemical cyclization process.
In contrast, more complex and extended heterocyclic electronrich π-systems such as compound 2j was obtained in 35% yield only, presumably as a result of electronic factors affecting the efficiency of the initial radical formation [47].
To further extend the scope of this methodology, the trans-1,2-diaminocyclohexane was replaced with (S)-1,1′-binaphthyl-2,2′-diamine. Under the optimized conditions, the corresponding cyclic product 2k was obtained in 34% yield, consistent with previous observations reporting reduced efficiency as the ring size increases [44].
To confirm the absolute configuration of the piperazine formed, a pure sample of compound 2b derived from (1R,2R)-trans-diaminocyclohexane was crystallized from a chloroform solution resulting in the formation of white crystals suitable for single crystal X-ray diffraction studies. The absolute configuration of the product was unambiguously determined, by XRD analysis, to be (2S,3S,4aR,8aR)-2b as shown in Figure 1.
Figure 1: X-ray determined structure of chiral piperazine 2b.
Figure 1: X-ray determined structure of chiral piperazine 2b.
Following the promising results obtained from the batch approach, the electroreductive coupling was investigated under continuous flow conditions [48-50], with the aim to improve process efficiency and in view of a possible scale up of the reaction [51,52]. Flow experiments were performed using the commercially available pre-configured Syrris-Asia electrochemical flow module, a user-friendly solution for continuous flow electrochemical synthesis which is part of the Asia platform [53].
A comprehensive optimization of the flow reaction conditions was carried out using the model substrate 1a systematically varying key parameters such as the working electrodes, the current intensity, the total charge, the flow rate, and the equivalents of the employed electrolyte. Following this in-depth screening, optimal conditions were identified: Carbon filled PPS electrodes, a constant current of 80 mA, a total charge of 4.15 F/mol, a flow rate of 96 μL/min, and 1.3 equivalents of NEt4BF4 as electrolyte. Under these optimized conditions, the desired tetrasubstituted piperazine 2a was obtained as single stereoisomer in 56% yield with a residence time of 2.34 minutes (Scheme 6).
Scheme 6: Continuous flow synthesis of piperazine 2a. The yield was determined by 1H NMR spectroscopy using 1,3,5-trimethoxybenzene as internal standard. Reactions were performed on a 0.1 mmol scale.
Scheme 6: Continuous flow synthesis of piperazine 2a. The yield was determined by 1H NMR spectroscopy using 1...
Although the yield appears lower compared to the batch process, it is important to highlight that the reaction time has been reduced by nearly a factor of 150, resulting in a substantial increase in terms of productivity, which is 6.2 times higher than the batch protocol. Moreover, the significantly shorter residence time in the flow setup led to an improved space-time yield (STY), a key metric for comparing reactors of different volumes. Under these conditions, the STY of the flow process was 112.2 times higher than that achieved in the batch reactor (Table 2).
Table 2: Comparison of productivity and space-time yield of batch and flow processes for the synthesis of piperazine 2a.
| Entry | Method |
Productivitya
(mmol/h) |
Productivity rel. factor |
STYb
(mmol/mL*h) |
STY rel. factor |
| 1 | batch | 0.065 | 1 | 0.016 | 1 |
| 2 | flow | 0.403 | 6.2 | 1.795 | 112.2 |
aProductivity: moles of product divided by the collection time required to collect the product obtained by the reaction of 0.5 mmol of diimine 1a. bSpace-time yield: moles of product in the reactor, divided by residence time and reactor volume (for details on calculations see Supporting Information File 1).
The scalability of the reaction was further demonstrated by performing the electroreduction of diimine 1d on a 2.5 mmol scale under flow conditions. Compound 2d was obtained in 52% isolated yield achieving productivity and STY values that were 10.4 and 184.7 times higher, respectively, compared to those obtained in the batch reaction (Table 3).
Table 3: Comparison of productivity and space-time yield of batch and flow processes for the synthesis of piperazine 2d.
|
|
|||||
| Entry | Method | Productivitya (mmol/h) | Productivity rel. factor |
STY b
(mmol/mL*h) |
STY rel. factor |
| 1 | batch | 0.036 | 1 | 0.009 | 1 |
| 2 | flow | 0.374 | 10.4 | 1.662 | 184.7 |
aProductivity: moles of product divided by the collection time required to collect the product obtained by the reaction of 0.5 mmol of diimine 1d. bSpace-time yield: moles of product in the reactor, divided by residence time and reactor volume (for details on calculations please see Supporting Information File 1).
To gain a deeper understanding of the mechanism behind the observed reaction, a plausible reaction pathway is proposed, as illustrated in Scheme 7. The process presumably involves the activation of the diimine substrate 1a by methanesulfonic acid, which acts as a strong Brønsted acid to selectively protonate the imine nitrogen atoms. This protonation step increases the electrophilicity of the adjacent imine carbons by inductive effect, leading to the formation of a highly reactive diiminium intermediate 4a. When formed, compound 4a is electrochemically reduced to give the carbon-centered diradical intermediate 5a and the spatial proximity of these two radical centers allows a rapid intramolecular radical–radical coupling resulting in the formation of the desired piperazine 2a. The feasibility of this mechanism is supported by literature precedents involving electroreduction of activated imines and diiminium species [19]. Furthermore, control experiments conducted in the absence of methanesulfonic acid (Table 1, entry 3) resulted in no observable product formation, highlighting the acid activation in driving this transformation.
Cyclic voltammetry measurements were also carried out in order to evaluate the electrochemical redox properties of the species involved in the process and to provide evidence for the behavior of the monoprotonated and the bisprotonated diimines, 3a and 4a (Scheme 8). The cyclic voltammogram of the starting diimine 1a shows one distinct reductive peak at −1.75 V. Upon addition of one equivalent of MsOH, monoprotonated species 3a is formed, and a shift of the reduction peak to −1.92 V was observed. Subsequent addition of a second equivalent of acid leads to the formation of the bisprotonated species 4a, and as consequence, a shift of the reduction peak to −1.82 V was observed. However, the addition of three equivalents of methanesulfonic acid (corresponding to the typical reaction conditions) results in a decrease in the intensity of the reduction peak at −1.81 V. This observation is presumably associated with the initiation of the SET reduction process, which leads to the consumption of the diiminium salt 4a and to the formation of the diradical intermediate 5a.
Scheme 8: Cyclic voltammetry investigation. Cyclic voltammetry of a 0.325 M solution of Et4NBF4 in DMF (light-blue line). Cyclic voltammetry of diimine 1a (10 mM) recorded in a 0.325 M solution of Et4NBF4 in DMF (dark-blue line). Cyclic voltammetry of diimine 1a (10 mM) in presence of 1 equiv of methanesulfonic acid (10 mM) recorded in a 0.325 M solution of Et4NBF4 in DMF (green line). Cyclic voltammetry of diimine 1a (10 mM) in presence of 2 equiv of methanesulfonic acid (20 mM) recorded in a 0.325 M solution of Et4NBF4 in DMF (dark-red line). Cyclic voltammetry of diimine 1a (10 mM) in presence of 3 equiv of methanesulfonic acid (30 mM) recorded in a 0.325 M solution of Et4NBF4 in DMF (light-red line). Glassy carbon as working, glassy carbon as counter, and Ag/AgCl as reference electrodes with 0.1 V/s as scan rate.
Scheme 8: Cyclic voltammetry investigation. Cyclic voltammetry of a 0.325 M solution of Et4NBF4 in DMF (light...
Conclusion
In conclusion, we have successfully developed a simple and mild electroreductive, stereoselective intramolecular coupling of aromatic diimines, performed under batch and flow conditions. The reaction provided good to excellent yields across a wide range of substrates with both electron-withdrawing and electron-donating groups being well tolerated. As a result, a series of chiral enantiopure tetrasubstituted piperazines was efficiently synthetized. Significantly higher productivities and space-time yields were achieved under continuous conditions compared to the batch process. Furthermore, the scalability of the reaction was successfully demonstrated, highlighting its potential for larger-scale applications.
Experimental
General procedure in-batch electrochemical imino-pinacol coupling
In a flame-dried, undivided cell equipped with two GC electrodes and a stirring bar, diimine (0.5 mmol, 1 equiv) and NEt4BF4 (1.3 mmol, 2.6 equiv) were added, followed by two vacuum–nitrogen cycles. Under nitrogen atmosphere, dry DMF (0.125 M) and methanesulfonic acid (1.5 mmol, 3 equiv) were then added to the electrochemical cell. The reaction mixture was degassed by bubbling with argon for 20 minutes under vigorous stirring. The undivided cell was then connected to the Autolab power supply and stirred at 25 °C under galvanostatic conditions at 5 mA (2.5 mA/cm2) until a total charge of 2.2 F/mol was delivered. The reaction mixture was poured into a beaker with 10 mL of distilled water, and a saturated solution of NaHCO3 was added to adjust the pH to ≈7. 10 mL of ethyl acetate were then added, and the two phases were separated. The aqueous phase was then extracted with CH2Cl2 (3 × 10 mL). The combined organic layers were dried over Na2SO4, filtered and concentrated under vacuum. The reaction crude was purified by flash column chromatography on silica gel (n-hexane/ethyl acetate 8:2) to give the desired pure product.
General procedure for in-flow electrochemical imino-pinacol coupling
Diimine (1 equiv) and NEt4BF4 (1.3 equiv) were added in a flame-dried Erlenmeyer flask with a stirring bar, followed by two vacuum–nitrogen cycles. Under nitrogen atmosphere, dry DMF (0.125 M) and methanesulfonic acid (3 equiv) were then added to the reaction mixture, which was degassed by bubbling with argon for 20 minutes under vigorous stirring. In-flow experiments were performed using the Asia (Syrris) modular system with two carbon filled PPS electrodes, a constant current of 80 mA, a flow rate of 96 μL/min, a residence time of 2.34 minutes, and 1.3 equivalents of NEt4BF4. The experiments were conducted until a total charge of 4.15 F/mol was delivered. The reaction mixture was then poured into a beaker with distilled water, and a saturated solution of NaHCO3 was added to adjust the pH to ≈7. Ethyl acetate was added, and the two phases were separated. The aqueous phase was then extracted three times with CH2Cl2. The combined organic layers were dried over Na2SO4, filtered and concentrated under vacuum. Yields were calculated on the reaction crude by 1H NMR spectroscopy using 1,3,5-trimethoxybenzene as internal standard. In the case of the large-scale reaction the crude was purified through flash column chromatography on silica gel to give the isolated pure product.
Supporting Information
| Supporting Information File 1: Synthetic procedures and physical data for the new compounds, copies of 1H and 13C NMR spectra of the prepared compounds. | ||
| Format: PDF | Size: 3.7 MB | Download |
Acknowledgements
The authors thank Prof. A. Puglisi (Università degli Studi di Milano) for valuable discussions. For the single-crystal X-ray diffraction analysis the Unitech COSPECT (Università degli Studi di Milano) for provision of beamtime and Prof. Leonardo Lo Presti for structure solution and refinement are acknowledged.
Funding
The authors thank MUSA – Multilayered Urban Sustainability Action – project, funded by the European Union – NextGenerationEU, under the National Recovery and Resilience Plan (NRRP) Mission 4 Component 2 Investment Line 1.5: Strengthening of research structures and creation of R&D “innovation ecosystems”, set up of “territorial leaders in R&D”. M. Benaglia thanks MUR for the project PRIN 2022 ““Flow chemistry, photo and organocatalysis: powerful tools for the development of technology-driven sustainable strategies to alfa and beta amination of carbonyls and carboxylic acids derivatives- TECHNO”, financed by EU - Next Generation EU, Mission 4 Component 1 CUP G53D23003280006. M. Gazzotti thanks Università degli Studi di Milano for a PhD fellowship.
Data Availability Statement
Data generated and analyzed during this study is available from the corresponding author upon reasonable request.
References
-
Lucet, D.; Le Gall, T.; Mioskowski, C. Angew. Chem., Int. Ed. 1998, 37, 2580–2627. doi:10.1002/(sici)1521-3773(19981016)37:19<2580::aid-anie2580>3.0.co;2-l
Return to citation in text: [1] -
Faugeroux, V.; Genisson, Y. Curr. Org. Chem. 2008, 12, 751–773. doi:10.2174/138527208784567188
Return to citation in text: [1] -
Eisch, J. J.; Kaska, D. D.; Peterson, C. J. J. Org. Chem. 1966, 31, 453–456. doi:10.1021/jo01340a024
Return to citation in text: [1] -
Smith, J. G.; Veach, C. D. Can. J. Chem. 1966, 44, 2497–2502. doi:10.1139/v66-376
Return to citation in text: [1] -
Smith, J. G.; Ho, I. J. Org. Chem. 1972, 37, 653–656. doi:10.1021/jo00969a027
Return to citation in text: [1] -
Mercer, G. J.; Sturdy, M.; Jensen, D. R.; Sigman, M. S. Tetrahedron 2005, 61, 6418–6424. doi:10.1016/j.tet.2005.03.124
Return to citation in text: [1] -
Baruah, B.; Prajapati, D.; Sandhu, J. S. Tetrahedron Lett. 1995, 36, 6747–6750. doi:10.1016/00404-0399(50)1331-b
Return to citation in text: [1] [2] -
Kalyanam, N.; Rao, G. V. Tetrahedron Lett. 1993, 34, 1647–1648. doi:10.1016/0040-4039(93)85031-q
Return to citation in text: [1] -
Vellemäe, E.; Tšubrik, O.; Mäeorg, S.; Mäeorg, U. J. Chem. Res. 2006, 149–150. doi:10.3184/030823406776330792
Return to citation in text: [1] -
Hatano, B.; Tachikawa, T.; Mori, T.; Nagahashi, K.; Kijima, T. Tetrahedron Lett. 2011, 52, 3467–3469. doi:10.1016/j.tetlet.2011.04.104
Return to citation in text: [1] -
Shimizu, M.; Iida, T.; Fujisawa, T. Chem. Lett. 1995, 24, 609–610. doi:10.1246/cl.1995.609
Return to citation in text: [1] -
Alexakis, A.; Aujard, I.; Mangeney, P. Synlett 1998, 873–874. doi:10.1055/s-1998-1790
Return to citation in text: [1] -
Hesemann, P.; Moreau, J. J. E.; Soto, T. Synth. Commun. 2003, 33, 183–189. doi:10.1081/scc-120015698
Return to citation in text: [1] -
Khan, N. H.; Zuberi, R. K.; Siddiqui, A. A. Synth. Commun. 1980, 10, 363–371. doi:10.1080/00397918008061825
Return to citation in text: [1] -
Shono, T.; Kise, N.; Oike, H.; Yoshimoto, M.; Okazaki, E. Tetrahedron Lett. 1992, 33, 5559–5562. doi:10.1016/s0040-4039(00)61145-0
Return to citation in text: [1] -
Sakai, T.; Korenaga, T.; Washio, N.; Nishio, Y.; Minami, S.; Ema, T. Bull. Chem. Soc. Jpn. 2004, 77, 1001–1008. doi:10.1246/bcsj.77.1001
Return to citation in text: [1] -
Dutta, M. P.; Baruah, B.; Boruah, A.; Prajapati, D.; Sandhu, J. S. Synlett 1998, 857–858. doi:10.1055/s-1998-1800
Return to citation in text: [1] -
Rieke, R. D.; Kim, S.-H. J. Org. Chem. 1998, 63, 5235–5239. doi:10.1021/jo971942y
Return to citation in text: [1] -
Mercer, G. J.; Sigman, M. S. Org. Lett. 2003, 5, 1591–1594. doi:10.1021/ol034469l
Return to citation in text: [1] [2] -
Shimizu, M.; Inayoshi, K.; Sahara, T. Org. Biomol. Chem. 2005, 3, 2237–2238. doi:10.1039/b505335h
Return to citation in text: [1] -
Kumar, A.; Samuelson, A. G. Eur. J. Org. Chem. 2011, 951–959. doi:10.1002/ejoc.201001256
Return to citation in text: [1] -
Talukdar, S.; Banerji, A. J. Org. Chem. 1998, 63, 3468–3470. doi:10.1021/jo9716892
Return to citation in text: [1] -
Mangeney, P.; Tejero, T.; Alexakis, A.; Grosjean, F.; Normant, J. Synthesis 1988, 255–257. doi:10.1055/s-1988-27536
Return to citation in text: [1] -
Periasamy, M.; Srinivas, G.; Karunakar, G. V.; Bharathi, P. Tetrahedron Lett. 1999, 40, 7577–7580. doi:10.1016/s0040-4039(99)01609-3
Return to citation in text: [1] -
Periasamy, M.; Srinivas, G.; Suresh, S. Tetrahedron Lett. 2001, 42, 7123–7125. doi:10.1016/s0040-4039(01)01480-0
Return to citation in text: [1] -
Arai, S.; Takita, S.; Nishida, A. Eur. J. Org. Chem. 2005, 5262–5267. doi:10.1002/ejoc.200500301
Return to citation in text: [1] -
Banik, B. K.; Zegrocka, O.; Banik, I.; Hackfeld, L.; Becker, F. F. Tetrahedron Lett. 1999, 40, 6731–6734. doi:10.1016/s0040-4039(99)01395-7
Return to citation in text: [1] -
Yanada, R.; Negoro, N.; Okaniwa, M.; Miwa, Y.; Taga, T.; Yanada, K.; Fujita, T. Synlett 1999, 537–540. doi:10.1055/s-1999-2695
Return to citation in text: [1] -
Kawaji, T.; Hayashi, K.; Hashimoto, I.; Matsumoto, T.; Thiemann, T.; Mataka, S. Tetrahedron Lett. 2005, 46, 5277–5279. doi:10.1016/j.tetlet.2005.06.038
Return to citation in text: [1] -
Annunziata, R.; Benaglia, M.; Caporale, M.; Raimondi, L. Tetrahedron: Asymmetry 2002, 13, 2727–2734. doi:10.1016/s0957-4166(02)00748-6
Return to citation in text: [1] -
Imamoto, T.; Nishimura, S. Chem. Lett. 1990, 19, 1141–1142. doi:10.1246/cl.1990.1141
Return to citation in text: [1] -
Kim, M.; Knettle, B. W.; Dahlén, A.; Hilmersson, G.; Flowers, R. A., II. Tetrahedron 2003, 59, 10397–10402. doi:10.1016/j.tet.2003.06.004
Return to citation in text: [1] -
Dahlén, A.; Prasad, E.; Flowers, R. A., II; Hilmersson, G. Chem. – Eur. J. 2005, 11, 3279–3284. doi:10.1002/chem.200401320
Return to citation in text: [1] -
Taniguchi, Y.; Kuno, T.; Nakahashi, M.; Takaki, K.; Fujiwara, Y. Appl. Organomet. Chem. 1995, 9, 491–503. doi:10.1002/aoc.590090519
Return to citation in text: [1] -
Nakajima, M.; Fava, E.; Loescher, S.; Jiang, Z.; Rueping, M. Angew. Chem., Int. Ed. 2015, 54, 8828–8832. doi:10.1002/anie.201501556
Return to citation in text: [1] -
Dang, V. Q.; Teets, T. S. Chem. Sci. 2023, 14, 9526–9532. doi:10.1039/d3sc03000h
Return to citation in text: [1] -
Gualandi, A.; Rodeghiero, G.; Della Rocca, E.; Bertoni, F.; Marchini, M.; Perciaccante, R.; Jansen, T. P.; Ceroni, P.; Cozzi, P. G. Chem. Commun. 2018, 54, 10044–10047. doi:10.1039/c8cc04048f
Return to citation in text: [1] -
Wang, H.; Qu, J.-P.; Kang, Y.-B. Org. Lett. 2021, 23, 2900–2903. doi:10.1021/acs.orglett.1c00537
Return to citation in text: [1] -
Kundu, S.; Roy, L.; Maji, M. S. Org. Lett. 2022, 24, 9001–9006. doi:10.1021/acs.orglett.2c03600
Return to citation in text: [1] -
Okamoto, S.; Kojiyama, K.; Tsujioka, H.; Sudo, A. Chem. Commun. 2016, 52, 11339–11342. doi:10.1039/c6cc05867a
Return to citation in text: [1] -
Okamoto, S.; Ariki, R.; Tsujioka, H.; Sudo, A. J. Org. Chem. 2017, 82, 9731–9736. doi:10.1021/acs.joc.7b01838
Return to citation in text: [1] -
Law, H. D. J. Chem. Soc., Trans. 1912, 101, 154–166. doi:10.1039/ct9120100154
Return to citation in text: [1] -
Tanaka, H.; Nakahara, T.; Dhimane, H.; Torii, S. Synlett 1989, 51–52. doi:10.1055/s-1989-34700
Return to citation in text: [1] -
Shono, T.; Kise, N.; Shirakawa, E.; Matsumoto, H.; Okazaki, E. J. Org. Chem. 1991, 56, 3063–3067. doi:10.1021/jo00009a026
Return to citation in text: [1] [2] [3] -
Siu, T.; Li, W.; Yudin, A. K. J. Comb. Chem. 2001, 3, 554–558. doi:10.1021/cc0100159
Return to citation in text: [1] -
Cui, W.; Xu, X.; Zhang, C.; Wang, D.; Yang, Y.; Wang, Q.; Wang, J. J. Org. Chem. 2025, 90, 3659–3664. doi:10.1021/acs.joc.4c02994
Return to citation in text: [1] -
When the reaction was attempted with the diimines derived from the 2-pyridinecarboxaldehyde and the 3-pyridinecarboxaldehyde, it resulted in extensive decomposition of the starting materials.
Return to citation in text: [1] -
Capaldo, L.; Wen, Z.; Noël, T. Chem. Sci. 2023, 14, 4230–4247. doi:10.1039/d3sc00992k
Return to citation in text: [1] -
Atobe, M.; Tateno, H.; Matsumura, Y. Chem. Rev. 2018, 118, 4541–4572. doi:10.1021/acs.chemrev.7b00353
Return to citation in text: [1] -
Elsherbini, M.; Wirth, T. Acc. Chem. Res. 2019, 52, 3287–3296. doi:10.1021/acs.accounts.9b00497
Return to citation in text: [1] -
Noël, T.; Cao, Y.; Laudadio, G. Acc. Chem. Res. 2019, 52, 2858–2869. doi:10.1021/acs.accounts.9b00412
Return to citation in text: [1] -
Cantillo, D. Curr. Opin. Electrochem. 2024, 44, 101459. doi:10.1016/j.coelec.2024.101459
Return to citation in text: [1] -
Asia premium flow chemistry system for modular efficiency in systems chemistry. http://www.syrris.com.
Return to citation in text: [1]
| 1. | Lucet, D.; Le Gall, T.; Mioskowski, C. Angew. Chem., Int. Ed. 1998, 37, 2580–2627. doi:10.1002/(sici)1521-3773(19981016)37:19<2580::aid-anie2580>3.0.co;2-l |
| 7. | Baruah, B.; Prajapati, D.; Sandhu, J. S. Tetrahedron Lett. 1995, 36, 6747–6750. doi:10.1016/00404-0399(50)1331-b |
| 40. | Okamoto, S.; Kojiyama, K.; Tsujioka, H.; Sudo, A. Chem. Commun. 2016, 52, 11339–11342. doi:10.1039/c6cc05867a |
| 41. | Okamoto, S.; Ariki, R.; Tsujioka, H.; Sudo, A. J. Org. Chem. 2017, 82, 9731–9736. doi:10.1021/acs.joc.7b01838 |
| 6. | Mercer, G. J.; Sturdy, M.; Jensen, D. R.; Sigman, M. S. Tetrahedron 2005, 61, 6418–6424. doi:10.1016/j.tet.2005.03.124 |
| 42. | Law, H. D. J. Chem. Soc., Trans. 1912, 101, 154–166. doi:10.1039/ct9120100154 |
| 3. | Eisch, J. J.; Kaska, D. D.; Peterson, C. J. J. Org. Chem. 1966, 31, 453–456. doi:10.1021/jo01340a024 |
| 4. | Smith, J. G.; Veach, C. D. Can. J. Chem. 1966, 44, 2497–2502. doi:10.1139/v66-376 |
| 5. | Smith, J. G.; Ho, I. J. Org. Chem. 1972, 37, 653–656. doi:10.1021/jo00969a027 |
| 35. | Nakajima, M.; Fava, E.; Loescher, S.; Jiang, Z.; Rueping, M. Angew. Chem., Int. Ed. 2015, 54, 8828–8832. doi:10.1002/anie.201501556 |
| 36. | Dang, V. Q.; Teets, T. S. Chem. Sci. 2023, 14, 9526–9532. doi:10.1039/d3sc03000h |
| 2. | Faugeroux, V.; Genisson, Y. Curr. Org. Chem. 2008, 12, 751–773. doi:10.2174/138527208784567188 |
| 37. | Gualandi, A.; Rodeghiero, G.; Della Rocca, E.; Bertoni, F.; Marchini, M.; Perciaccante, R.; Jansen, T. P.; Ceroni, P.; Cozzi, P. G. Chem. Commun. 2018, 54, 10044–10047. doi:10.1039/c8cc04048f |
| 38. | Wang, H.; Qu, J.-P.; Kang, Y.-B. Org. Lett. 2021, 23, 2900–2903. doi:10.1021/acs.orglett.1c00537 |
| 39. | Kundu, S.; Roy, L.; Maji, M. S. Org. Lett. 2022, 24, 9001–9006. doi:10.1021/acs.orglett.2c03600 |
| 18. | Rieke, R. D.; Kim, S.-H. J. Org. Chem. 1998, 63, 5235–5239. doi:10.1021/jo971942y |
| 19. | Mercer, G. J.; Sigman, M. S. Org. Lett. 2003, 5, 1591–1594. doi:10.1021/ol034469l |
| 26. | Arai, S.; Takita, S.; Nishida, A. Eur. J. Org. Chem. 2005, 5262–5267. doi:10.1002/ejoc.200500301 |
| 9. | Vellemäe, E.; Tšubrik, O.; Mäeorg, S.; Mäeorg, U. J. Chem. Res. 2006, 149–150. doi:10.3184/030823406776330792 |
| 10. | Hatano, B.; Tachikawa, T.; Mori, T.; Nagahashi, K.; Kijima, T. Tetrahedron Lett. 2011, 52, 3467–3469. doi:10.1016/j.tetlet.2011.04.104 |
| 11. | Shimizu, M.; Iida, T.; Fujisawa, T. Chem. Lett. 1995, 24, 609–610. doi:10.1246/cl.1995.609 |
| 12. | Alexakis, A.; Aujard, I.; Mangeney, P. Synlett 1998, 873–874. doi:10.1055/s-1998-1790 |
| 13. | Hesemann, P.; Moreau, J. J. E.; Soto, T. Synth. Commun. 2003, 33, 183–189. doi:10.1081/scc-120015698 |
| 14. | Khan, N. H.; Zuberi, R. K.; Siddiqui, A. A. Synth. Commun. 1980, 10, 363–371. doi:10.1080/00397918008061825 |
| 15. | Shono, T.; Kise, N.; Oike, H.; Yoshimoto, M.; Okazaki, E. Tetrahedron Lett. 1992, 33, 5559–5562. doi:10.1016/s0040-4039(00)61145-0 |
| 16. | Sakai, T.; Korenaga, T.; Washio, N.; Nishio, Y.; Minami, S.; Ema, T. Bull. Chem. Soc. Jpn. 2004, 77, 1001–1008. doi:10.1246/bcsj.77.1001 |
| 17. | Dutta, M. P.; Baruah, B.; Boruah, A.; Prajapati, D.; Sandhu, J. S. Synlett 1998, 857–858. doi:10.1055/s-1998-1800 |
| 27. | Banik, B. K.; Zegrocka, O.; Banik, I.; Hackfeld, L.; Becker, F. F. Tetrahedron Lett. 1999, 40, 6731–6734. doi:10.1016/s0040-4039(99)01395-7 |
| 28. | Yanada, R.; Negoro, N.; Okaniwa, M.; Miwa, Y.; Taga, T.; Yanada, K.; Fujita, T. Synlett 1999, 537–540. doi:10.1055/s-1999-2695 |
| 29. | Kawaji, T.; Hayashi, K.; Hashimoto, I.; Matsumoto, T.; Thiemann, T.; Mataka, S. Tetrahedron Lett. 2005, 46, 5277–5279. doi:10.1016/j.tetlet.2005.06.038 |
| 30. | Annunziata, R.; Benaglia, M.; Caporale, M.; Raimondi, L. Tetrahedron: Asymmetry 2002, 13, 2727–2734. doi:10.1016/s0957-4166(02)00748-6 |
| 31. | Imamoto, T.; Nishimura, S. Chem. Lett. 1990, 19, 1141–1142. doi:10.1246/cl.1990.1141 |
| 32. | Kim, M.; Knettle, B. W.; Dahlén, A.; Hilmersson, G.; Flowers, R. A., II. Tetrahedron 2003, 59, 10397–10402. doi:10.1016/j.tet.2003.06.004 |
| 33. | Dahlén, A.; Prasad, E.; Flowers, R. A., II; Hilmersson, G. Chem. – Eur. J. 2005, 11, 3279–3284. doi:10.1002/chem.200401320 |
| 34. | Taniguchi, Y.; Kuno, T.; Nakahashi, M.; Takaki, K.; Fujiwara, Y. Appl. Organomet. Chem. 1995, 9, 491–503. doi:10.1002/aoc.590090519 |
| 7. | Baruah, B.; Prajapati, D.; Sandhu, J. S. Tetrahedron Lett. 1995, 36, 6747–6750. doi:10.1016/00404-0399(50)1331-b |
| 8. | Kalyanam, N.; Rao, G. V. Tetrahedron Lett. 1993, 34, 1647–1648. doi:10.1016/0040-4039(93)85031-q |
| 20. | Shimizu, M.; Inayoshi, K.; Sahara, T. Org. Biomol. Chem. 2005, 3, 2237–2238. doi:10.1039/b505335h |
| 21. | Kumar, A.; Samuelson, A. G. Eur. J. Org. Chem. 2011, 951–959. doi:10.1002/ejoc.201001256 |
| 22. | Talukdar, S.; Banerji, A. J. Org. Chem. 1998, 63, 3468–3470. doi:10.1021/jo9716892 |
| 23. | Mangeney, P.; Tejero, T.; Alexakis, A.; Grosjean, F.; Normant, J. Synthesis 1988, 255–257. doi:10.1055/s-1988-27536 |
| 24. | Periasamy, M.; Srinivas, G.; Karunakar, G. V.; Bharathi, P. Tetrahedron Lett. 1999, 40, 7577–7580. doi:10.1016/s0040-4039(99)01609-3 |
| 25. | Periasamy, M.; Srinivas, G.; Suresh, S. Tetrahedron Lett. 2001, 42, 7123–7125. doi:10.1016/s0040-4039(01)01480-0 |
| 45. | Siu, T.; Li, W.; Yudin, A. K. J. Comb. Chem. 2001, 3, 554–558. doi:10.1021/cc0100159 |
| 43. | Tanaka, H.; Nakahara, T.; Dhimane, H.; Torii, S. Synlett 1989, 51–52. doi:10.1055/s-1989-34700 |
| 44. | Shono, T.; Kise, N.; Shirakawa, E.; Matsumoto, H.; Okazaki, E. J. Org. Chem. 1991, 56, 3063–3067. doi:10.1021/jo00009a026 |
| 53. | Asia premium flow chemistry system for modular efficiency in systems chemistry. http://www.syrris.com. |
| 19. | Mercer, G. J.; Sigman, M. S. Org. Lett. 2003, 5, 1591–1594. doi:10.1021/ol034469l |
| 48. | Capaldo, L.; Wen, Z.; Noël, T. Chem. Sci. 2023, 14, 4230–4247. doi:10.1039/d3sc00992k |
| 49. | Atobe, M.; Tateno, H.; Matsumura, Y. Chem. Rev. 2018, 118, 4541–4572. doi:10.1021/acs.chemrev.7b00353 |
| 50. | Elsherbini, M.; Wirth, T. Acc. Chem. Res. 2019, 52, 3287–3296. doi:10.1021/acs.accounts.9b00497 |
| 51. | Noël, T.; Cao, Y.; Laudadio, G. Acc. Chem. Res. 2019, 52, 2858–2869. doi:10.1021/acs.accounts.9b00412 |
| 52. | Cantillo, D. Curr. Opin. Electrochem. 2024, 44, 101459. doi:10.1016/j.coelec.2024.101459 |
| 47. | When the reaction was attempted with the diimines derived from the 2-pyridinecarboxaldehyde and the 3-pyridinecarboxaldehyde, it resulted in extensive decomposition of the starting materials. |
| 44. | Shono, T.; Kise, N.; Shirakawa, E.; Matsumoto, H.; Okazaki, E. J. Org. Chem. 1991, 56, 3063–3067. doi:10.1021/jo00009a026 |
| 46. | Cui, W.; Xu, X.; Zhang, C.; Wang, D.; Yang, Y.; Wang, Q.; Wang, J. J. Org. Chem. 2025, 90, 3659–3664. doi:10.1021/acs.joc.4c02994 |
| 44. | Shono, T.; Kise, N.; Shirakawa, E.; Matsumoto, H.; Okazaki, E. J. Org. Chem. 1991, 56, 3063–3067. doi:10.1021/jo00009a026 |
© 2025 Gazzotti et al.; licensee Beilstein-Institut.
This is an open access article licensed under the terms of the Beilstein-Institut Open Access License Agreement (https://www.beilstein-journals.org/bjoc/terms), which is identical to the Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0). The reuse of material under this license requires that the author(s), source and license are credited. Third-party material in this article could be subject to other licenses (typically indicated in the credit line), and in this case, users are required to obtain permission from the license holder to reuse the material.